Human cardiac 31P magnetic resonance spectroscopy at 7 tesla
Abstract
Purpose
Phosphorus magnetic resonance spectroscopy (31P-MRS) affords unique insight into cardiac energetics but has a low intrinsic signal-to-noise ratio (SNR) in humans. Theory predicts an increased 31P-MRS SNR at 7T, offering exciting possibilities to better investigate cardiac metabolism. We therefore compare the performance of human cardiac 31P-MRS at 7T to 3T, and measure T1s for 31P metabolites at 7T.
Methods
Matched 31P-MRS data were acquired at 3T and 7T, on nine normal volunteers. A novel Look-Locker CSI acquisition and fitting approach was used to measure T1s on six normal volunteers.
Results
T1s in the heart at 7T were: phosphocreatine (PCr) 3.05 ± 0.41s, γ-ATP 1.82 ± 0.09s, α-ATP 1.39 ± 0.09s, β-ATP 1.02 ± 0.17s and 2,3-DPG (2,3-diphosphoglycerate) 3.05 ± 0.41s (N = 6). In the field comparison (N = 9), PCr SNR increased 2.8× at 7T relative to 3T, the Cramer-Ráo uncertainty (CRLB) in PCr concentration decreased 2.4×, the mean CRLB in PCr/ATP decreased 2.7× and the PCr/ATP SD decreased 2×.
Conclusion
Cardiac 31P-MRS at 7T has higher SNR and the spectra can be quantified more precisely than at 3T. Cardiac 31P T1s are shorter at 7T than at 3T. We predict that 7T will become the field strength of choice for cardiac 31P-MRS. Magn Reson Med 72:304–315, 2014. © 2013 The Authors. Magnetic Resonance in Medicine Published by Wiley Periodicals, Inc. on behalf of International Society of Medicine in Resonance. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Phosphorus magnetic resonance spectroscopy (31P-MRS) makes a unique and valuable contribution to our understanding of metabolism 1-4. Applied in the human heart 5, 31P-MRS reveals the biochemistry of ATP, ADP, and phosphocreatine (PCr), which are critical to the supply of energy for contractile work in the myocardium. For example, derangement of the ratio of concentrations of PCr to ATP measured by 31P-MRS (“the PCr/ATP ratio”) predicts mortality 2; diminution of the creatine-kinase flux is seen in patients with myocardial infarction 6; and changes in the PCr/ATP concentration ratio during pharmacological stress are associated with disease 7. However, clinical applications 8 of 31P-MRS have yet to see widespread acceptance, principally as a result of the method's low intrinsic signal-to-noise ratio (SNR) (31P-MRS has ∼10−5x lower
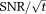 than 1H MRI). This has meant that scan times of ∼30 min have been necessary to obtain the important metabolic insights listed above. Even a relatively modest increase in 31P-MRS SNR could bring scan times below 10 min and make the method significantly more suitable for clinical studies. Increases in SNR may also permit single-subject comparisons where grouped analysis has previously been required.
than 1H MRI). This has meant that scan times of ∼30 min have been necessary to obtain the important metabolic insights listed above. Even a relatively modest increase in 31P-MRS SNR could bring scan times below 10 min and make the method significantly more suitable for clinical studies. Increases in SNR may also permit single-subject comparisons where grouped analysis has previously been required.
Theory predicts that the quality of the raw 31P-MRS signal (
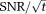 ) increases approximately in proportion to the scanner's magnetic field strength B0 9, 10. Increasing B0 thus offers the potential for significant increases in 31P-MRS performance, so long as the anticipated gains in 31P Boltzmann equilibrium magnetization and radiofrequency (RF) receive sensitivity outweigh any exacerbation of the effects of B0-inhomogeneity, RF-induced heating, increased RF thermal noise at the higher field strength and the increased RF power needed to excite the same chemical shift range by the same flip angle 11. At 3T, 31P-MRS typically takes ∼30 min to acquire metabolite concentrations in the interventricular septum 12. The spectral SNR at 3T is 2.1× greater than was the case using equivalent methods at 1.5T 13, which has driven a move from 1.5T to 3T for cardiac 31P-MRS in recent years. Yet, even at 3T, wider application of 31P-MRS has remained seriously constrained by its limited SNR.
) increases approximately in proportion to the scanner's magnetic field strength B0 9, 10. Increasing B0 thus offers the potential for significant increases in 31P-MRS performance, so long as the anticipated gains in 31P Boltzmann equilibrium magnetization and radiofrequency (RF) receive sensitivity outweigh any exacerbation of the effects of B0-inhomogeneity, RF-induced heating, increased RF thermal noise at the higher field strength and the increased RF power needed to excite the same chemical shift range by the same flip angle 11. At 3T, 31P-MRS typically takes ∼30 min to acquire metabolite concentrations in the interventricular septum 12. The spectral SNR at 3T is 2.1× greater than was the case using equivalent methods at 1.5T 13, which has driven a move from 1.5T to 3T for cardiac 31P-MRS in recent years. Yet, even at 3T, wider application of 31P-MRS has remained seriously constrained by its limited SNR.
