Novel retrospective, respiratory-gating method enables 3D, high resolution, dynamic imaging of the upper airway during tidal breathing
Abstract
Purpose
A retrospective, respiratory-gated technique for measuring dynamic changes in the upper airway over the respiratory cycle was developed, with the ultimate goal of constructing anatomically and functionally accurate upper airway models in obstructive sleep apnea patients.
Methods
Three-dimensional cine, retrospective respiratory-gated, gradient echo imaging was performed in six adolescents being evaluated for polycystic ovary syndrome, a disorder with a high obstructive sleep apnea prevalence. A novel retrospective gating scheme, synchronized to flow from a nasal cannula, limited image acquisition to predefined physiological ranges. Images were evaluated with respect to contrast, airway signal leakage, and demonstration of dynamic airway area changes.
Results
Two patients were diagnosed with obstructive sleep apnea. Motion artifacts were absent in all image sets. Scan efficiency ranged from 48 to 88%. Soft tissue-to-airway contrast-to-noise ratio varied from 6.1 to 9.6. Airway signal leakage varied between 10 and 17% of soft tissue signal. Automated segmentation allowed calculation of airway area changes over the respiratory cycle. In one severe apnea patient, the technique allowed demonstration of asynchronous airway expansion and contraction above and below a severe constriction.
Conclusions
Retrospective, respiratory gated imaging of the upper airway has been demonstrated, utilizing a gating algorithm to ensure acquisition over specified ranges of respiratory rate and tidal volume. Magn Reson Med 70:1580–1590, 2013. © 2013 Wiley Periodicals, Inc.
Obstructive sleep apnea syndrome (OSAS) is a growing public health problem affecting children and adolescents 1, and is linked to the rapidly rising prevalence of pediatric obesity 2. It affects as many as 1–4% of children 3, with an odds ratio of 4.5 in obese children 2. Transient upper airway obstruction during sleep leads to cardiovascular and cognitive morbidity in children with OSAS 4. Adenotonsillectomy and weight loss may reduce the clinical manifestations of OSAS in some cases, but their effectiveness remains unclear and the identification of patients likely to benefit continues to be a challenge 4.
Recent studies have identified several important anatomical and physiological factors which contribute to the development and progression of airway constriction in children with OSAS 5-10. It is clear from these studies that a comprehensive understanding of the underlying pathophysiology of the disorder will require precise temporal and anatomic characterization of upper airway obstruction. A number of investigators, including our group, have developed techniques for quantifying changes in upper airway anatomy over the respiratory cycle 11-15. Previous studies utilizing prospectively gated, single-shot, fast imaging techniques suffer from various technical limitations. For example, CT-based techniques require radiation exposure 11, 16, optical coherence tomography is limited to studies of the airway lumen itself with no information on the surrounding soft tissues 17, 18, and MRI techniques tend to be signal-starved, requiring the use of nonisotropic voxel dimensions, and often provide limited anatomical coverage 13, 15, 19-22 unsuited to comprehensive airway modeling. In addition, most techniques have used prospective gating for respiratory synchronization, thus not allowing for rejection of respiratory events outside the range of the airway dynamics being studied.
Here, we describe a novel method for retrospective, respiratory-gated MRI with high, isotropic spatial resolution of the entire upper airway. The technique follows the general methodology of retrospective gating used in cardiac imaging, in which arrhythmia detection is used to reject invalid cycles whose length lie outside a predetermined acceptable range. For respiratory gating, we utilized the flow waveform from a nasal cannula to retrospectively reject cycles during which the tidal volume was outside a predetermined acceptable range for normal tidal breathing. Although only providing pseudo-real time imaging of upper airway motion, the retrospective rejection technique allows careful selection of a predefined range of respiratory parameters, potentially allowing exploration of respiratory dynamics during different types of breathing, such as during an apneic event.
We show feasibility of the technique for producing high quality, dynamic imaging of the upper airway across the respiratory cycle in six young female subjects being evaluated for polycystic ovary syndrome (PCOS). PCOS is a disorder affecting girls and women of reproductive age and is associated with irregular or lack of menstruation, polycystic ovaries, clinical signs of hyperandrogenism, obesity, and cardiometabolic abnormalities 23. Research has shown a high prevalence of OSAS in this disorder 24-27.
