Integration of the computational fluid dynamics technique with MRI in aortic dissections
Abstract
Short-term and long-term prognosis and their determining factors of Type III/Stanford B aortic dissections (TB-AD), which separate the aorta distal at the origin of the subclavian artery into a true lumen and false lumen, have been elusive: One quarter of patients thought to be treated successfully, either by medical or by surgical means, do not survive 3 years. Unfavorable hemodynamic conditions are believed to lead to false lumen pressure increases and complications. A better characterization of TB-AD hemodynamics may therefore impact therapeutic decision making and improve outcome. The large variations in TB-AD morphology and hemodynamics favor a patient-specific approach. Magnetic resonance imaging with its capability to provide high-resolution structural images of the lumen and aortic wall and also to quantify aortic flow and kinetics of an exogenous tracer is a promising clinical modality for developing a deeper understanding of TB-AD hemodynamics in an individual patient. With the information obtained with magnetic resonance imaging, computational fluid dynamics simulations can be performed to augment the image information. Here, an overview of the interplay of magnetic resonance imaging and computational fluid dynamics techniques is given illustrating the synergy of these two approaches toward a comprehensive morphological and hemodynamic characterization of TB-AD. Magn Reson Med, 2013. © 2012 Wiley Periodicals, Inc.
CLINICAL OBSERVATIONS
Aortic dissection is an uncommon disease with an incidence of approximately several thousand cases per year (1). DeBakey Type III/Stanford B aortic dissections (dissections of the descending aorta, TB-AD, Fig. 1a) are less lethal than aortic dissection involving the ascending aorta at onset (Stanford A aortic dissections). Given the variable prognosis of TB-AD (2, 3), predictors of poor outcomes are urgently needed. Versatile imaging techniques, such as magnetic resonance imaging (MRI), can potentially assist in providing surrogate markers: Medically treated chronic TB-AD (2 weeks after onset) have the particular risk of progression in aortic diameter thereby significantly increasing incidence of complications, surgical repair or risk of fatal aortic rupture (4-6). While both computed tomographic angiography (CTA) and MRI can display TB-AD with high spatial resolution (7), cine MRI studies providing time-resolved images have high potential for improving accuracy of this imaging marker by resolving the dynamics of the intra-arterial septum and thereby quantifying variations in diameter of the true lumen (TL) and false lumen (FL), as they occur during the cardiac cycle. Branch vessel involvement is a common complication of TB-AD responsible for malperfusion and insufficiency of visceral organs as well as the lower leg (8) eventually leading to surgical interventions (9, 10) and increased hospital mortality (6). Time-resolved MRI techniques may help to distinguish dynamic (necessitating reduction of the interluminal pressure gradient) from static (necessitating revascularization of the affected artery) branch vessel compromise, a distinction that is clinically important for endovascular treatment planning (11, 12). MRI may also be used for visualizing aortic remodeling, such as TL expansion and FL shrinkage or thrombosis in patients that tolerated the initial vascular event (13, 14). During disease progression, the intra-arterial septum thickens and stiffens. Dynamic MRI methods can be utilized to characterize this change in mobility (15) (Fig. 1b).
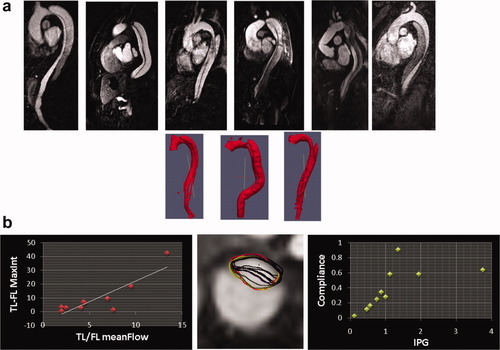
a: TB-AD present with a large variability. Top row shows selected slices from 3D contrast-enhanced magnetic resonance angiographic acquisitions, bottom row 3D reconstructions for three selected cases. Not only differences in TB-AD morphology can be appreciated but also variation in TL/FL gray scale intensities, which may be related to varying TL/FL contrast-filling dynamics. b: On left: Delay in maximum luminal image intensity (from time-resolved 3D magnetic resonance angiography, in seconds) versus ratio of TL/FL mean flow ratio (from 2D pcMRI). A linear relationship can be recognized (R2 = 0.76). Center: TL boundaries segmented at different time points in the cardiac cycle overlaid onto 2D pcMRI magnitude image. Right: Compliance (per unit area) versus interarterial pressure gradient (IPG, from 2D pcMRI flow velocities in mmHg). A linear (left, <2 mmHg) and a nonlinear range (right, >2 mmHg) may be recognized, which may have implications for TB-AD FL expansion. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The magnitude of flow-induced pressure impingement onto the arterial wall is relatively small (in the order of 2–4 mmHg) compared to the average blood pressure (100 mmHg). Instead of direct injury by this impact, repeated tensile strain may cause microstructural damage in arterial tissue (16), and changes in the temporal pattern of shear stress may cause modulation of the endothelial cell phenotype (17).