Whole-body 7T MRI scanners with 31P capability have recently become available commercially, leading several groups to pioneer methods for cardiac MR at 7T (see Moser et al for a review) 14. Theory predicts that cardiac 31P-MRS will show a further dramatic (approximately 2.3×) increase in
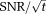 compared with the performance available today at 3T. This gain in
compared with the performance available today at 3T. This gain in
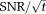 could permit a reduction in scan times allowing the study of dynamic processes; or it could lower variability to allow reliable individual subject comparisons; or it could give sufficient spatial resolution to study focal disease; or it could make visible metabolites that cannot be detected at lower fields, such as inorganic phosphate. With a growing range of techniques having been demonstrated for 7T cardiac magnetic resonance 14, it is now timely to give a proof-of-principle for 7T human cardiac 31P-MRS and to test whether 7T 31P-MRS truly delivers this predicted data quality in normal volunteers.
could permit a reduction in scan times allowing the study of dynamic processes; or it could lower variability to allow reliable individual subject comparisons; or it could give sufficient spatial resolution to study focal disease; or it could make visible metabolites that cannot be detected at lower fields, such as inorganic phosphate. With a growing range of techniques having been demonstrated for 7T cardiac magnetic resonance 14, it is now timely to give a proof-of-principle for 7T human cardiac 31P-MRS and to test whether 7T 31P-MRS truly delivers this predicted data quality in normal volunteers.
We also introduce a novel Look-Locker inversion recovery (IR) chemical shift imaging (CSI) pulse sequence and associated analysis methods to determine the longitudinal relaxation times (T1s) of 31P-containing metabolites for the first time in the heart at 7T. These T1s are required to correct for the effects of partial saturation during postprocessing of data in the main comparison study. We compare the 31P T1s at 7T with literature values at lower field strengths; and we discuss the origins of the changes we observe.
Thus, we aim to demonstrate a proof-of-principle of 7T human cardiac 31P-MRS, to compare the performance at 7T against an established protocol at 3T, and to characterize the longitudinal relaxation times of 31P-containing metabolites in the heart at 7T.
METHODS
Materials
Scans at 3T used a Trio MRI scanner (Siemens, Germany) equipped with a 10-cm 31P Tx/Rx loop coil (PulseTeq, UK). Scans at 7T used a Magnetom 7T scanner (Siemens, Germany), with a T/R switch and preamplifier module (Virtumed, MN), and a purpose-built 10-cm 31P Tx/Rx loop coil whose housing has the same subject-coil distance as the 3T coil. Localizer images were acquired at 7T with a separate 10-cm 1H Tx/Rx loop coil (Rapid Biomedical, Germany).
The B1+ profiles and receive sensitivities of the two 31P coils were measured using a purpose-built cuboidal phantom comprising 18 L of saline surrounding a height-adjustable 2 × 2 × 2-cm3 cube of KH2PO4(aq) 15. At a depth of 10 cm [approximately that of the heart 16] and at maximum voltage in vivo, B1+ was 15.5 μT for 222 V at 3T compared with a B1+ of 11.1 μT for 270 V at 7T (see Supplementary Information Figs. SI1–3 for details, which are available online). Both coils were fitted with a 31P fiducial reference, comprising an 18-mm outer diameter plastic sphere (The Precision Plastic Ball Company Ltd, UK) filled with a solution of phenylphosphonic acid (PPA) and chromium (III) acetylacetonate [Cr(acac)3] dissolved in ethanol and sealed with epoxy resin. The concentration of Cr(acac)3 was adjusted until initial calibration inversion recovery experiments gave T1 ≈500 ms. Both coils also had position markers: 4× cod liver oil capsules at 3T and, at 7T, 2× further plastic spheres of PPA but dissolved in acetone instead to alter the 31P chemical shift 17 so as not to interfere with the fiducial reference signal. To confirm that the receive SNR performance of the two coils was comparable, sets of 90° free induction decays (FIDs) with TR ≫ T1 were acquired from the cube phantom with the KH2PO4 source at the same depth (shown in Figure SI4).
Before scanning human subjects, RF heating due to the 31P loop coil was calculated following the procedure recommended by the manufacturer (i.e., the procedure in section 4.2.1.2.2 of the document entitled “SAR Parameter N4 B 03” supplied by Siemens.): the quasi-static approximation for a semi-infinite volume of uniformly conductive material was used to determine the ratio of input power to maximum local specific absorption rate (SAR) over a 10-cm3 region. This k-value of 5 kg−1 (i.e., input in W → SAR in W kg−1) was also checked against manufacturer-supplied data for coils of a similar geometry. Finally, following an established protocol 18, these SAR calculations were validated on a meat phantom using fiber-optic temperature probes (Neoptix, UK) to ensure compliance with IEC guidelines during human scans 19.
Basic Protocol
Normal volunteers were recruited and scanned in compliance with ethical and legal requirements. Spectroscopy acquisitions used the ultra-short-TE chemical shift imaging (UTE-CSI) pulse sequence 13, 20 shown in Figure 1. The protocol and sequence parameters were adapted to accommodate the more restrictive limits on peak B1+ and SAR at 7T, starting from what has been used for several years at 3T 13.
