A comparison of static and dynamic ∆B0 mapping methods for correction of CEST MRI in the presence of temporal B0 field variations
Abstract
Purpose
To assess the performance, in the presence of scanner instabilities, of three dynamic correction methods which integrate ∆B0 mapping into the chemical exchange saturation transfer (CEST) measurement and three established static ∆B0-correction approaches.
Methods
A homogeneous phantom and five healthy volunteers were scanned with a CEST sequence at 7 T. The in vivo measurements were performed twice: first with unaltered system frequency and again applying frequency shifts during the CEST acquisition. In all cases, retrospective voxel-wise ∆B0-correction was performed using one intrinsic and two extrinsic [prescans with dual-echo gradient-echo and water saturation shift referencing (WASSR)] static approaches. These were compared with two intrinsic [using phase data directly generated by single-echo or double-echo GRE (gradient-echo) CEST readout (CEST-GRE-2TE)] and one extrinsic [phase from interleaved dual-echo EPI (echo planar imaging) navigator (NAV-EPI-2TE)] dynamic ∆B0-correction approaches [allowing correction of each Z-spectral point before magnetization transfer ratio asymmetry (MTRasym) analysis].
Results
All three dynamic methods successfully mapped the induced drift. The intrinsic approaches were affected by the CEST labeling near water (∆ω < |0.3| ppm). The MTRasym contrast was distorted by the frequency drift in the brain by up to 0.21%/Hz when static ∆B0-corrections were applied, whereas the dynamic ∆B0 corrections reduced this to <0.01%/Hz without the need of external scans. The CEST-GRE-2TE and NAV-EPI-2TE resulted in highly consistent MTRasym values with/without drift for all subjects.
Conclusion
Reliable correction of scanner instabilities is essential to establish clinical CEST MRI. The three dynamic approaches presented improved the ∆B0-correction performance significantly in the presence of frequency drift compared to established static methods. Among them, the self-corrected CEST-GRE-2TE was the most accurate and straightforward to implement.
1 INTRODUCTION
High and ultrahigh static magnetic fields (B0) provide advantages that are critical for the quality of CEST MRI results. Not only the SNR, but also the chemical specificity are significantly improved, because the spectral separation between the resonances of interest increases. The CEST also benefits from a prolonged storage of saturation in bulk water due to longer T1, which facilitates its sensitivity. Furthermore, CEST works particularly well in the slow to intermediate exchange regime (ksw < ∆ωS, where ksw is the exchange rate from the labile proton pool to the bulk water pool and ∆ωS the solute proton pool frequency offset (which is proportional to B0)). Under this condition, spectral resonances of interest can be much better distinguished from the direct saturation of water. This regime is increasingly met at higher B0 even for rapidly exchanging protons.1, 2
On the other hand, local B0 inhomogeneities (∆B0) are more severe at higher B0, causing regionally dependent frequency shifts in Z-spectra. This complicates quantification in CEST. The applied frequency-selective CEST saturation pulses at ∆ωRF are shifted away from the targeted nominal frequency offset (∆ω) by δω = ∆ωRF - ∆ω = ∆B0/γ (γ being the gyromagnetic ratio), which is proportional to the local ∆B0.2 Even with optimized B0 shimming protocols prior to the experiments, a retrospective ∆B0 correction is generally needed, in which the Z-spectrum of each voxel is centered at the water resonance (0 ppm). A highly accurate ∆B0 correction is particularly important when studying exchanging compounds very close to water (e.g., glucose, lactate, myo-inositol).3-6 Zaiss et al. recently reported substantial pseudo-CEST effects for dynamic CEST at 3 T from B0 alterations (~1% per 7-Hz drift).7