HEMODYNAMIC FACTORS IN TYPE B AORTIC DISSECTIONS
TB-AD represent a complex system with two pressurized lumens connected by usually one entry tear and one or several exit tears. Its hemodynamics is not fully understood. In vitro results suggest that exit tear occlusion during disease progression may increase FL pressure promoting TL collapse and consequent malperfusion or even FL rupture (18). Complete remodeling (thrombosis) of the FL has been reported to be an indicator of good outcome (19-21). Remnant FL flow (retrograde through the exit tear) resulted in a 2.7-fold increase of death (3). However, only small studies so far investigated the effects of FL thrombosis. In addition, Clough et al. (22) recently demonstrated that many first pass contrast-enhanced imaging studies (MRI and CTA) may overestimate FL thrombosis due to incomplete filling of the FL. 4D magnetic resonance angiography providing several phases every few seconds may be able to reduce image filling artifacts caused by incorrect timing, provided image acquisition is extended into the late enhancement phase.
COMPUTATIONAL FLUID DYNAMICS AND TYPE B AORTIC DISSECTIONS
Computational fluid dynamics (CFD), which uses a numerical approach to solve problems that involve fluid flows, has been applied by several groups to qualitatively and quantitatively describe hemodynamic parameters in cerebral aneurysms (23-27) and—focusing on tensile stresses by combining CFD with fluid structure interactions—in abdominal aortic aneurysms to assess rupture risk. A caveat is that fluid structure interactions require assumptions about elastic moduli that usually cannot be derived from clinical imaging data (28-30). Boundary conditions for CFD include the geometry of the volume of interest (Fig. 1a) as well as inflow and outflow conditions. Validation of the computational solutions is a topic of particular interest, which has to be addressed should this technology gain broad acceptance in clinical pretreatment planning. Small, preliminary studies comparing computational results for the velocity vectors with direct measurements of the components of the velocity vectors of the flowing blood using phase contrast MRI (pcMRI) have been reported for cerebral aneurysms (31-33) and lately for TB-AD (34) emphasizing the need for patient-derived geometries and inflow boundary conditions to arrive at realistic results.
If the full 3D geometry of a TB-AD is obtained, a computational mesh can be created and CFD simulations can be performed. Simulation results should be compared to measurements to ensure that the simulations have succeeded in a realistic description of TB-AD hemodynamics.
Although non-electrocardiography (ECG) gated computed tomographic angiography yields 3D image information, it is in general not possible to obtain flow information with this imaging technique. Similarly, ultrasound techniques succeed in providing real-time flow information with high temporal resolution, but 3D geometry information is not easily obtained. On the other hand, there are now MRI techniques clinically available, which provide 3D geometry as well as time-resolved 2D and 3D flow information. Using time-resolved 3D magnetic resonance angiography and 2D pcMRI, a correlation of TL and FL pressures with the intra-arterial septum motion was demonstrated (Ref.20; Fig. 1b,c) and a reduction of intra-arterial septum motion was consequently demonstrated in a longitudinal study (15). A comprehensive characterization of TB-AD morphology and hemodynamics may prove beneficial in developing a new characterization scheme toward improving treatment outcome once its clinical value has been evaluated.
CFD simulations can complement MRI imaging and provide access to hemodynamic parameters that are not (yet) accessible to clinical imaging technologies, such as pressures and wall shear stress (WSS). As a first application of this concept, Karmonik et al. (35) have shown that CFD can visualize differences in velocities, dynamic pressure, and WSS before and after thoracic endovascular treatment (thoracic endovascular aortic/aneurysm repair or TEVAR) of a TB-AD (Fig. 2a). In a similar approach, the same group investigated the influence of entry or exit tear coverage on TL and FL pressures (36), a complimentary study to the ex vivo experiments of Tsai et al. (3), which yielded similar results. These hemodynamic parameters are derived from the calculated velocity field at the nodes of the computational grid. For an accurate calculation, numerical errors have to be minimized. For a Newtonian fluid, WSS is defined as the value of the first derivative of the velocity component along the wall taken perpendicular to the wall multiplied by the viscosity. Including a boundary layer, i.e., a layer of a fine mesh at the wall of the computational model, will enhance the accuracy of the WSS value (37). Tradeoffs are an increase in the complexity of creating the computational mesh and a longer simulation time.