To facilitate the exchange of 1H and 31P coils at 7T, subjects were scanned supine at both field strengths. Spectroscopy sequences used no gating to avoid the possibility of bias due to increased mis-triggering at 7T 21. “Tune up” shim settings were used for 31P spectroscopy at both field strengths.
Localization at 3T was performed with bSSFP and CINE images acquired using the body coil. However, 7T scanners do not have built-in body coils, so localization at 7T was performed using a separate 10-cm 1H loop surface coil to record CINE FLASH images with pulse oximeter gating (Siemens, Germany). The 1H coil was then removed and replaced with the 10-cm 31P loop coil in the same position above the interventricular septum.
At the start of each 31P scan, the 31P coil was adjusted (match at 3T, tune and match at 7T) for each subject using an RF Sweeper (Morris Instruments Inc., Ottowa, Ontario, Canada) with the coil in situ on the subject. Then, a set of nonlocalized inversion recovery FIDs and a set of images (1H bSSFP over 21 slices at 3T, 31P FLASH projection images covering three orthogonal planes at 7T) were recorded. From these, the coil location, transmit efficiency (i.e., reference voltage at the primary fiducial) and the coil's B1 spatial profile were computed with custom Matlab (MathWorks, Natick, MA) code. Sample output from this code in phantoms is shown on the left of Figures SI2 and SI3.
T1 Determination
The primary focus of this work is to compare the performance of 31P-MRS at 7T against 3T. Yet before we can analyze 7T spectra, we need first to determine T1s of 31P-containing metabolites in the heart at 7T.
Matlab calculations using calibrated B1+ values demonstrated that the conventional dual-angle method (DAM) 22 would not be appropriate at 7T because B1+ is insufficient to generate acceptably short BIR-4 or BIRP pulses, and because B1+ is too inhomogeneous to use hard pulses. Therefore, we created the following novel spectroscopy Look-Locker inversion recovery pulse sequence with adiabatic inversion pulses and chemical shift imaging (CSI) localization.
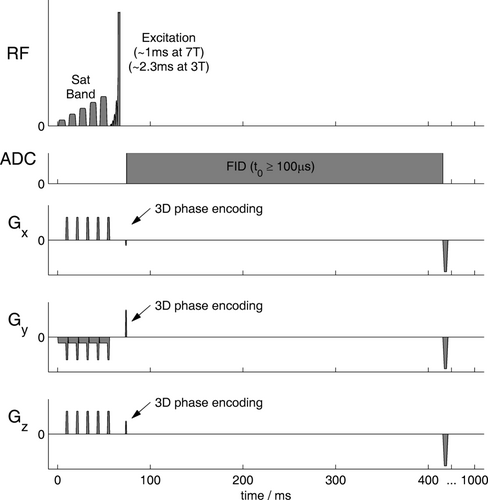
Keeping the excitation pulse and three-dimensional (3D) acquisition-weighted k-space sampling strategy from the UTE-CSI sequence (Fig. 1), we extended each TR to include a 4th dimension: inversion time TI. To prepare the magnetization, we added an adiabatic inversion pulse, with parameters optimized in Matlab, and a spoiler gradient. For readout, we acquired data with the same phase encoding and flip angle at TI = 50 ms and at 20× 656 ms intervals thereafter following a Look-Locker scheme 23. We then left a 5.2-s gap to allow magnetization recovery 24, followed by three additional excitations. Pulse voltages were set to run at the SAR limit, prioritizing effective inversion subject to adequate readout SNR. This “LL-CSI” pulse sequence is summarized in Figure 2. The acquisition-weighted k-space sampling pattern is illustrated in Figure SI6.
The LL-CSI sequence was validated by Monte Carlo simulation (see Figure SI8), in phantoms (see Figure SI9) and in human calf muscle where the T1s at 7T are known 25, 26. Validation data were acquired from the calf muscle of two subjects (male, 21 and 31 years, 70 and 85 kg, 21.6 and 22.8 body mass index [BMI]) over a transverse 6 × 6 × 4 matrix (4 in the HF direction) and a 180 × 180 × 200-mm3 FOV, using acquisition weighting with three averages at k = 0. Data were interpolated to an 8 × 8 × 8 matrix by zero-filling in k-space during reconstruction. The pulse voltages were 250 V for inversion and 50 V (15° nominal FA) for readout. The inversion pulse was a 29.7 ms HS8 27 with time-bandwidth product R = 18. To cover all metabolites in a total of 34 min, we performed five repetitions with inversion centered at 836, 47, −566, −1339, and −1589 Hz relative to the readout central frequency, i.e., 586 Hz from PCr. (In units of chemical shift relative to PCr = 0 ppm, these frequencies are equal to +2.1, −4.5, −9.6, −16.0, and −18.1 ppm.)
In the heart, data were acquired from 6 subjects (male, 22–43 years, 68–87 kg, BMI 20.3–26.9) over a transverse 8 × 8 × 6 matrix (6 in the HF direction) over a 240 × 240 × 200-mm3 FOV, using acquisition weighting with four averages at k = 0, interpolated to an 8 × 8 × 8 matrix by zero-filling in k-space. The inversion pulse was a 29.7 ms HS8 with R = 24. This long pulse was used to permit inversion of each metabolite despite the considerably weaker B1+ at the heart. The pulse voltages were 160 V for inversion and 200 V (15° nominal FA) for readout. A saturation band was placed in the coronal plane to suppress signal from skeletal muscle. The saturation voltage was maximized subject to SAR limits, typically at 40 V. In a total of 81 min for each subject, we performed two repetitions with inversion centered at 774 Hz and −827 Hz relative to the readout central frequency, i.e., 586 Hz from PCr. (In units of chemical shift relative to PCr = 0 ppm, these frequencies are equal to +1.6 and −11.7 ppm.)