METHODS
Subjects
Subjects were recruited from a pool of patients being evaluated at Montefiore Medical Center for PCOS, and were all females between 14- and 18-years-old. Following overnight polysomnography, subjects underwent an awake, upper airway MRI without sedation. The study was approved by the Institutional Review Board at the Albert Einstein College of Medicine, and informed consent was obtained from each subject (or subject's parent for minors; subject assent was obtained from minors), prior to enrollment in the study.
Gating System
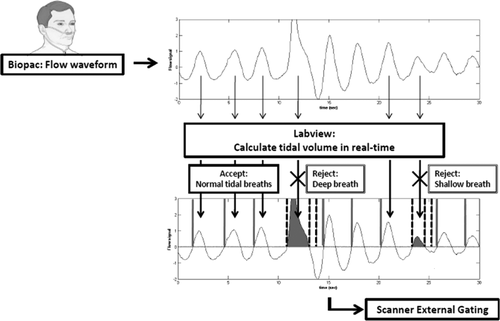
Qualitative breathing airflow waveforms were collected from a nasal cannula coupled to a pressure transducer (TSD-160B, Biopac Systems, Inc., Goleta, CA). The Biopac signal was digitized with a National Instruments data acquisition board (DAQCard-6036E coupled to a SCB-68 board) and displayed on a custom graphical user interface written for Labview (National Instruments, Austin, TX). The cannula signal was initially zeroed for each subject to the no-flow condition. A gating signal was generated at the upward-going zero crossing at end expiration, and fed into the scanner external trigger input. Because a nasal cannula was used, calibrated tidal flow-volume information was not available. However, based on 2–3 min of flow data from the patient, mean and normal variation of relative tidal volume was calculated and used to limit MRI data acquisition to within the normal range of tidal breathing. Although an exact algorithm was not used, the limits were generally set from 50 to 200% of the mean relative tidal volume. Subjects were instructed to breathe through their nose and keep their mouth closed during the duration of the scan.
MRI retrospective gating systems are typically designed to accept or reject input waveforms based on timing information, i.e., periods that are unusually short or long. This type of rejection system is optimized for cardiac imaging, where the beat-to-beat variation in heart rate is usually small, and large changes in rate are indicative of an arrhythmia which should be rejected from image acquisition. In contrast, in respiratory physiology the tidal volume is an equally important parameter which can potentially alter respiratory dynamics. A shallow breath, a very deep inspiration or breathing during a swallow will have very different respiratory dynamics compared to normal tidal breathing.
Thus, it was necessary to transform the standard arrhythmia rejection software on the scanner into an abnormal-tidal-breath rejection system. This was accomplished by measuring relative tidal volume in real-time within the Labview software, and producing gating signals based on the calculated relative inspiratory tidal volume for each breath; a flowchart of this rejection scheme can be found in Figure 1. As noted above, a gating trigger was generated at end expiration for each breath, independent of relative tidal volume. At end inspiration (i.e., the downward-going zero crossing), relative tidal volume was calculated and compared to the acceptable relative tidal volume range. If the relative tidal volume was within range, this constituted a valid breath, nothing further was generated during the cycle and the scanner regarded this as an “accepted” respiratory cycle. If the calculated relative tidal volume was out of range, however, an additional gating signal was generated at end inspiration and again every 500 ms for the remainder of the expiratory period. Thus, the scanner receives two consecutive gating pulses separated by the inspiratory period alone; the scanner's timing-based rejection algorithm judges the entire cycle as a rejected respiratory period and reacquires the data until a valid respiration is detected. The additional gating pulses every 500 ms during expiration ensure that the expiratory portion of this invalid cycle is not judged as a valid respiratory period on its own. Because rejection was still ultimately based on timing information, an abnormally deep, and long, inspiratory period could still be judged as a valid respiratory period. However, the lower acceptance bound for arrhythmia rejection could usually be set high enough to prevent this from happening.