The simplest approach for a ∆B0 correction is to determine the water resonance frequency from the Z-spectrum (from here on this approach is termed “CEST-minZ”).8-10 This works well only when the applied saturation power is low and the magnetization transfer contrast from semisolid macromolecules and CEST effect close to the water resonance can be neglected.8, 9, 11 In addition to this limitation, CEST-minZ requires the full sampling of a high-spectral-resolution Z-spectrum, which increases the scan time (i.e., the spectral range covering the water peak cannot be excluded). In vivo CEST measurements with higher saturation power and more accurate ∆B0 correction can be achieved by acquiring an external ∆B0 map. Water saturation shift referencing is probably the most widely used method to acquire such an external ∆B0 map for CEST data processing. In WASSR, a matching CEST pulse sequence is acquired as a prescan, but with low saturation power and targeting only a narrow range of offsets around 0 ppm with high spectral resolution.12 Alternatively, a prescanned ∆B0 map can be obtained from the phase difference of at least two gradient-echo images acquired at different echo times (here termed “GRE-2TE”).13-18
All these three established ∆B0 correction methods, (A) CEST-minZ, (B) WASSR, and (C) GRE-2TE, estimate the water resonance frequency for a single time point and correct every z-spectral point by applying the same shift (δω). This shift may not necessarily be representative for all points of the CEST spectrum, as they are acquired at different times. Consequently, all three methods share the drawback of being prone to errors due to temporal changes in the B0 field over the course of a CEST experiment. Such temporal ∆B0 fluctuations may arise from system instabilities [primarily heating of magnet’s gradient coils by heavy duty cycles19 or heating of passive shims20], from cardiac or respiratory effects21 or subject movement.22 Some previous studies performed at 3 T have reported drifts ranging from of 1.2 Hz/min to 5 Hz/min after a series of functional MRI or diffusion weighted imaging scans.23-25 Larger drifts are expected at higher B0 (e.g., at 7 T periodic B0 fluctuations of up to ~4 Hz due to respiration are already detectable even far away from the lungs).26-29
In this paper, three dynamic ∆B0 correction methods, which integrate the ∆B0 mapping as part of the CEST sequence, are proposed; two of them via the phase generated by the CEST readout itself and the third by an interleaved 2D EPI navigator. They allow temporal fluctuations in B0 to be mapped and compensated for each individual Z-spectral point separately. The accuracy of this B0 mapping and its impact on CEST correction are compared for these dynamic approaches and to three established static methods (i.e., CEST-minZ, WASSR, and GRE-2TE) for CEST analysis close to water.
2 THEORY
2.1 Chemical exchange in the presence of B0 inhomogeneities
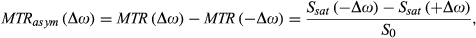 (1)
(1)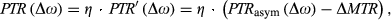 (2)
(2)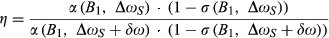 (3)
(3)2.2 Static ∆B0 correction
The three state-of-the-art static approaches for ∆B0 correction compared in this study are:
(A) CEST-minZ
(B) WASSR
(C) GRE-2TE
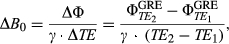 (4)
(4)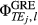 is the phase image acquired at echo time (j), by channel (l), in this case from the gradient-echo pre-scan (see Figure 1). For multichannel coils, the coil combination can be performed calculating the sum over the channels of the weighted channel-wise phase difference (i.e., the sum Hermitian-inner product)14:
is the phase image acquired at echo time (j), by channel (l), in this case from the gradient-echo pre-scan (see Figure 1). For multichannel coils, the coil combination can be performed calculating the sum over the channels of the weighted channel-wise phase difference (i.e., the sum Hermitian-inner product)14:
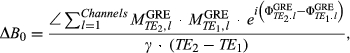 (5)
(5)
where  symbolizes the angle of the complex data,
symbolizes the angle of the complex data,  and
and  are the magnitude images, and
are the magnitude images, and 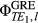 and
and 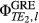 are the phase images for a given channel acquired at echo times TE1 and TE2.
are the phase images for a given channel acquired at echo times TE1 and TE2.