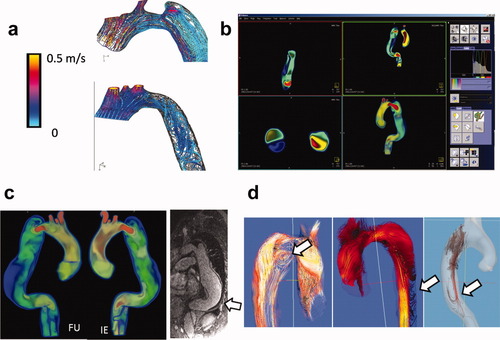
a: Streamlines during systole calculated with CFD before (top) and after (bottom) thoracic endovascular (TEVAR) treatment of a TB-AD. High velocity in entry tear can be appreciated. After TEVAR, velocities are lower but disturbed flow in descending aorta (disordered streamlines) may indicate mismatch in vascular impedance of the endograft. b: Screenshot of a research plugin (not yet for clinical use) for Inspace 3D on the Siemens Leonardo workstation displaying volume velocity data in a TB-AD. Integration of CFD data into the clinical workflow in this fashion may eventually be beneficial for evaluating this technique. c: Velocity data (displayed in same fashion as in B) for a TB-AD with initial examination (IE) and 6 months follow-up (FU) examination. Largest FL expansion at distal posterior wall coincided with low velocities (blue) and FL partial thrombus as visualized in postcontrast 3D contrast-enhanced magnetic resonance angiographic data (arrow). d: 4D pcMRI is capable to visualize vortices in FL flow (left), flow toward intercostal arteries (center) and retrograde FL filling. Information obtained with this technology may be useful for the validation of CFD simulations (arrows). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For a broader acceptance of CFD technologies, results have to be validated with clinical outcome. To facilitate this, CFD results have to be made more accessible, i.e., by conversion into image data for integration into the clinical workflow (Fig. 2b; Ref.38). In this way, the simulated image data can then be compared with clinical image data: slow FL flow was correlated with FL thrombus formation as visible in 3D contrast-enhanced magnetic resonance angiographic images (Fig. 2c).
Recent advances in 4D pcMRI have made it possible to measure the 3D velocity vector field in TB-AD directly (39). Not only primary flow patterns such as the predominately superior–inferior main flows in the TL and FL but also secondary flow patterns such as vortices in the FL or flow entering the intercostal arteries can be visualized with this technique (Fig. 2d). As the full 3D velocity field is available from 4D pcMRI, WSS can be calculated. The low in-plane resolution of MRI relative to the computational mesh used by CFD and partial volume effects impede the calculation of the first derivate of the velocity and the accurate determination of the location of the artery wall. Jiang et al. recently compared 4D pcMRI with CFD simulations in a canine aneurysm model and obtained good agreement of the velocity field (0.6 on a scale from 0 to 1) but poor correlations of WSS (0.22 and 0.31; Ref.33). Until higher image resolutions can be obtained in shorter imaging times, CFD will be better suited to calculate these hemodynamic parameters. In addition, CFD might be useful for pretreatment planning by evaluating virtual scenarios corresponding to different kinds of interventions. Information currently obtainable by 4D pcMRI for TB-AD was demonstrated by Clough et al. (40): Stroke volume, velocity, distal dominant entry tears, and helical flow were related to the rate of aortic expansion. Although CFD may be helpful for developing a better understanding of the mechanics of the blood flow in vascular pathologies, it should be pointed out that the dominant factor in the outcome of TB-AD may be determined by biological processes. The CFD technique should therefore be considered as an additional tool providing additional information about mechanical processes only.
CONCLUSIONS
Type B aortic dissections with its large variations in geometry and flow represent a complex hemodynamic system. CFD simulations with boundary conditions from clinical image data may be able to provide additional information about the underlying mechanical processes of its hemodynamics. If validated with measurements and verified by clinical outcome, CFD may become an additional tool for pretreatment planning. Such a validation may be provided by MRI due to its versatility in visualizing and quantifying structural, dynamic, and flow information.