MRI Acquisition
All MRI images were collected on a 3T Philip Achieva scanner (Philips Medical Systems, Best, The Netherlands). The patient was positioned in the RF coil (described below) with padding on both sides of the head to prevent motion during the scans. A three-dimensional (3D), T1-weighted, inversion-prepared gradient echo sequence, acquired in the sagittal plane and reconstructed in the axial and coronal planes, was used for planning of the 3D dynamic study. The dynamic study used a 3D-retrospectively gated, turbo gradient echo sequence with the following parameters: echo time/pulse repetition time = 3.3/6.9 ms, flip angle 8°, field of view 24 (foot-head) × 18 (anterior-posterior) cm, matrix 220 × 164, bandwidth 209 Hz/pixel. Thirty-six 1.1-mm thick sagittal slices were acquired (with 30% oversampling in the slice encode direction), a SENSE acceleration factor of 2.0 in the primary phase encode direction, and 42 phase encode lines were collected per frame, resulting in a total of 91 valid breaths required for image reconstruction. The 18-cm field of view in the anterior-posterior direction was chosen to minimize scan time, but would normally result in a SENSE-related artifact in the center of the field of view. SENSE-based fold-over suppression 28 was used to minimize these effects utilizing a SENSE fold-over factor of 2.0. Ten respiratory phases were reconstructed across the respiratory cycle, independent of individual subject respiratory rate. Minimum scan time, assuming a respiratory period of 3 s, was approximately 4.5 min.
Our new, 3D retrospective technique was compared qualitatively to 2D, prospectively gated, single shot imaging, which has been used previously for real-time imaging of airway motion 10, 14, 29. 2D, axial images used a balanced steady-state free precession sequence with the following parameters: echo time/pulse repetition time = 2.57/5.14 ms, flip angle 35°, field of view 18 × 14 cm, matrix 164 × 128, slice thickness 3 mm/slice gap 0.3 mm, bandwidth 362 Hz/pixel, SENSE factor 2.0 and temporal resolution of 345 ms/frame. Eleven to fourteen frames were collected following the initial gating pulse at end expiration to cover at least one full respiratory cycle. Total scan time for 50 slices, assuming a respiratory period of 3 s, was 5 min.
Optimal coverage of the upper airway with adequate spatial and temporal resolution, and specifically for the study of OSAS involving a typically obese patient population, places constraints on the RF coil arrangement. The standard 16-channel neurovascular (NV) coil available on most MRI systems should be the best option with respect to airway coverage and SNR, but has two significant limitations: 1 the larger, obese patients being studied either do not fit into the coil or are extremely uncomfortable, and 2 accurate quantification of airway dynamics ideally requires collection of the entire flow-volume using a mask and pneumotachometer, which would not fit inside of the full NV coil for any patient. Although a mask and pneumotach was not used for the current work, future work requiring quantitative flow information will utilize this option. To overcome these limitations, we utilized the lower portion of the NV coil, coupled with a 2-element flexible surface coil pair (16.5 × 14 cm diameter) to recover signal loss from the removal of the anterior NV elements. One element of the flex-coil pair was placed over the face and the second over the neck. Typical SNR loss was 10–20% relative to the full NV coil in the tissues surrounding the upper airway.
Image Quality and Scan Performance Evaluation
Performance of the dynamic sequence was based on the following measures: 1 temporal efficiency of the sequence, calculated as the minimum scan time (based on the patient's mean respiratory rate) divided by actual scan time, 2 presence of artifacts in the images and their proximity to the airway and surrounding tissue, 3 contrast-to-noise ratio (CNR), with contrast calculated as the signal difference between the airway and surrounding tissue, with the surrounding tissue signal taken as the mean of signal within three regions-of-interest, in adenoid, soft palate and esophageal wall (CNR will determine the ease with which the airway can be reliably segmented from the surrounding tissue, a requirement for reliable airway size measurements; airway SD was calculated within the same three ROI's listed in item 4, and assumes a Rayleigh noise distribution), 4 mean airway signal and temporal coefficient of variance of signal within the airway, (both measures taken as an indication of gating quality, because poor gating will produce motion artifact and spurious signal within the airway), measured within three airway ROI's (in velopharynx, oropharynx and trachea) and measured relative to mean tissue signal for comparison across all subjects, 5 timing consistency of the “accepted” respiratory flow pattern over the 91 breaths required for image acquisition, reported as the standard deviation of the position of peak inspiration and expiration flows.