2.3 Dynamic ∆B0 correction
(D) CEST-GRE-2TE
(E) CEST-GRE-1TE
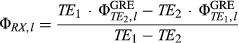 (6)
(6)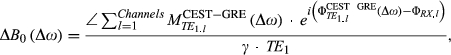 (7)
(7) and
and  are the magnitude and phase images per channel, acquired from the CEST readout at a single echo time for each frequency offset.
are the magnitude and phase images per channel, acquired from the CEST readout at a single echo time for each frequency offset.
(F) NAV-EPI-2TE
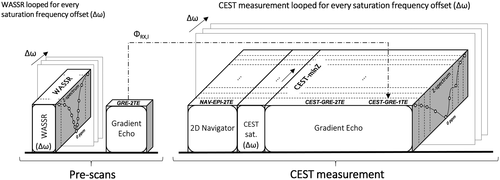
In contrast to using the intrinsic magnitude/phase information of the CEST-weighted images, the NAV-EPI-2TE method uses dual-echo data in Equation (5), which are additionally acquired before each saturation module via a 2D multishot EPI navigator (see Figure 1).
3 METHODS
The accuracy of all six ∆B0 correction methods and any possible bias on the intrinsic dynamic mapping methods (i.e., CEST-GRE-2TE and CEST-GRE-1TE) was first investigated in a homogeneous polydimethylsiloxane oil phantom (Siemens AG, Munich, Germany). In such a phantom, MTRasym should ideally be 0% in the absence of magnetization transfer (MT) from semisolid or CEST agents. Subsequently, each method was tested on five healthy volunteers (three males, two females; mean age 34 ± 4 years) after Ethics Committee approval by the Medical University of Vienna and informed consent was obtained.
All phantom scans were performed for a single slice with 1.7 × 1.7 × 6 mm3 spatial resolution over a field of view of 220 × 220 mm2 with a spectral resolution of 0.11 ppm. For the volunteer scans, the field of view was 270 × 270 mm2 with a resolution of 2.1 × 2.1 × 6 mm3, and the frequency offset increments were 0.15 ppm. Details on imaging parameters are listed in the following and in Table 1. Imaging parameters were matched wherever possible.
| ∆B0 mapping | TR [ms] | TE1 [ms] | TE2 [ms] | BW [Hz/Px] | k-space lines / shot | Miscellaneous | |
|---|---|---|---|---|---|---|---|
| STATIC | CEST-minZ | 9.5 | 1.74 | - | 780 | 1 | Ts = 700 ms, B1rms = 2.0 µT, ∆ω = 0.15/0.11 ppm |
| WASSR | 4.5 | 1. 74 | - | 780 | 1 | Ts = 100 ms, B1rms = 0.2 µT, ∆ω = 0.05/0.01 ppm | |
| GRE-2TE | 9.5 | 1.74 | 5.16 | 780 | 1 | ||
| DYNAMIC | CEST-GRE-2TE | 9.5 | 1.74 | 5.16 | 780 | 1 | Ts = 700 ms, B1rms = 2.0 µT, ∆ω = 0.15/0.11 ppm |
| CEST-GRE-1TE | 9.5 | 1.74 | - | 780 (390)* | 1 | Ts = 700 ms, B1rms = 2.0 µT, ∆ω = 0.15/0.11 ppm | |
| NAV-EPI-2TE | 15 | 5.4 | 9.0 | 2442 | 4 |
- * The experimentally used readout BW of 780Hz/Px stated here for CEST-GRE-1TE, could be in practice halved to 390Hz/Px, thereby matching the readout duration of two echoes of the CEST-GRE-2TE readout. Averaging these two echoes of the CEST-GRE-2TE readout with 780Hz/Px BW, should result in very similar SNR as the single CEST-GRE-1TE readout with 390Hz/Px BW.
3.1 Static ∆B0 corrections
- To minimize the sensitivity to temporal instabilities, the Z-spectral points were sampled with alternating saturation frequency offsets, decreasing from the maximum frequency to those close to water.