For each subject, the mid-septal voxel in the most basal short-axes plane showing the papillary muscles was chosen for further analysis. Spectra from that voxel at every TI and from every acquisition on that subject were fitted simultaneously, using the Matlab “lsqcurvefit” routine, to a model function comprising the Fourier transform of a Bloch simulation of the system. The model includes exact RF pulse waveforms and exact timings for RF and ADC events. To remain tractable, the model assumes: that there is perfect spoiling after the inversion pulse and after each readout; that the signal arises from a single point; that J-couplings may be treated with separate peaks that are constrained to have the appropriate relative amplitude and frequency differences for each multiplet component; and that B0-inhomogeneity for all the peaks is equivalent to a single exponential damping term exp(-t/ T2*). In summary, the model has the following adjustable parameters: T1, T2, M0 and Δν for each of PCr, γ-ATP, α-ATP, β-ATP, and either Pi (leg) or 2,3-DPG (2,3-diphosphoglycerate) (heart) (except PCr where Δν is fixed at 0 Hz); a global T2* exponential damping factor; a global phase; and a global B1+ (Hz V−1) factor. The two peaks from 2,3-DPG have separate variable frequency offsets Δν but are constrained to have identical T1, T2 and M0. Fitting required ∼30 min on a Dell Precision T1500 desktop computer with an Intel Core i7 quad-core CPU and 16 GB RAM. Pseudo-code for this algorithm is given in the Supplementary Information.
Field-Strength Comparison
The performance of 31P-MRS at 3T and 7T was then compared in a paired study on nine normal volunteers (male, 22–53 years, 52–88 kg, BMI 19.6–28.7). For each subject, both scans were performed in mornings and within 10 days, to minimize physiological variation. Spectra were recorded using the UTE-CSI pulse sequence (Fig. 1) with a 16 × 16 × 8 matrix, 15 × 15 × 25-mm3 (i.e., 5.6 mL) nominal voxel size, acquisition weighting with 10 averages at k = 0 and TR = 1 s. At 7T, excitation was always at the full power supported by the coil (270 V) giving a flip angle of ∼20° in the inter-ventricular septum. At 3T, flip angles were matched approximately to those at 7T using the subject-specific B1+ maps (akin to the left of Figures SI2 and SI3). Excitation was centered at 250 Hz (i.e., 5.0 ppm) relative to PCr at 3T to ensure a uniform flip angle from 2,3-DPG (at ∼6 ppm) to β-ATP (at ∼−16 ppm) 10. Excitation pulses at 7T were adapted from 3T by reducing their duration by the ratio of field strengths (6.96T / 2.89T = 2.41×) and centering now at 586 Hz (i.e., 4.9 ppm) relative to PCr. A 25-mm-thick saturation band was placed to suppress signal from skeletal muscle in the anterior chest wall. Given the surface coil's inhomogeneous B1+, we implemented a BISTRO-style saturation scheme 28 comprising 5× HS8 pulses with pulse duration Tp = 8 ms and time-bandwidth product R = 11 27 whose amplitude ramped linearly up to a final pulse at ∼70 V. The exact voltage was set to the maximum permissible given the SAR limits for each subject. The HS8 excitation pulse R and Tp were selected to minimize the chemical shift displacement artifact while still giving effective saturation. Bloch simulations, shown in Figure SI7, give a ∼4 kHz bandwidth for the HS8 excitation pulse. Thus, for a saturation band with 25-mm nominal thickness, this equates to a chemical shift displacement of ± 8 mm for Pi and β-ATP.
After each scan, the following analysis was performed using a purpose-made Matlab program. First, the voxel lying at the centre of the interventricular septum (see Figure 4) was extracted for analysis. The spectrum there was fitted using the AMARES 29 implementation in jMRUI v4 30, together with prior knowledge specifying 11 Lorentzian peaks (α,β,γ-ATP multiplet components, PCr, PDE, and 2× 2,3-DPG) and fixed amplitude ratios and scalar couplings for the multiplets. The fitted amplitudes were then corrected for blood contamination by subtracting 30% of the average of the two 2,3-DPG signals from each of the ATP amplitudes 31. The remaining PCr and ATP signals were corrected for the effects of partial saturation 32 using the flip angle at the centre of the voxel, assuming no motion effects and with the T1s shown in Table 1. The final PCr/ATP ratio is taken as the ratio of the blood and saturation-corrected values of PCr / γ-ATP, discounting the α-ATP peak because it has contributions from NADPH+ and the β-ATP peak because it had a phase artifact in some subjects. Finally, the spectral SNR was determined by applying a matched filter and then measuring the SNR as the peak height/baseline SD 33. The final uncertainty in metabolite concentrations was expressed using Monte Carlo error propagation to calculate the Cramer-Ráo lower bounds (CRLB) 34. Statistical comparisons were made in Matlab using paired comparisons wherever possible 35.