Demonstration of Dynamic Imaging
The quality of the dynamic images was evaluated according to the ability to extract airway cross sectional area changes over the respiratory cycle from axial reconstructed images, as follows: Histogram analysis of a 20 × 20 pixel region centered on the airway was used to identify a cut-off threshold for airway signal intensity; in most cases, identified as a low-intensity-value peak in the histogram. Based on this threshold, the airway region was automatically segmented and airway area was measured in mm2 as the total number of pixels below the threshold times the pixel area. Waveforms of airway cross-sectional area over time were also evaluated qualitatively for demonstration of periodic changes in airway size over the respiratory cycle and consistency of temporal variation from slice to slice along the length of the airway.
A distinct advantage of the retrospective gating technique is the ability to collect the entire 3D volume under conditions representing the same respiratory dynamics, as opposed to single slice techniques where each slice is collected over a different cycle with potentially different respiratory dynamics. To demonstrate this strength, airway area changes over the cycle were extracted on a slice-by-slice basis over the entire upper airway and evaluated in terms of timing of the area changes. Mean waveforms of airway cross-sectional area over time were first compared qualitatively at the mid-velopharynx and mid-oropharynx levels. A quantitative measure of the timing of airway motion was extracted from the phase of the fundamental harmonic of the Fourier transform of the airway size change waveform.
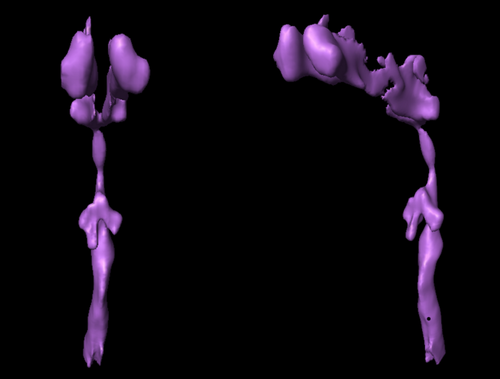
3D surface renderings of the airway were constructed with Amira 4.1 (Visage Imaging Inc., San Diego, CA) from MR image stacks at each phase of tidal breathing. The upper airway was segmented by the identification of voxels in selected slices using a single threshold value taken from top of the velopharynx to the upper tip of the epiglottis.
RESULTS
Patient Clinical Assessments
Patient demographics and clinical assessment data are summarized in Table 1. Only two of the patients had a confirmed PCOS diagnosis (OSA2 and Cntrl4). Overnight polysomnography confirmed sleep apnea as moderate in one patient (apnea-hypopnea index, AHI, of 8.1, the number of apnic/hypopneic events per hour), and severe in one patient (AHI 36.9), labeled subjects OSA1 and OSA2, respectively. All other subjects had normal values (AHI ranging from 0 to 4.6) and were considered controls for the purposes in this study.
| OSA1 | OSA2 | Cntrl1 | Cntrl2 | Cntrl3 | Cntrl4 | |
|---|---|---|---|---|---|---|
| Age, y | 15 | 15 | 18 | 14 | 18 | 17 |
| BMI, kg/m2 | 34.1 | 41.5 | 36.6 | 46 | 24.8 | 30.9 |
| AHI, events/h | 8.1 | 36.9 | 0 | 4.6 | 0.9 | 0 |
| Snoring | Moderate | Severe | Moderate | Mild | Mild | Moderate |
| Resp. rate, min−1 | 19.3 | 19.9 | 23.7 | 30.8 | 15.3 | 17.6 |
| Rate, min/max | 15/25 | 15/39 | 16/30 | 25/40 | 12/20 | 12/30 |
| rTV, min/max | 44/154 | 49/225 | 46/195 | 32/173 | 45/195 | 25/214 |
- AHI (apnea-hypopnea index) is a commonly used index for assessing severity of OSA, and is measured as the total number of apneic (cessation of respiration for >8 s) or hypopneic (O2 sat drop of >4%) events per hour. rTV (relative tidal volume) was measured from the flow waveform collected the nasal cannula, and thus was only a relative measure of net tidal volume, quoted as a percentage of the mean relative tidal volume over the scan. The large range for this variable indicate the amount of variability in relative tidal volume in these patients, which can be further limited through the gating rejection algorithm but obviously will impact scan efficiency and total scan time.