- For WASSR, a high spectral resolution with ∆ω steps of 0.05 ppm (≈15 Hz) over a frequency range of ±0.8 ppm was chosen, resulting in a total acquisition time of <4 min for in vivo and ∆ω = 0.01 ppm (≈3.6 Hz) with a total acquisition time <6 min for phantom measurements.
- For GRE-2TE, the prescan was acquired in <1 s. To prevent possible phase errors from mistiming of readout gradients and the acquisition, both echoes were sampled under gradients of the same polarity (a.k.a. “monopolar”).
3.2 Dynamic ∆B0 corrections
- For CEST-GRE-2TE, the more commonly used single-echo readout of the CEST sequence was replaced by two readouts with doubled receiver bandwidth (e.g., 780 Hz/Px instead of 390 Hz/Px).
- The CEST-GRE-1TE could be acquired with no change to the conventional single-echo readout of the CEST sequence. However, to prevent bias in the comparison, data from TE2 of the GRE readout post CEST labeling were simply ignored and the ∆B0 mapping used only data from TE1. The required coil offset maps, which were assumed to be time-invariant,39, 40 were obtained from the GRE pre scan.
- For NAV-EPI-2TE, a navigator was placed before each CEST-labeling module as shown in Figures 1 and 2. The number of k-space lines (i.e., echoes) collected in each shot of the EPI navigator was set to 4 to minimize geometric distortions and a readout bandwidth of 2442 Hz/Px used to reduce fat-water chemical shift to 0.4 mm, resulting in a ~1-s navigator duration. This could be further shortened by increasing the number of k-space lines per shot (e.g., from 4 up to 128). The delay between the ∆B0 mapping with the navigator and CEST data sampling illustrated in Figure 2 was assumed to be negligible.
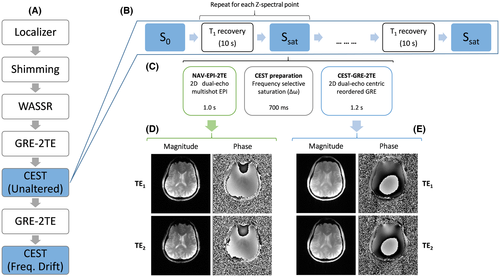
3.3 Measurement protocols
All experiments were performed on a whole-body 7 T MR Magnetom system (Siemens Healthcare, Erlangen, Germany) with a 1H 32-channel head coil (Nova Medical, Wilmington, Massachusetts, USA). As illustrated in Figure 2A, after localization and B0 shimming, three prescans were performed: 1) One WASSR measurement, in which the magnetization preparation was performed using one saturation pulse (B1rms = 0.2 µT) and 2) two monopolar dual-echo gradient-echo scans (duration <1 s), one prior to each CEST measurement, which generated magnitude and phase images for each channel and echo time.
To investigate the performance of the dynamic ∆B0 correction methods versus the static ones in the presence of scanner instabilities, a scan-rescan experiment was defined. The first CEST acquisition was performed with no deliberate changes to the imaging system, while in the second one a linear frequency drift of 60 Hz [consistent with previously reported drifts up to 5 Hz/min at 3 T 23-25]) was induced over the duration of the scan. The sequence was modified to apply a drift by updating the reference frequency in the analog-to-digital converters (ADCs) blocks of the navigator and CEST readouts for each ∆ω loop.
