Axonal damage in the spinal cord of multiple sclerosis patients detected by magnetic resonance spectroscopy
Abstract
Axonal damage is a major factor contributing to permanent disability in patients with multiple sclerosis (MS); it has been extensively investigated in the brain using magnetic resonance spectroscopy (MRS). In this study, MRS was used to investigate the degree of neuronal damage in the cervical spinal cord in MS. Spectra were acquired from spinal cord and brain in 11 patients with MS (expanded disability status score [EDSS], range 2.5–7.0) and 11 controls. Brain lesion volume and spinal cord cross-sectional area were measured. Concentration of the neuronal metabolite N-acetyl-aspartate ([NAA]) was reduced in the spinal cord in MS patients relative to controls (reduced by 32%, P < 0.05), indicating significant neuronal damage. Additionally, the spinal cord was significantly atrophied in MS patients (15%, P < 0.001). No significant reduction in brain [NAA] was seen in the MS group. There were no correlations between clinical measures and cord atrophy or brain lesion volume on MRI; however, spinal cord [NAA] correlated with the cerebellar subscore of the neurological assessment (P < 0.005), while brain [NAA] correlated with disease duration (P < 0.05). MRS demonstrated cellular damage within the cord over and above the tissue atrophy seen by MRI. Combining MRI and MRS may therefore give a more complete picture of neurodegeneration in the spinal cord. Magn Reson Med 58:880–885, 2007. © 2007 Wiley-Liss, Inc.
Axonal damage is a well-recognized component of multiple sclerosis (MS) that has only rarely been quantitatively assessed using conventional histopathology, (1-3) but which has been extensively investigated using proton magnetic resonance spectroscopy (MRS) (for a review, see Ref. 4). MRS allows noninvasive quantitation of the metabolite N-acetyl-aspartate (NAA), which is found exclusively within neurons (5, 6). MRS studies of the brain in MS have reported a reduction in NAA not just within discrete lesions (7, 8), but also within the normal appearing white matter (NAWM) (9, 10), indicating widespread axonal involvement. This diffuse axonal damage as measured by MRS is strongly correlated with disability (8, 9, 11), which is in contrast to conventional MRI measures, such as focal lesion volume, which only weakly correlate with clinical symptoms (12, 13).
The role of spinal cord damage in MS has been investigated to a lesser degree. Histological examination of spinal cord specimens has demonstrated both significant levels of cord atrophy and substantial axonal loss (3, 14, 15). However, there does not seem to be a strong relationship between these two measures, i.e., cord atrophy is not strongly correlated with total axonal number (3). This suggests that measures of spinal cord atrophy using MRI (16-18) may substantially underestimate the true extent of axonal loss, and may not be the most sensitive marker of disease progression in the spinal cord. The additional measurement of axonal damage using MRS in the spinal cord may therefore provide a more complete picture of cord involvement in the individual MS patient.
MRS of the spinal cord is more demanding than in the brain and has received little attention (19-22). We have previously developed a robust protocol for MRS measurement in the spinal cord MRS (20). Here, we apply this method in a cohort of MS patients to explore the potential role of spinal cord MRS in assessing spinal cord damage; specifically, we hypothesize that the spinal cords of even moderately disabled MS patients will have significant levels of neuronal damage detectable as loss of NAA.
PATIENTS AND METHODS
A total of 11 patients with MS (five female, mean age 44 ± 9 years, range 26–55 years) were recruited from the local neurological service and compared with 11 healthy controls (four female, mean age 38 ± 11 years, range 24–59 years). Clinical assessment at the time of MR investigation comprised full history, neurological examination, medication, expanded disability status score (EDSS), timed walk over 10 m, and nine-hole peg test (see Table 1). The study was approved by the local ethical committee and informed consent was obtained from all volunteers.