Image Quality and Scan Performance Evaluation
The NV-flex coil combination worked well, with all patients being comfortable in the coil setup, the coil providing excellent signal recovery in the anterior regions, with signal levels similar to those obtained with the full NV coil. The dynamic 3D sequence completed successfully in all patients. Image quality measures are summarized in Table 2. Overall, scan times varied considerably depending on respiratory rate and scan efficiency, ranging from 3.3 to 10.1 min (mean 7.0 min). SENSE-related artifacts were detected in three patients but were only severe in one of these. However, even in these cases, the artifacts were only notable within the brain and did not affect image quality in the vicinity of the upper airway in any case.
| OSA1 | OSA2 | Cntrl1 | Cntrl2 | Cntrl3 | Cntrl4 | |
|---|---|---|---|---|---|---|
| Scan efficiency | 88.35 | 65.47 | 47.64 | 90.10 | 58.71 | 65.00 |
| SENSE artifacts | No | Faint | No | Faint | No | Yes |
| CNR | 7.71 | 6.10 | 9.14 | 6.42 | 9.32 | 9.56 |
| Mean airway signal | 15.1 | 17.8 | 12.1 | 16.1 | 12.3 | 13.6 |
| Temporal COV | 16.29 | 14.31 | 14.95 | 17.85 | 15.65 | 12.31 |
| Flow consistency | 4.8/6.3 | 12.6/7.2 | 2.7/4.2 | 9.6/5.7 | 5.3/7.1 | 5.6/5.8 |
- All measures except for CNR are quoted as percentages. Scan efficiency indicates the length of time for collecting the image compared to the theoretical minimum scan time given the patient's respiratory rate, and was reduced from 100% due to scan rejections based on respiratory rate or relative tidal volume being outside of a predetermined acceptance window. CNR, airway signal, and temporal coefficient of variance (COV) were all measured within three airway ROIs (see definition in Methods). Flow consistency is the standard deviation of the timing of peak inspiration and peak expiration, quoted as a percentage of the average length of the respiratory cycle.
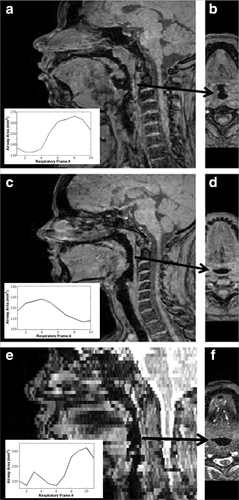
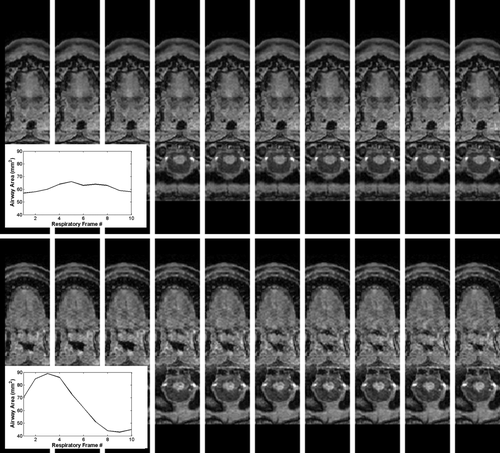
Figure 2 shows examples illustrating the typical airway-to-soft tissue contrast achieved with the dynamic sequence, with a comparison to the 2D technique illustrating the improvement in airway-tissue contrast, along with the obvious isotropic resolution advantage of the 3D technique allowing high quality display along multiple orientations. Although not seen in this example, the 2D technique occasionally suffered from SSFP-related banding artifacts which often intersected the airway and were difficult to avoid because of the expected susceptibility difference between the airway and surrounding tissue. For the 3D technique, CNR varied from subject-to-subject, although this was likely due to an effect on coil performance in the larger patients; CNR was negatively correlated with both patient weight and BMI (P < 0.05). Nonetheless, automated airway segmentation was successful even in the cases with lowest CNR, allowing slice-by-slice calculation of airway dynamics (see insets). Mean airway signal and its temporal coefficient of variation were low in all cases. Mean airway signal was correlated with both BMI and with the flow consistency measure (variation in peak inspiration across all acquisitions, discussed below), indicating contributions to airway noise from coil and gating performance.