Each CEST scan (of duration 13 min) comprised three blocks (Figure 2): 1) a multishot EPI navigator with dual-echo readout “NAV-EPI-2TE”; followed by 2) the CEST-labeling module; and 3) a train of monopolar dual-echo gradient-echo readouts that covered the entire k-space in one step (i.e., “CEST-GRE-2TE”). A subsequent delay of 10 s ensured T1 recovery of the water signal between acquisitions of different Z-spectral points. The CEST labeling was executed by a train of four Gaussian pulses of 100-ms duration, duty cycle of 50%, and B1rms = 2.0 µT. To study the bias of inaccurate ΔB0 correction (which should be particularly strong closer to water) 61 spectral offsets were equidistantly distributed in the range from −4.5 ppm to 4.5 ppm (−4.5 ppm, +4.5 ppm, −4.35 ppm, +4.35 ppm, …, 0 ppm) for in vivo and 41 spectral offsets from −2.2 ppm to 2.2 ppm for phantom measurements. Magnitude and phase images were saved separately for each channel and echo.
3.4 Data analysis
All MR images were saved in DICOM format and data processing and evaluation were conducted retrospectively with MATLAB (R2017b, MathWorks, Natick, MA USA).
The resonance frequency of bulk water was determined voxel-wise for the static methods CEST-minZ and WASSR, as the minimum of the spline-interpolated z-spectra,9 and by least squares Lorentzian fitting [MATLAB function b0wassr.m available at http://godzilla.kennedykrieger.org/CEST/], respectively. For the ∆B0 mapping methods (C)–(F) phase images from different coils were combined by applying Eqs. 5-7 and subsequently unwrapped via fast 2D phase unwrapping41 available at https://github.com/mfkasim91/unwrap_phase/. The phase offset maps required for the method CEST-GRE-1TE were masked and smoothed using a discretized spline smoother [MATLAB function smoothn.m42] to provide reliable results even at the brain’s boundaries, as has been shown previously for coil combination and distortion correction.40, 43-45 Finally, as the ∆B0 maps were not masked, they were smoothed by a spatial Hamming filter before being used for CEST correction.
Each pair of magnitude images (i.e., from the CEST double-echo readout) were averaged and ∆B0 correction and subsequent MTRasym analysis [Equation (1)] were performed voxel-wise. For static ∆B0 correction methods (A)–(C), the estimated frequency shift δω was applied to center the entire Z-spectra to 0 ppm, whereas for the dynamic methods (D)–(F) a time-dependent δω(t) was applied to correct each Z-spectral point independently.
To evaluate the B0 estimation performance between different methods, a region of interest (ROI) was defined to compute ROI-averaged B0 and MTRasym curves (Figure 6). To evaluate the effect of ∆B0 on the MTRasym maps, a ROI was manually drawn along phantom and volunteer’s brain boundaries and MTRasym mean and standard deviation were derived from voxels contained within these ROIs.
4 RESULTS
The accuracy of dynamic B0 estimation via CEST-GRE-2TE and CEST-GRE-1TE was compromised when the CEST-labeling pulses were applied close to water. Figure 3 shows how the ∆B0 maps of phantom experiments were apparently corrupted by saturation RF trains applied at ∆ω < |0.33| ppm. On the other hand, NAV-EPI-2TE presented unaffected ∆B0 maps over the whole saturation ∆ω range.
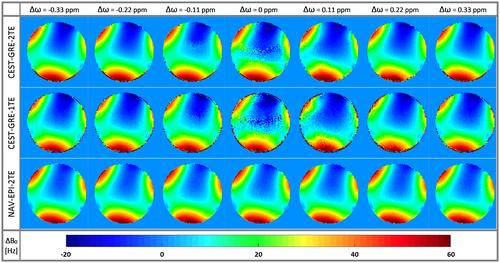
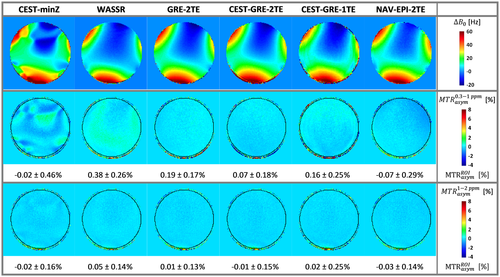
Figure 4 presents the accuracy of ∆B0 maps for the different correction methods by evaluating deviations from the 0% MTRasym that was expected for a phantom containing no MTC or CEST agents. The GRE-2TE generated the most homogeneous MTRasym map (i.e., although comparable to WASSR, the one that had the lowest spatial variability) among the static methods with minimal offset from 0% MTRasym compared to WASSR. Among the dynamic methods, CEST-GRE-2TE resulted in the most accurate MTRasym maps, with spatial variability similar to GRE-2TE. The methods, CEST-GRE-1TE and NAV-EPI-2TE, on the other hand, led to slight spatial gradients in MTRasym maps, and ~40% to 60% higher variability than for CEST-GRE-2TE. The third row of Figure 2 illustrates that most of these differences become negligible for ∆ω = ±[1–2] ppm.