| Patient | Sex | Age | Clinical category | EDSS | Years since progression | Years since diagnosis | Years since first symptoms | Time to walk 10 m (s) | Nine-hole peg test average (L + R) (s) | Brain lesion volume (cm3)a | Medication |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 40 | RR | 2.5 | – | 15 | 15 | 7 | 18 | 31.6 | Tsabri |
| 2 | M | 47 | RP | 2.5 | – | 11 | 11 | 5 | 16 | 10.4 | Monoclonal antibody to IL12 (trial agent) |
| 3 | M | 49 | SP | 3.0 | 10 | 14 | 14 | 7.5 | 18 | 6.3 | – |
| 4 | M | 38 | RR | 3.0 | – | 4 | 4 | 8.5 | 16 | 8.5 | Glatiramer acetate |
| 5 | F | 46 | SP | 3.0 | 5 | 9 | 24 | 9 | 16 | 12.0 | – |
| 6 | M | 57 | SP | 3.5 | 4 | 23 | 29 | 7.5 | 17 | 18.9 | – |
| 7 | M | 26 | RR | 3.5 | – | 5 | 6 | 7 | 14 | 12.7 | Interferon beta |
| 8 | F | 34 | RR | 5.0 | – | 5 | 12 | 10.5 | 15 | 9.0 | Interferon beta |
| 9 | M | 52 | SP | 6.0 | 9 | 12 | 26 | 15 | 19 | 0.4 | – |
| 10 | F | 44 | SP | 6.5 | 5 | 19 | 19 | 26 | 26 | 5.9 | – |
| 11 | F | 55 | SP | 7.0 | 4 | 23 | 25 | – | 25 | 12.7 | – |
- * Control age 38 ± 11 years, not significantly different from patients (t-test, P = 0.15).
- a Lesion volume is the volume of hyperintense lesions within the brain seen on T2-weighted MRI.
- EDSS = expanded disability status score, M = male, F = female, RR = relapsing remitting, SP = secondary progressive, IL= interleukin, L = left, R = right.
Spinal Cord Measurements
MRI and MRS were performed in a 2 Tesla (2T) magnet, using a Bruker Avance spectrometer (Bruker Medical, Ettlingen, Germany) and a quadrature surface coil. Following collection of midline sagittal scout images, T1-weighted images were acquired in oblique-transverse orientation with slices angled perpendicular to the cord axis (TR = 500 ms, TE = 10 ms, field of view = 17 cm, matrix size = 256, 16 × contiguous slices = 5 mm). Proton spectra were acquired from the spinal cord adjacent to the C3 vertebral body using our published protocol (20) (9 × 7 × 35 mm3 point-resolved spectroscopy sequence (PRESS) voxel, cardiac gated, TR = 3 s, TE = 30 ms, chemical shift selective (CHESS) water suppression, 4 blocks of 64 averages). For quantitation of metabolite concentrations, a series of 24 water spectra were collected with increasing echo times (TR = 10 s, TE = 35–2235 ms, gated) from the same PRESS localized voxel.
Brain Measurements
Following spinal cord measurements, subjects were removed from the magnet and the RF coil exchanged for a quadrature birdcage head coil. Transverse T2-weighted images were acquired (TSE sequence, TR = 3 s, TE = 80 ms, contiguous slices = 20 × 5 mm, in-plane resolution = 1 mm), followed by an MRS spectrum from normal- appearing frontoparietal white matter (PRESS voxel = 20 × 20 × 20 mm3, TR = 3 s, TE = 30 ms, averages = 128, ungated). The voxel was positioned to exclude any areas of hyperintensity on the T2-weighted MRI. Water spectra were again collected to determine the relative contributions of cerebrospinal fluid (CSF) and tissue water within the voxel. Total scanning time was 1 h.
Data Processing, Analysis, and Statistics
Processing of the water-suppressed metabolite spectra included removal of residual water signal using a Hankel-Lanczos singular value decomposition (HLSVD) filter java Magnetic Resonance User Interface (jMRUI (23)), Fourier transformation with 1-Hz exponential line-broadening and fitting of the spectral peaks using an in-house developed variant of the LCmodel technique (24). Processing of the water data used Fourier transformation with 20-Hz line-broadening and spectral peak quantification using a single lineshape using the same software. The degree of contamination of the MRS voxel with CSF was quantified by fitting the peak areas from the multiple water spectra to a biexponential decay to determine the contributions of fast decaying (intracellular) and slow decaying (extracellular water and CSF) signal. Metabolite concentrations (N-acetyl-aspartate [NAA], creatine [Cre], choline [Cho], and myoinositol [myo-I]) were then calculated by referencing the metabolite data to the tissue water fraction (fast decaying fraction) alone (25). Using this process, absolute metabolite concentrations were determined that were independent of cord atrophy and automatically corrected for partial volume effects of CSF within the MRS voxel.
Brain lesion volume was quantified on the T2-weighted images using a semiautomated threshold outlining approach (Jim, Version 3; Xinapse Software) to identify lesion borders and determine the total lesion volume. Repeated measures of the same subject over a period of several days showed that mean intrarater variability was 6.2%. Cross-sectional area of the cervical cord was determined on a single transverse image at the level of C3 with mean intrarater variability of 3.9%. Lesion load within the spinal cord voxel could not be quantified using the images collected for MRS localization as MS plaques are not conspicuous on T1-weighted imaging (16). However the T2 of tissue water with the spinal cord voxel was quantified from the multiple TE water data as part of the spectral quantitation process. Water T2 was compared between patient and controls as an indicator of T2-weighted lesions within the spinal cord.