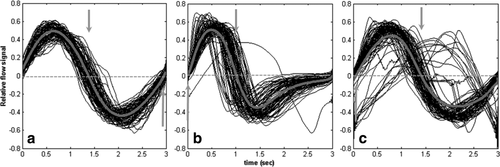
Because retrospective gating relies on the collection of k-space across multiple respiratory cycles, an important consideration is the reproducibility of the respiratory pattern over time. In fact, the consistency varied from subject to subject (Table 2). Three different cases, representing the different types of variation seen, are illustrated in Figure 3. All waveforms have been scaled in time and amplitude to illustrate changes in flow pattern only. In some subjects, flow waveforms were very consistent across all acquisitions, as seen with the very tight distribution of the plots in Figure 3a. In others, there was considerable variability, with both peak inspiration and peak expiration varying in time from shot-to-shot, as seen in Figure 3b. As discussed above, there were occasional, erroneous acquisitions with the entire cycle being acquired during inspiration only; one such acquisition can be seen in Figure 3b. Data from three patients contained one such error each. In one extreme case, where the rejection timing window was set too large, 10 such erroneous acquisitions were encountered, as illustrated in Figure 3c. There was an association between the peak inspiration timing variability and either image CNR (P = 0.04) or mean airway signal (P = 0.02).
Demonstration of Dynamic Information
Figure 4 demonstrates the image quality obtained over the respiratory cycle with the dynamic sequence, showing both one patient with near-perfect gating performance and another with suboptimal gating performance (OSA2, improper setting of the rate rejection window, as discussed above). The suboptimal gating is presumed to lead to the signal leakage into the airway seen. Nonetheless, the CNR of 6.1 was still adequate for automated segmentation of the airway from the surrounding soft tissue and calculation of airway size over the respiratory cycle (figure inset). Although the images were acquired in the sagittal plane, allowing full coverage of the upper airway, the isotropic resolution of the 3D technique allows these high-quality reconstructions across the respiratory cycle for accurate calculations of airway size irrespective of local airway orientation.
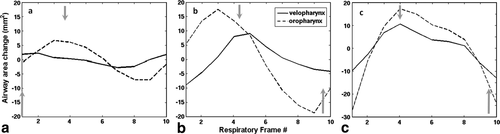
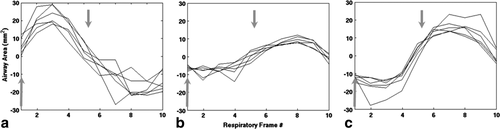
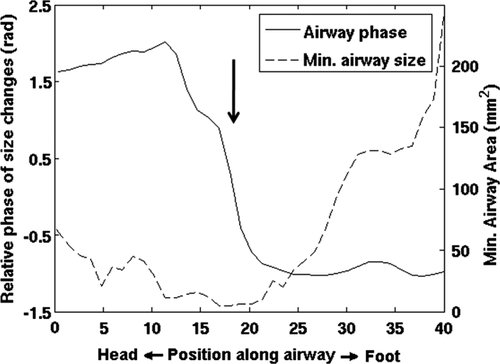
Calculations of airway area changes over the cycle are shown in three non-OSA subjects (Fig. 5), revealing inter-subject variation in the relative timing of size changes along the airway. However, in all cases airway motion was roughly synchronous along the entire length of the upper airway. Irrespective of sleep apnea symptoms (i.e., absent in these subjects), these dynamic changes may be a sensitive gauge of respiratory physiology and dynamics. In contrast to these cases, patient OSA2 with severe sleep apnea (AHI = 36.9) had a significant narrowing of the airway in the lower velopharynx with a complete phase reversal of the airway area motion from above to below the airway constriction (i.e., asynchronous motion of the airway above vs. below). These, likely pathologic, dynamics are demonstrated in Figures 6 and 7, and can best be appreciated from the cine movie available in the Supporting Information. 3D reconstructions yielded closed, contiguous surface-rendered maps of the upper airway in all cases (Fig. 8).
DISCUSSION
We have demonstrated a novel method for dynamic upper airway imaging. Retrospective, respiratory-gated MR imaging was controlled based on flow through a nasal cannula, to generate dynamic, 3D, isotropic resolution images of the entire upper airway over the respiratory cycle. The technique can be used to collect images during normal tidal breathing, limiting acquisition to a specific, predetermined range of respiratory rate and relative tidal volume, depicting changes in airway size with 300 ms temporal resolution at ∼1 mm isotropic spatial resolution. The isotropic resolution facilitates multiplanar reconstruction and airway size determinations based on optimal oblique cross-sectional slice orientation, even at the abrupt 90° bend of the nasopharyngeal airway.