| Calf muscle 7T (this work, N = 2) | Calf muscle 7T (from (25), N = 8) | Heart muscle 7T (this work, N = 6) | Heart muscle 3T or 2T (literature in Figure 6) | |||
|---|---|---|---|---|---|---|
| T1 / s | ||||||
| Pi | 6.65 ± 0.23 | No value | – | No value | No value | – |
| 2,3-DPG | No value | No value | – | 3.05 ± 0.41 | No value | – |
| PCr | 3.96 ± 0.07 | 4.0 ± 0.2 | NS | 3.09 ± 0.32 | 5.3 ± 1.7 (N = 50)b | ** |
| γ-ATP | 4.12 ± 0.15 | 3.3 ± 0.4 | * | 1.82 ± 0.09 | 2.8 ± 1.1 (N = 50)b | * |
| α-ATP | 1.70 ± 0.02 | 1.8 ± 0.1 | NS | 1.39 ± 0.09 | 2.0 ± 0.9 (N = 5)c | NS |
| β-ATP | 1.42 ± 0.12 | 1.8 ± 0.1 | ** | 1.02 ± 0.17 | 2.5 ± 0.6 (N = 5)c | ** |
| T2 / ms | ||||||
| Pi | 58 ± 7 | 109 ± 17 | ** | No value | No value | – |
| 2,3-DPG | No value | No value | – | 10 ± 6 | No value | – |
| PCr | 138 ± 15 | 217 ± 14 | ** | 220 ± 195 | No value | – |
| γ-ATP | 26.3 ± 0.3 | 29.0 ± 3.3 | NS | 105 ± 127 | No value | – |
| α-ATP | 22.1 ± 0.8 | No value | – | 14 ± 4 | No value | – |
| β-ATP | 18 ± 1 | No value | – | 70 ± 140 | No value | – |
| + T2* / ms | 31 ± 2 | – | – | 10 ± 4 | – | – |
- a Summary of the 31P T1s and T2s obtained in this study from the calf and heart at 7T. These are compared to literature values also for the calf at 7T to validate our method, and to heart muscle at 3T to demonstrate the significant difference in cardiac 31P T1s between the field strengths. Statistically significant differences (two-tailed t-test) are denoted * for P ≤ 0.05 and ** for P ≤ 0.01.
- b These values were recorded at 3T.
- c These values were recorded at 2T, no 3T data are available.
RESULTS
The 31P spectra measured from a small phantom (Fig. SI4) show 2.8× higher SNR at 7T compared with 3T, which is close to the ∼2.4× (i.e., 6.96T / 2.89T) B0-induced improvement predicted by theory 36. The calculated coil field maps agree with those measured with the adjustable phantom to within 10% (see Figs. SI1–3). These preliminary data confirm that the hardware performs acceptably for 31P-MRS at both 3T and at 7T.
Table 1 presents a summary of fitted T1, T2 and T2* values from our Look-Locker CSI method applied in the calf muscle alongside pertinent comparators from the literature. Our T1s from calf muscle are consistent with those in the literature, giving us confidence that our cardiac T1s will be reliable enough for use in saturation correction of the main 3T versus 7T comparison.
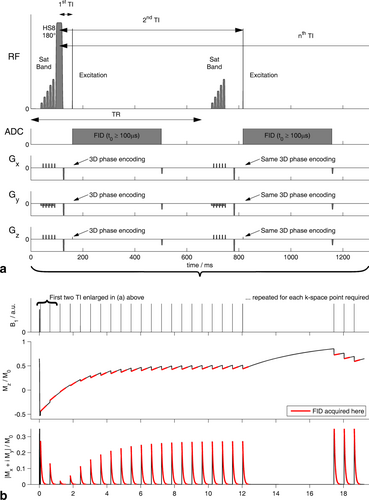
To further illustrate the quality of Look-Locker CSI data in the heart, Figure 3 shows the spectra recorded in one subject to determine cardiac 31P T1s. Each column corresponds to one execution of the LL-CSI pulse sequence from Figure 2. The inversion and recovery of PCr with inversion centered at 774 Hz (i.e., 1.6 ppm, in the left-hand panel) is evident. The corresponding trace in Figure 3d shows clearly that PCr inversion is reasonably effective. It also shows that there is substantial readout-induced saturation, which confirms the need to include the gap and three additional excitations. The residuals show very little structure, except around the 2,3-DPG peak at short TI, which we attribute to blood flow, and around γ-ATP after the gap, which we attribute to chemical exchange processes (see the Discussion for details).
Figure 4 illustrates the 3T versus 7T comparison study with the data from one subject. The 7T CINE localizers in Figure 4c,d demonstrate that the quality of localizers attainable at 7T even with a simple 10-cm 1H loop coil is quite adequate for cardiac localization. The pulse profile in Figure 4b–note the expanded y-axis scale–shows the uniform excitation generated by the shaped pulse we used. The spectra are shown here after application of a matched filter and normalisation to the baseline SD so that height is proportional to SNR. The increased SNR at 7T is evident. We see also that there is acceptable linewidth and baseline at both field strengths. There is, however, a pronounced signal from blood (2,3-DPG), which Matlab simulations (not shown) lead us to attribute to in-flow effects in the right ventricular blood pool proximal to the coil.