All data processing and analysis was performed blinded to clinical status of the subject (patient/control) and to all the other assessments (brain atrophy, lesion volume, MRS). Statistical analysis was performed using SPSS software (version 11.0; SPSS Inc. Chicago, IL, USA). The Mann-Whitney test was used for comparisons between the patient and control groups (metabolite concentrations, spinal cord area). Nonparametric correlation was used to explore the relationship between metabolite concentrations in the patients and clinical measures. Specifically, we tested for correlation between [NAA] and EDSS, functional system scores (pyramidal, cerebellar, brainstem, and sensory), disease duration, and nine-hole peg test. The relationship between spinal cord metabolite concentrations and cord atrophy were also tested. Bonferroni correction was applied to all tests to compensate for multiple comparisons and P < 0.05 was considered as significant.
RESULTS
Spectra were successfully obtained from the brain and spinal cord in all subjects. Figure 1 illustrates MRS voxel locations and spectra from the spinal cord and brain for MS patient 5 (Fig. 1b and d) and a control subject (Fig. 1a and c).
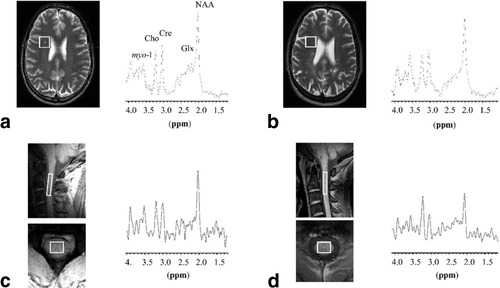
Sampled voxels and typical spectra in a control (a and c) and MS patient (b and d).
Spinal Cord Metabolites
The [NAA] in the spinal cord voxel was significantly reduced in patients compared to controls (P < 0.05, Mann Whitney, corrected for multiple comparisons) (Table 2). Although [Cre] appeared reduced, this was not significant (P = 0.16). The [Cho] and [myo-I] were not significantly different from controls.
| [NAA] (mM) | [Cre] (mM) | [Cho] (mM) | [myoI] (mM) | Cross-sectional area (cm2) | |
|---|---|---|---|---|---|
| Spinal cord | |||||
| Patients | 8.5 ± 2.8* | 4.9 ± 2.1 | 2.3 ± 0.8 | 6.3 ± 2.3 | 0.84 ± 0.08** |
| Control | 12.4 ± 2.3 | 7.1 ± 1.8 | 2.7 ± 0.3 | 6.1 ± 1.9 | 0.99 ± 0.04 |
| Brain (NAWM) | |||||
| Patients | 12.6 ± 1.8 | 8.9 ± 1.3 | 2.5 ± 0.3 | 5.5 ± 1.4*** | |
| Control | 13.4 ± 1.6 | 9.1 ± 1.3 | 2.5 ± 0.3 | 4.0 ± 1.2 |
- * P = 0.036, Mann Whitney U test, Bonferroni corrected.
- ** P < 0.001, Mann Whitney U test, Bonferroni corrected.
- *** P = 0.046 (uncorrected).
- NAWM = normal-appearing white matter; myoI = myoinositol.
Brain Metabolites
The mean [myo-I] was higher in the patients than in controls (Table 2, P < 0.05, uncorrected), but there were no significant differences in any brain metabolite concentrations when corrected for multiple comparisons.
Imaging Changes
The MS patients had significant spinal cord atrophy compared to controls (P < 0.001, Table 2). There was no significant difference in spinal cord water T2 relaxation time (126 ± 39 ms in patients, 124 ± 11 ms in controls, P = 0.46) although the spread of values in patients was larger than in controls. MS lesions within the voxel would increase apparent tissue T2 so this increased variance in patients may indicate the presence of such lesions. The difference in spinal cord [NAA] was therefore retested by analysis of variance (ANOVA) with spinal cord water T2 as covariate, which demonstrated that water T2 was not a significant factor (P = 0.43). Total brain lesion load on T2-weighted imaging varied considerably between patients, with a median volume of 10.4 cm3 (range, 0.4–31.6 cm3, Table 1).