Historically, retrospective MRI gating techniques were developed for cardiac imaging 30, and have not been applied to imaging of respiratory-related airway motion. In cardiac imaging, heart rate and stroke volume variations are assumed to have minor impact on retrospectively gated cine MRI image quality. For airway imaging, in contrast, respiratory rate and tidal volume vary considerably, potentially invalidating the basic assumptions of retrospective gating. In this study, we assumed that respiratory rate variations do not impact airway dynamics, using very conservative rate rejection windows. Tidal volume, however, may play an important role in respiratory dynamics; we therefore developed a technique to retrospectively reject acquisitions outside of a predetermined relative tidal volume range. For this initial study, relative tidal volume acceptance ranges were deliberately broad to ensure reasonable scan times. Nonetheless, dynamic images in all cases had no evidence of motion-related artifacts. Future study will investigate narrower acceptance ranges for improving image quality. Our rejection scheme will allow for future studies exploring the effect of tidal volume on airway dynamics (e.g., limiting acquisition to high vs. low tidal volume ranges), potentially offering valuable insight into the ongoing question of the relationship between airway stiffness and parenchymal tethering 31, 32, which can be affected by tidal volume and airway resistance.
The retrospective gating technique used in this study differs from those used in prior airway dynamics studies which almost exclusively utilized rapid, single slice scanning, with each slice gated on a different breath 10-13, 33, 34. In comparison to the technique developed here, these prior studies suffer from three potential drawbacks: 1 the acquisition is inherently prospective, so that no information about the respiratory pattern is available until after image acquisition, 2 breath-to-breath variations in airway dynamics allow for the possibility that different slices in the image dataset will record changes in the airway relating to different airway dynamics, and 3 single slice techniques are inherently lower in signal-to-noise compared to true 3D volume technique and thus are limited in their ability to acquire images with high isotropic resolution which can later be reconstructed in an orientation optimal for airway segmentation. The retrospectively gated, 3D technique described here overcomes these issues by: 1 collecting real-time respiratory rate and relative tidal volume information to ensure that image acquisition occurs over breaths with a similar respiratory pattern, 2 using a 3D acquisition, so that all slices in the dataset relate to the identical collection of respiratory dynamics, and 3 collecting high isotropic resolution images allowing multiplanar reconstruction tailored to the local airway geometry, a particularly important issue in the nasopharyngeal region.
This first demonstration of the technique was in a group of patients being evaluated for PCOS, a condition with a significant prevalence of obesity and concomitant obstructive sleep apnea. OSAS was confirmed by polysomnography in two subjects. The MRI technique was sensitive enough to detect airway area changes as small 2 mm2 over the cycle. In the patient with severe sleep apnea, the technique proved extremely useful; we demonstrated a unique and likely pathologically based asynchrony in the motion of different parts of the airway. Although the airway motion below the constriction follows the expected pattern for a passive airway (i.e., motion dictated by the flow-related pressure changes), the reverse pattern above the constriction would seem to indicate active airway motion, possible evidence of neuromotor activation of the surrounding tissue. Although it is possible that this particular patient drifted into sleep during the scan leading to this unique motion pattern, we should note that all patients were carefully instructed to remain awake for the duration of the scan. In addition, given the high AHI for this patient, apnic-like respiratory periods would have been likely had she fallen asleep and none were recorded during the scan. This particular case highlights a specific advantage of the retrospective approach noted above; because the entire airway is imaged using the same set of tidal breaths, quantitative comparison of both amplitude and timing characteristics of airway motion at multiple levels within the image volume is possible.
Our technique, as demonstrated here, is an ideal method for obtaining isotropic, high spatial and temporal resolution dynamic images of the upper airway during normal tidal breathing. However, the interesting dynamics in this population occurs either preceding or during an apnea. Because of the flexibility of our novel gating approach, we believe that it would be possible to adapt our methodology for studying apneas as well. Considering that each apnic event is preceded by an inspiratory period, gating with the flow waveform may still be feasible in these cases. Alternatively, a respiratory bellows could be substituted for the nasal cannula to measure respiratory effort, as described previously 10. In either case, additional temporal bounds would confine accepted cycles to the long apnic periods. Of course, because of the infrequent occurrence of apnic events, addition acceleration techniques such as keyhole or kt-BLAST 35 need to be explored to allow for 3D reconstruction within a reasonable scan time.