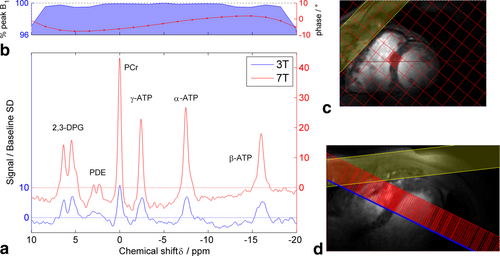
Table 2 summarizes the results of AMARES fitting, which completed successfully for all subjects and which detected all the expected metabolites at 3T and at 7T. There is a statistically significant 2.8× increase in PCr SNR and a 2.4× decrease in the Cramer-Ráo lower bounds on PCr concentration. As we would expect in a paired study on normal volunteers, the difference in mean PCr/ATP values (2.1–1.7 = 0.4) is not statistically significant even at the P = 0.05 level. Importantly, the PCr/ATP ratio does have a smaller SD at 7T (0.3 at 7T versus 0.5 at 3T) and the CRLB uncertainty in the final calculated PCr/ATP ratio decreases 2.7× at 7T. However, the mean PCr linewidth increases from 16 Hz (i.e., 0.32 ppm) to 37 Hz (i.e., 0.31 ppm) at 7T, which suggests there is scope for further improvement through better optimized shimming.
| Field strength B0 | 3T (mean ± SD) | 7T (mean ± SD) | Ratio 7T/3T | |
|---|---|---|---|---|
| PCr SNR | 11 ± 3 | 31 ± 13 | 2.8 | ** |
| PCr amplitude CV/% | 2.8 ± 1.2 | 0.6 ± 0.4 | 0.2 | ** |
| Linewidth/Hz | 16 ± 2 | 37 ± 11 | 2.3 | ** |
| Linewidth/ppm | 0.32 ± 0.05 | 0.31 ± 0.09 | 1.0 | NS |
| Flip angle/ ° | 20 ± 9 | 20 ± 5 | 1.0 | NS |
| PCr SNR (90°, TR ≫ T1)b | 44 ± 15 | 105 ± 35 | 2.4 | ** |
| Blood corrected PCr/ATP | 1.7 ± 0.5 | 2.1 ± 0.3 | 1.2 | NS |
| (Mean CRLB on PCr/ATP) | ± 0.67 | ± 0.25 | 0.4 | – |
- a Mean values were compared with a paired t-test. Significance is denoted * at P = 0.05, ** at P = 0.01.
- b This is an extrapolation to the SNR that could hypothetically have been achieved by using 90° excitation pulses and a long repetition time (TR >> T1). It removes any effects due to changing 31P T1s from the comparison.
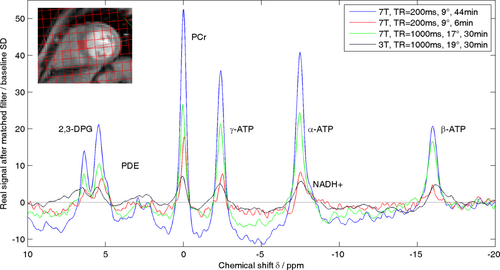
Finally, Figure 5 compares cardiac 31P spectra recorded in the same subject with TR = 200 ms in long and short protocols against the 7T and 3T data described above. This spectrum demonstrates some of the further potential for cardiac 31P-MRS at 7T as the methods are refined. The 44-min TR = 200 ms spectrum shows pronounced shoulders to the right of 2,3-DPG and α-ATP which we believe are Pi and NADH+. Meanwhile, the 6-min TR = 200 ms protocol gives a spectrum with SNR comparable to that obtained at 3T in our standard 30-min protocol. This shows that cardiac 31P-MRS at 7T has great potential to follow dynamic processes such as the response to exercise or pharmacological stressors.
DISCUSSION
Validation of LL-CSI T1 Method
The SNR in cardiac data that can be acquired in a bearable scan duration (<2 h) is relatively low, so fitting each individual spectrum becomes impractical and it is essential to use our prior knowledge of the metabolite peaks to connect the repeated scans. Our fitting algorithm uses this prior knowledge to give adequate Cramer-Ráo bounds on the fitted T1s even with the relatively low SNR data available. Our fitting approach also handles automatically the partial inversion/saturation that is inevitable given the relatively weak B1+ at 7T.
We validated our acquisition and fitting methods on skeletal muscle, where the T1s measured here (see Table 1) agree well with those recently reported 25. The exception to this agreement is our longer γ-ATP T1 (4.12 ± 0.15 s versus 3.3 ± 0.2 s), which we believe is due to differences in acquisition and a decision in both cases to neglect magnetization transfer in the CK shuttle 37.