For in vivo experiments, MT effects and other confounding factors cannot be neglected, so ∆B0 mapping accuracy cannot be evaluated. However, rows 2 to 4 of Figure 5 show how the differences between correction methods gradually diminish the further the integration ranges ∆ω are from the water resonance. This indicates that in vivo CEST is sensitive to ∆B0 over a wider range of ∆ω than the phantom experiments. The ∆B0 corrections via static methods were generally inferior to those using the dynamic methods for ∆ω = ±[0.3–1.0] ppm (Figure 5). The CEST-GRE-1TE slightly underestimated B0 compared to CEST-GRE-2TE, thereby producing artificial MTRasym increases, while the MTRasym maps corrected by NAV-EPI-2TE showed a gradient from posterior left to anterior right direction (consistent with the phantom experiments).
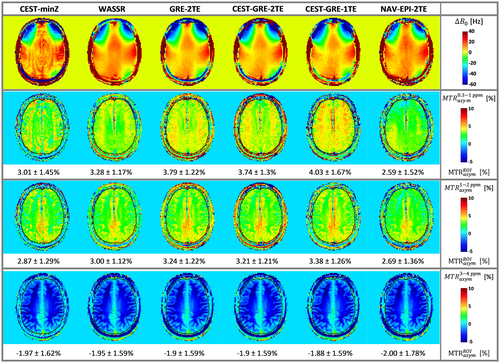
Figure 6 shows the variation in B0 over time and the effect of this on the corrected MTRasym curves within a ROI placed in a white matter (WM) region of volunteer V3, for both minimum field change and the imposed frequency drift. The dynamic mapping methods successfully followed the B0 evolution and corrected each Z-spectral point independently before the MTRasym analysis. All static methods, on the other hand, resulted in severely underestimated (~1/3 for CEST-minZ) or overestimated (~3 times for WASSR and GRE-2TE) MTRasym values.
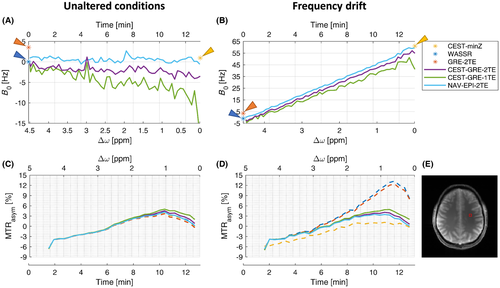
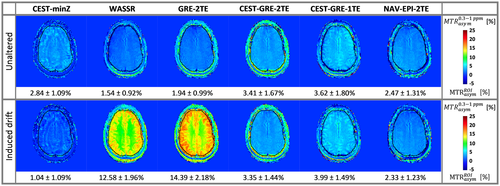
The ΔB0-corrected color-coded MTRasym maps derived from the scan-rescan experiment are presented in Figure 7 for volunteer V4. For the CEST sequence with induced frequency drift, the MTRasym contrast after ΔB0 correction via CEST-minZ was underestimated, while WASSR and GRE-2TE ΔB0 correction led to the opposite effect. On the contrary, all dynamic methods compensated for this drift efficiently. The CEST-GRE-2TE and NAV-EPI-2TE achieved MTRasym maps with the most similar contrasts with and without induced frequency drift (ROI-averaged errors of 0.001%/Hz and 0.002%/Hz drift, respectively), while CEST-GRE-1TE showed slightly higher deviation (e.g., error of 0.006%/Hz), but was still superior to all the static approaches.