A prior study of airway caliber changes over the respiratory cycle reported a consistent pattern across both control subjects and patients, with maximum airway size occurring at early- to mid-expiration and little change in size during inspiration 11. In fact, this might be expected in a purely passive airway where caliber is determined by airway pressure—positive pressure during expiration should expand the airway, while the negative pressure generated during inspiration should cause airway collapse. Our results, in contrast, show significant variability in this pattern, although peak airway size generally occurred toward the beginning of expiration (Fig. 5). However, a recent series of studies using MR tagging to track tissue motion clearly shows anterior motion of the genioglossus during inspiration, i.e., motion which would tend to increase airway caliber, as well as subject-to-subject variability in the direction of the tissue motion in the more superior portions of the upper airway 21, 22. These results are in concert with earlier studies of electromyographic activity of the genioglossus during inspiration 36, 37. Thus, in addition to the expected passive pressure response of the airway, there are clearly neuromuscular activation-related changes in airway caliber affecting airway dynamics, which can vary over the length of the airway and from subject-to-subject.
A few limitations of the study should be addressed. Our RF coil used the lower half of the standard NV coil coupled with a 2-element flex coil pair over the face and neck, to ensure that all patients could fit into the same coil setup. Our finding of a negative correlation between image quality and patient weight is unfortunate because it indicates that in larger patients, those most likely to have OSAS, this more open coil configuration is suboptimal. The flex coil elements are much larger than the individual NV elements and in fact comparable in size to the patient's head, so that their noise performance will be most affected by patient head size 38. Given that the added flex coil over the face is the one which most effects image upper airway CNR, the lower CNR in larger patients is therefore not surprising; further work will be needed to investigate alternate coil options less sensitive to patient size.
Although our analysis focused on airway segmentation, one of the primary applications for airway imaging in OSAS is for visualization of surrounding soft tissue motion for CFD and fluid structure interaction simulations to understand airway-soft tissue interactions and their effect on obstruction dynamics 10, 39-42. For this first demonstration, sagittal slices covered a limited region of the surrounding soft tissues. However, we still need to determine the degree of motion of the lateral tissues not covered, and adequate models might be constructed from the available dynamic information coupled with static images of the remaining anatomy. In addition, higher acceleration factors along two dimensions of the 3D volume could be used to increase anatomical coverage without loss of temporal resolution.
Finally, the strategy for limiting data acquisition to predetermined ranges for relative tidal volume worked well in most cases, but was subject to occasional erroneous gating in some of the subjects. This was due to the fact that our tidal volume rejection scheme relied on the timing based rejection algorithm of the native scanner software and could erroneously accept an abnormally long inspiration as a full respiratory cycle. Nonetheless, these erroneously gated events were rare; dynamic image quality was not significantly affected even in the one case with a few occurrences. Future implementations of the sequence will utilize an improved rejection algorithm to recognize such erroneous gating in real-time and reacquire the data.
In conclusion, we have demonstrated a technique for 3D, isotropic resolution, retrospectively gated, cine imaging of the upper airway based on both respiratory rate and relative tidal volume acceptance criteria, which limits image acquisition to a desired range of tidal breathing. The contrast between the airway and the surrounding soft tissue allows for automated, slice-by-slice airway segmentation and depiction of very small changes in airway cross sectional area over the respiratory cycle. The proposed technique will permit construction of high-resolution 3D dynamic models of the upper airway and surrounding tissues, which can facilitate improved understanding of the mechanical and functional mechanisms leading to airway obstruction and apneic events. Moreover, this methodology, coupled with sophisticated CFD and fluid structure interaction models, may allow better insight into the above mechanisms and may better predict treatment outcomes.
ACKNOWLEDGMENTS
The authors would like to thank Joseph McDonough, MS, and Jayaram Udupa, PhD, of the University of Pennsylvania, and David Wootton, PhD, of the Cooper Union for the Advancement of Science and Art for reviewing and commenting on the manuscript.