We also obtained a value for skeletal muscle γ-ATP T2 that agrees well with the literature 25 and report for the first time values for α- and β-ATP T2. However, our value for PCr T2 is shorter than previously reported 25. Sensitivity analysis of the Bloch simulator shows that both B1 and T2 govern the efficiency of the inversion pulse while B1 also governs the readout-induced saturation that recovers during the “gap” before the final three excitations. Hence, T2 is determined by the balance between inversion efficiency and readout-induced saturation; the additional T2* factor accounts for any observed linewidth that is not already accounted for by the fitted T2. Together, these factors mean that we are sensitive only to T2s that are comparable to the duration of the inversion pulse (i.e., 29.7 ms) and also significantly longer than the additional T2* factor, which explains our anomalously low value for PCr T2 in skeletal muscle and our failure to obtain consistent T2s in the heart.
LL-CSI T1 in the Heart
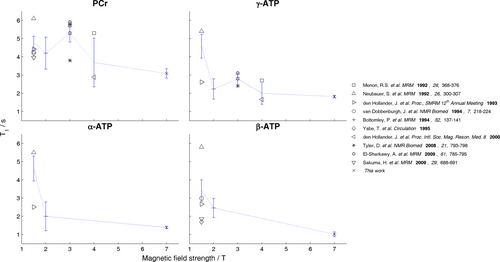
At 7T, T1s are shorter in the heart than in skeletal muscle (Table 1). Our 7T T1s are also significantly shorter than average values reported in the literature for PCr and γ-ATP at 3T and for α,β-ATP at 2T (see Figure 6). This decrease in T1 continues a trend tentatively described at lower field strengths 38 and is consistent with animal studies 39.
Proton T1s typically increase with field strength B0, so the decrease of 31P metabolite T1s may at first seem surprising. To understand this, we recall that T1 relaxation is driven by various mechanisms. Typically dipole-dipole (DD) relaxation dominates (T1 ∼ B03/2), then chemical shift anisotropy (CSA) (T1 ∼ B0−2), followed by smaller contributions from spin-rotation, scalar coupling, etc. 40, 41. The relative contributions from these mechanisms depend on the electronic structure and rotational motion of the molecule in question. The decrease in 31P T1s with increasing B0 suggests a substantial contribution from CSA-driven relaxation. The exact explanation for the T1s of PCr and ATP in vivo has been the subject of some debate, with the CSA mechanism being proposed as early as 1985 in rat muscle 39 and investigated in vitro in the early 1990s 42-44 before being invoked to explain data for the T1 of human skeletal muscle at 3T 45. The current situation is summarized by Nabuurs et al 46, 47.
The analysis above did not consider the significant effects of chemical exchange in the creatine-kinase cycle on T1 48, 49. In our cardiac studies, we chose long Tp = 29.7 ms, R = 24, HS8 inversion pulses for LL-CSI to maximize inversion efficiency across all metabolites despite the low peak B1+ at the heart. This meant that we did not invert PCr and γ-ATP selectively, and therefore that there were multiple sets of CK-flux and intrinsic T1 values that fitted the data, between which we could not distinguish. Nevertheless, our observed T1s are appropriate for saturation correction.
Limitations of LL-CSI T1
Finally, our Look-Locker spectroscopy method is not without its limitations. The current protocol requires an 80-min acquisition. In common with other approaches to determine 31P T1s, we assume in postprocessing that all signal arises from a region with uniform B1, T1s, T2s, etc., except for an exponential T2* relaxation term to account for B0-inhomogeneity. The cardiac data sets have insufficient SNR to determine precisely this T2* value and the individual metabolite T2s, essentially because the data do not sufficiently constrain the fitted linewidths. Also, it was not feasible to consider the in-flow of blood, so the “T1” we observed for 2,3-DPG is likely to be an underestimate and there may be small artifacts due to blood contamination of the ATP resonances.
3T versus 7T Comparison
This study reports the first human 7T cardiac 31P spectra. It also provides the first quantitative matched comparison of the performance of human cardiac 31P-MRS at a field strength exceeding previous reports at 3T 12, 13 and at 4T 50, 51. Figure 4 and Table 2 show the marked superiority of cardiac 31P spectra at 7T relative to 3T. The SNR of every peak increases and therefore the quality of the ensuing AMARES fits also increases, e.g., the CV of the PCr amplitude decreases by 2.4×.
Table 2 shows an extrapolation of the PCr SNR to a hypothetical fully relaxed acquisition with 90° excitation flip angle, revealing a 2.4× gain at 7T. Meanwhile, in this study, PCr SNR increased by 2.8×. Together, these values demonstrate that, with this protocol, there is not only a 2.4× improvement arising from the increase in B0 alone as predicted by theory 9, 36 but also a further 1.2× improvement because of better Mz recovery due to the decrease in PCr T1 (see also Table 1).
The 10-cm Tx/Rx coil used in this study was chosen to facilitate a fair comparison between field strengths. However, it is unlikely to be optimal design for cardiac 31P-MRS either at 3T or at 7T. In particular, a 10-cm loop may not be appropriate for female subjects or those with a high BMI. A loop coil has optimal SNR at a depth of approximately 1× its diameter, making a 10-cm coil most appropriate for lean male subjects. A more sophisticated 7T coil, e.g., using a larger Tx loop combined with one or more <10-cm Rx elements 13, 52-56, should give even better data and facilitate the transition of 7T 31P-MRS to clinical populations.