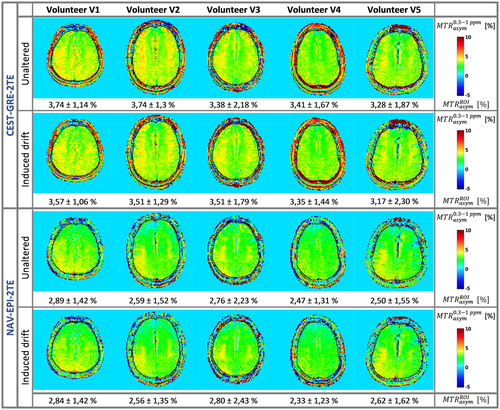
The results of the comparison among all five healthy volunteers (V1-V5) for the dynamic ΔB0 correction methods CEST-GRE-2TE and NAV-EPI-2TE are presented in Figure 8. The MTRasym maps derived by CEST-GRE-2TE and NAV-EPI-2TE were highly consistent between acquisitions with/without artificially induced frequency drift for all subjects.
5 DISCUSSION
In this study, we have investigated the applicability of three dynamic ∆B0 mapping methods for correction of CEST, which integrate ∆B0 mapping in the CEST measurement. In contrast to static correction methods, each Z-spectral point can be adjusted independently to compensate for temporal B0 changes such as those arising from system instabilities. Static and dynamic correction performance were evaluated and compared focusing on the ΔB0 mapping accuracy and bias of MTRasym maps in the human brain at 7 T, first in the absence and then in the presence of a frequency drift.
Previous studies have evaluated the accuracy of static ∆B0 correction of CEST-weighted maps by multiecho methods,15-18 while studies proposing dynamic methods to correct for temporal ∆B0 changes have only recently emerged.46, 47
Windschuh et al. proposed a method to correct each Z-spectral point independently retrospectively, combining phase images from a single-echo GRE CEST readout and a prescan to calculate relative ∆B0 maps (WASABI).46 However, development work is still needed to improve the stability of this approach, which was significantly affected by the RF saturation pulses even at ∆ω ≈ 1 ppm from the water resonance. In contrast, the intrinsic dynamic correction methods we propose here (CEST-GRE-2TE and CEST-GRE-1TE) were affected by the CEST labeling only within a narrow range of ∆ω < |0.3| ppm, most likely because of low SNR of the saturated CEST images.
Windschuh et al. estimated the error of the MTRasym (1.1 ppm) to be on average 0.18%/Hz drift. This is in good agreement with the deviations of MTRasym (0.3–1.1 ppm) between acquisitions with and without induced B0 drift found in our study (e.g., 0.21%/Hz and 0.18%/Hz corrected by WASSR and GRE-2TE, respectively). The spatial inhomogeneity cannot be directly compared, since the surface of the ROI over the measured cartilage on the knee in the study by Windschuh et al. was much smaller than that covered here, in the brain.
Simegn et al. proposed a prospective motion and ∆B0 correction of glycoCEST by updating the zero-order and first-order shim gradients for each CEST offset acquisition using a 3D version similar to that of our navigator.47 However, they did not show any ∆B0 map or provide any information about the remaining local inhomogeneities of higher than first-order within the CEST volume after correction, and hence did not attempt to apply any further postprocessing steps.
The metric MTRasym, which is highly sensitive to frequency shift errors close to the water resonance,12 has been used to assess the quality of the ∆B0 correction methods in a similar way to the previously proposed Symmetric Analysis of Z-Spectra (SAS).48 The static GRE-2TE and the dynamic CEST-GRE-2TE methods resulted in MTRasym maps with lowest spatial variability and mean values closest to 0% for the phantom experiments. This indicates that these two methods lead to the most accurate ∆B0 correction among the static and dynamic approaches.