Correlations
No significant correlations were found between spinal cord [NAA], EDSS, disease duration, or performance on the nine-hole peg test. Unexpectedly, the [NAA] in the spinal cord was inversely correlated with the cerebellar functional system score (Fig. 2a, Spearman's rho = –0.927, P < 0.005, corrected), but not with the pyramidal functional score (Fig. 2b, Spearman's rho = 0.126, P = 0.713, uncorrected). No correlation was found between reduced spinal cord [NAA] in the MS patients and spinal cord cross-sectional area (Spearman's rho = 0.310, P = 0.35).
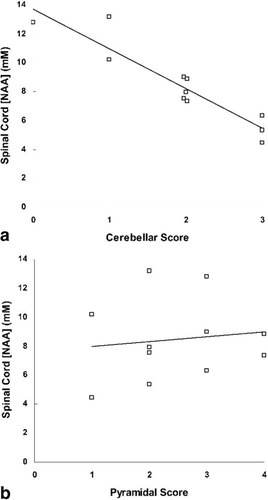
Correlations between subscales of the individual patient neurological score and concentration of neuronal metabolite NAA in the spinal cord. a: Spearman's rho = –0.927, P < 0.005 corrected. b: Spearman's rho = 0.126, P = 0.71.
Although group comparison showed that mean [NAA] was not significantly reduced in the brain, the concentration in individual patients was inversely correlated with disease duration (Spearman's rho = –0.74, P = 0.045, corrected). There was no correlation between spinal cord [NAA] and brain [NAA] or between cord and brain MRI measures.
DISCUSSION
Histopathology provides the gold-standard for assessment of neuronal damage, but requires invasive tissue sampling, and is therefore generally confined to end-stage disease or atypical cases of MS. The ability to measure neuronal damage in vivo is important for understanding the pathogenesis of MS, for assessing neuroprotective treatments and understanding the relationship with clinical disability. Since NAA is localized only within neurons (6), it is commonly used as a MRS biomarker for neuronal injury. In this study we observed a significant reduction in [NAA] in the spinal cord in MS patients with only moderate disease burden. The decrease in spinal cord [NAA] was much greater than in the NAWM within the brain. These results also suggest that reduction in spinal [NAA] cannot be explained by spinal cord atrophy alone (and therefore suggests loss of the metabolite from within remaining axons).
The precise biochemical role of NAA within the CNS remains unclear. Loss of NAA is most simply interpreted as direct physical loss of neurons or axons. However, Bates et al. (26) have shown that NAA is a mitochondrial product related to oxygen consumption and adenosine triphosphate (ATP) synthesis and that inhibition of electron transport (particularly complexes I and III) leads to reduced NAA synthesis by isolated rat brain mitochondria. Thus reduced NAA can be more generally interpreted as neuronal dysfunction rather than loss, and this is demonstrated by reversible changes in NAA seen in the brain is some MS lesions (10, 27). A further proposed role for NAA is as a donor of acetyl groups in the formation of myelin (28). Increased turnover of myelin requires increased amounts of precursors and hence leads to more rapid breakdown of NAA and lower steady state levels. Other hypotheses suggest additional roles for NAA as a source of glutamate (29) and also as an antiinflammatory agent (30). Based on these main proposed functions of NAA, three potential mechanisms can be postulated that would influence the measured concentration within the remaining cord tissue and the relationship to cord atrophy: 1) Complete loss of axons, associated myelin, and oligodendrocytes would result in tissue atrophy but [NAA] (which is corrected for volume loss) would remain unchanged. 2) Neuronal dysfunction leads to reduced NAA, but since the axons remain physically present there would be no atrophy, and hence [NAA] would be reduced. 3) Loss of neurons or axons without immediate loss of associated myelin (as has been reported by Buss et al. (31)) would lead to both atrophy and reduced [NAA] and these measures would be correlated. Our data are consistent with a combination of primarily the first two of these simple models, since we observed significant atrophy, reduced [NAA], but no correlation between these measures.
Unlike MRS of the brain, where relatively homogeneous white matter regions can be sampled, MRS of the spinal cord is limited by the anatomy of the cord to a voxel of mixed gray and white matter content (Fig. 1). Directly attributing NAA loss to a particular tract or tissue type is therefore difficult. Within the sampled spinal cord white matter, our voxel included approximately equal amounts of both the corticospinal and sensory tracts. In the brain, both white matter and cortical gray matter volumes are reduced in MS and decrease with disease progression (32). Further, brain [NAA] in MS has been shown to be significantly reduced in both gray and white matter within the normal appearing tissue (33, 34). In the spinal cord, however, atrophy is largely due to white matter loss (35) with gray matter volume remaining unchanged, suggesting that gray matter may be involved to a lesser degree. Postmortem studies of the spinal cord in MS have reported reductions in axonal density of 33% in the upper cervical corticospinal tract, and 17% in the posterior sensory tract (3), clearly in more advanced cases of MS. Our observed 32% reduction in [NAA] is therefore larger than might be expected due to white matter involvement alone and therefore suggests that gray matter changes also occur in the spinal cord.