In this study, we chose to match the flip angle at 3T to that at 7T because our maximum flip angle at 7T was limited by the maximum rated power of the coil T/R switch. Furthermore, there is no direct link during coil design between achievable B1+ and RF heating or peak RF power requirements. Hence, it would not have been possible to guarantee an “optimal” coil design at both field strengths, precluding such a comparison. Similarly, we chose not to use gating in this study, primarily to avoid potential difficulties at 7T. As we gain more experience at 7T, the gating “problem” is becoming less acute with better subject preparation and ECG positioning, etc. We know that there is scant difference between ECG gated and nongated 3D CSI 31P-MRS data quality at 3T (unpublished data), so we opted to eliminate this potential complication, because it does not inexorably diminish the long-term capabilities at 7T.
The SNR improvement with field strength varies noticeably between subjects (e.g., PCr SNR increased 0.8, 1.1, 2.4, 2.9, 3.5, 3.9, 4.1, 4.2, and 4.3×). We attribute this to the challenge of accurate coil positioning with a small loop coil. When performing the comparison study, we checked the coil position images and accepted a position within 1 cm of the target position on the chest. Even if optimally placed, a 10-cm Tx/Rx loop will show significant variation in receive sensitivity and B1+ homogeneity at the interventricular septum due to variations in body shape and in the coil, septum distance. The fraction of the voxel filled with myocardium will also vary between subjects. These effects are consistent with previous reports regarding the positioning of 31P-MRS surface coils 13, 54.
Cardiac 31P spectra typically contain a contribution from blood in addition to the desired signal from the myocardium. Blood contamination gives rise to the two 2,3-DPG peaks at ∼6 ppm and to additional ATP signals which overlap myocardial ATP. We therefore subtract 30% 31 of the mean 2,3-DPG peak amplitude from each ATP peak to remove contributions to ATP from blood. It is not immediately apparent whether this blood correction should be performed before or after saturation correction. The correct choice depends on the degree of replacement of blood by in-flow during each TR: there is almost 100% in-flow in the ventricular blood pool, but much less in the myocardial capillary bed. Given the size of our voxels, we believe ventricular blood dominates and, therefore, we perform blood correction before saturation correction. This also avoids the difficulties in obtaining a meaningful T1 for 2,3-DPG which were discussed above.
Linewidths previously reported at 3T for PCr are on the order of 22 ± 12 Hz (i.e., 0.44 ± 0.24 ppm) 13, which compares favorably with the data reported in Table 2. In this study we only used “tune up” B0 shims because of difficulties with 1H image-based shimming at 7T due to the inhomogeneous B1 from the 1H loop. Therefore, our 7T 31P linewidths represent an (encouragingly good) worst case for future studies.
Finally, Figure 5 gives a glimpse of the great promise for cardiac 31P-MRS at 7T. Being able to obtain spectra with acceptable SNR in 6 min will open the way to more detailed study of the response of 31P energetics to stressors such as exercise or infusion with dobutamine. Three 6-min scans could easily be incorporated into a more general protocol. Alternatively, one could follow the time course of recovery in 6-min intervals allowing more detailed characterization of the recovery curve than current paradigms. At the other extreme, the 44-min 200-ms TR spectrum suggests that it may soon be possible to quantify further metabolites in a cardiac 31P-MRS scan, extending the options available for clinical research. Finally, the almost 10× improved SNR comparing the 44-min 200-ms TR spectrum against the 30-min 3T spectrum could be used to increase the resolution of the 3D CSI matrix by a factor of exp(ln(10) / 4.5) = 1.67 while still obtaining adequate SNR for further analysis. This would offer greater chances of detecting regional energetic anomalies as opposed to the global changes we are sensitive to today.
CONCLUSIONS
We have demonstrated proof-of-principle for cardiac 31P-MRS at 7T. We also quantified the significant improvements in spectral SNR relative to the previous gold-standard field strength of 3T (e.g., PCr SNR increased 2.8×). We have also developed a new Look-Locker CSI spectroscopy method and used it to determine for the first time the T1s of high energy phosphate metabolites in the human heart at 7T. These cardiac 7T T1s were found to be lower than values reported in the literature at 3T and lower than in skeletal muscle (e.g., PCr T1 is 3.09 ± 0.32 s at 7T in the heart versus 3.96 ± 0.07 s in skeletal muscle at 7T, and versus 5.3 ± 1.7 s at 3T in the heart). Together, the increases in Boltzmann magnetization and spectral bandwidth at 7T coupled with the decreases in T1, provide a substantial boost to the sensitivity of cardiac 31P-MRS at ultra-high field strengths. We were able to acquire spectra in 6 min of a quality that took 30 min at 3T. Alternatively, we would be able to increase the CSI resolution by ∼1.67× while maintaining adequate spectral SNR.
To close, we have found that cardiac 31P-MRS shows great potential at 7T. We recommend 7T as the field strength of choice for applications of 31P-MRS to clinical research.
ACKNOWLEDGMENTS
We thank G. Keith for assistance with the coil housing and D.J. Tyler for helpful discussions. This work was funded by the NIHR Oxford Biomedical Research Centre; the Medical Research Council (UK); Merton College. C.T.R. holds a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society.