The in vivo results showed comparable corrected CEST-weighted signal distribution between the static methods GRE-2TE and WASSR. In contrast, the dynamic methods showed the following divergences: 1) the MTRasym maps corrected by GRE-CEST-2TE achieved similarly homogeneous distribution to the static methods; and 2) corrections performed by CEST-GRE-1TE and NAV-EPI-2TE resulted in an overall positive MTRasym offset and a slight spatial gradient in the anterior-posterior direction (frequency encoding direction) relative to GRE-CEST-2TE, respectively. These effects could arise from delays of the applied gradients and could be corrected by acquiring the same scan with opposite image readout orientation.49, 50
The scan-rescan experiment revealed very high consistency between ΔB0-corrected MTRasym maps with and without frequency drift for all subjects when using GRE-CEST-2TE and NAV-EPI-2TE for all volunteers. The B0 estimation by GRE-CEST-1TE corrected the frequency drift, but less efficiently than the other two dynamic methods. Further investigations would be necessary to identify the source of this deviation; however, the known nonlinear phase evolution in white matter (due to specific tissue microstructure) could be a potential contributor.51
In our study, the accuracy of multiecho ∆B0 mapping was dependent not only on the ∆TE between the two echoes, as reported previously,16 but also on the actual values set for each TE and their receiver bandwidths. We had to optimize the TE settings experimentally to eliminate erroneous B0 offsets and spatially linear B0 gradients (mostly in the readout encoding direction) in phantoms prior to the CEST experiments. For routine use, it will be important to achieve accurate ∆B0 mapping for any CEST sequence setting without previous optimization. For similar reasons we also used only monopolar readout gradients, although GRE-CEST-2TE and GRE-2TE should benefit from bipolar readout gradients. We have also refrained from performing additional corrections for any other confounding effects such as motion, B1 inhomogeneities, semisolid MT, water relaxation, T2-dependent spillover or nuclear Overhauser enhancement exchange to prevent introducing factors that could make it difficult to isolate B0-related effects.52-60 Of course these should be used when applying accurate CEST quantification in (patient) studies.
In the future, the proposed dynamic methods could be additionally combined with real-time motion correction by extending the navigator to 3D as previously applied in MRI and MRSI.47, 52-55 Thereby, artifacts due to motion and B0-instabilities could be simultaneously mitigated. Although not investigated here, other CEST quantification routines such as Lorentzian or Bloch fitting61-63 would also be likely to benefit from the presented dynamic ∆B0 correction, since B0 is usually included as a fitting parameter.
6 CONCLUSION
We have presented three dynamic ∆B0 correction methods for CEST MRI that successfully mapped and compensated for B0 changes for each individual Z-spectral point. Improved correction performance in the presence of frequency drift was demonstrated by comparison with established static approaches. Among them, the self-correcting properties of CEST-GRE-2TE made it the most reliable and easiest to implement. Implementation of an interleaved navigator (NAV-EPI-2TE) was more complicated, but allowed improved dynamic ∆B0 correction even close to water, but not better than CEST-GRE-2TE for typically investigated frequency ranges.
Dynamic B0 corrections for CEST are another important step toward more reliable clinical CEST MRI without the need for (lengthy) prescans or for acquisition of additional Z-spectral points near water (as required for CEST-minZ), lending itself particularly to dynamic CEST MRI.
ACKNOWLEDGMENTS
This study was supported by the Austrian Science Fund (FWF): KLI-718, the Austrian National Bank (OeNB Jubiläumsfonds) under Grant 16911, and the Christian Doppler Laboratory for Clinical Molecular MR Imaging. Barbara Dymerska was additionally supported by a Marie Skłodowska-Curie Individual Fellowship. We thank Vladimir Mlynarik for his invaluable advice.