Previous studies in MS have reported reduced NAA/Cre ratios within the NAWM in the brain (9-11, 36). We did not observe any significant group reduction in [NAA] (or the NAA/Cre ratio; data not shown) in the frontoparietal NAWM voxel we sampled. Another recent study using quantitative MRS methods (37) has also reported normal concentrations of NAA in MS patients, although that study did report alterations in the NAA/Cre ratio, which was normal in our study (data not shown). In the current work, brain [NAA] within the patient group did correlate with disease duration (Spearman's rho = –0.74, P = 0.045, corrected), suggesting a link between the interpatient variation in brain concentrations and their disease and indicating that patient heterogeneity probably limited the power to detect group differences relative to controls.
The large reduction in spinal cord [NAA] in the absence of similar loss in the brain may be due to a number of factors. Reduced [NAA] in the cord did not correlate with disease duration, suggesting that spinal cord involvement may more extensive than in the brain. Alternatively, since the spinal cord contains axons traveling to and from all regions of the brain, it is possible that the observed NAA changes in the cord represent the sum of axon damage, Wallerian degeneration, or transection occurring in areas of the brain not sampled by the chosen brain voxel but feeding into pathways in the spinal cord. Such a mechanism would lead to enhancement of the NAA change within the cord. T2-weighted images were not acquired within the spinal cord due to time constraints in collecting paired data from both brain and spinal cord in each patient. The conspicuity of small cord lesions on T1-weighted MRI is not high (16) and so we cannot entirely exclude the presence of some lesions within the spinal cord voxel, which would contribute to reduced [NAA]. However, spinal cord tissue water T2 was not different between patients and controls suggesting that the voxels did not contain large volumes of lesions. This is further supported by the lack of significant effect when [NAA] was retested by ANOVA with water T2 as a covariate.
The lack of correlation between cord metabolite levels and clinical measures such as EDSS is most likely due to the relatively small numbers of patients in this study and the heterogeneity of clinical status. As already described, the mixed content of the voxel complicates assignment of changes to specific tracts, but given the importance of motor disability in MS, it was surprising that spinal cord [NAA] was strongly correlated with cerebellar functional score but not with pyramidal score in these patients. Davie et al. (38) demonstrated a negative correlation between cerebellar score and the level of NAA within the white matter of the cerebellum itself and significantly lower cerebellar NAA levels were seen in patients with cerebellar score ≥3. Our observation of a similar correlation with cord NAA may again suggest that the spinal cord measurements are sensitive to remote changes elsewhere in the brain, including the cerebellum. This requires further confirmation in larger, more homogeneous patient groups including comparison of cord and cerebellar levels and more detailed clinical assessment.
This preliminary study using MRS in the spinal cord reports the first clinical findings using this method and demonstrates the clinical and research potential for the technique. Significant differences were observed between patients and controls despite heterogeneity of the patient group. In addition to the clinical status of each patient, ongoing or previous use of disease modifying medication is another potential confound within our patient group. More than half of our patients were not using (or had not used in the past) any form of medication. Two patients were using interferon beta, which has been shown to have variable effect on NAA levels using MRS. In a 12-month longitudinal study we did not find any effect of interferon beta on progressive NAA loss within the brain (39), while Narayanan et al. (40), however, has reported small increases in NAA in a similar study. One patient was using glatiramer acetate, which has been shown to increase NAA levels in MS (41). In either case, if the prescribed medications were acting to increase NAA levels in these three patients, then an entirely untreated patient cohort would be expected to show even greater differences than we have reported. This suggestion applies equally to all patients on medication.
MRS of the spinal cord requires careful positioning of the voxel and the somewhat unusual approach in neuro-MR exams of cardiac gating, in order to obtain high quality data (20). The method was robust for clinical investigation and good quality data was obtained in all subjects investigated. We believe that further development of clinical protocols are therefore justified to perform more detailed investigation in larger and more uniform cohorts of patients.
In summary, our MRI and MRS measures were able to detect greater axonal density changes in the spinal cord than in brain frontal white matter. The lack of correlation between spinal cord [NAA] and cord atrophy or brain [NAA] suggests that spinal cord measurements may provide additional information when investigating neurodegeneration.
Acknowledgements
We thank Dr. Mark Horsfield, Leicester University for providing a demonstration package of the Jim software.