Bio-Inspired Polydiacetylene Vesicles for Controlling Stimulus Sensitivity
Abstract
Polydiacetylene (PDA) is a kind of photopolymerizable polymer, which exhibits a unique color transition in response to external stimuli such as heat, pH, and solvent. PDAs are attractive as eye-detection stimulus sensors with excellent time performance; however, the sensitivity of PDAs should be improved. Considering the biological membrane-like structure of diacetylene (DA) vesicles, their modification by incorporating membrane lipids (e.g., diacylphosphocholine, PC) can be used to control the membrane fluidity, and consequently molecular ordering of DAs in the vesicle. Inspired by biological membrane systems, lipid vesicles are employed as platforms to generate PDA, and essential factors that influence the sensitivity of PDA are investigated. By lowering the polymerization temperature, the generation of PDA becomes slower, while the sensitivity improves. By adding PCs at the molar ratio of lipid:DA = 1:1, the sensitivity of PDA can be varied: the PCs with lower phase transition temperatures (Tm) made PDA insensitive, while the PCs with higher Tm improved the sensitivity as compared to pure poly(PCDA). It is concluded that the photopolymerization of DAs with a lower membrane fluidity induces highly sensitive PDA, while the photopolymerization of DAs with a higher membrane fluidity induces insensitive PDA with robustness toward stimuli.
1 Introduction
Various kinds of stimuli-responsive sensors have been developed. Generally, a sensor system is composed of a detection unit and a signal amplification unit, and each unit is independently designed to achieve high sensitivity. Polydiacetylenes (PDAs) are a group of conjugated polymers exhibiting a vivid color change via conformational change, which enables the detection of external stimuli by the naked eye.[1-3] PDAs and their composites are attractive as colorimetric sensors for the detection of heat,[4, 5] solvent,[6] and alkaline chemicals (pH[7, 8]). In addition, PDAs are responsive to more complicated stimuli such as volatile organic compounds,[9] biological species,[10-12] and mechanical stress.[13-15] According to the type of analyte, the chromism of PDA can be classified into thermochromism, solvatochromism, chemochromism, biochromism, gasochromism, and mechanochromism (Figure 1). These stimuli disturb the ordered structure of PDA, which leads to a color transition. Therefore, the sensitivity of PDA is strongly affected not only by the configuration of PDA itself but also by the support surrounding PDA's chromophore.
PDAs are generated via topochemical polymerization of diacetylene (DA) monomers.[16] Self-assembled structures of DAs, such as solid crystals, Langmuir films, micelles, and vesicles, can be utilized as platforms to obtain PDA.[17-20] DAs such as 10,12-pentacosadiynoic acid (PCDA) and 10,12-tricosadiynoic acid (TCDA) are the most widely-used PDA precursors; however, the sensitivities of poly(PCDA) and poly(TCDA) are insufficient. The sensitivity of PDA is a matter of the stability of PDA-embedded membrane[21] and the interaction selectivity of the analyte.[22] Thus, chemical modifications of the PDA precursor are one of the most successful approaches to improve sensitivity,[8, 23-26] while a tailor-made design of DA at the molecular level might lose the versatility of PDA, leading to a limitation in applications. The sensitivity of PDA is correlated to its structure: the sensitivity of crystalline PDA is lower because the external stimulus gradually penetrates from surface to interior.[20] In comparison, the sensitivity of vesicular PDA is higher because most of the PDA units exist at the interface. Within PDA assemblies, a vesicular configuration is preferred to develop highly sensitive PDA.
As mentioned above, a synthetic approach of novel DA monomers is a promising way to improve PDA sensitivity, but it is a costly and time-consuming process. Instead, a more promising option is the fabrication of biomimetic PDA vesicles by incorporating lipid molecules such as diacylphosphocholine lipids (PC) or cholesterol.[27, 28] The added lipids form a phase-separated structure in the lipid/DA membrane,[29] which promotes or inhibits the photopolymerization of DA. Focusing on the structure of DA or lipid/DA membrane, its structure can be considered a biological cell membrane-like bilayer. The chain length of PC, the number of unsaturated C═C bonds, and the existence of sterols are essential factors to regulate the ordered state of lipid molecules in bilayer membranes. The topochemical polymerization of DA requires an ordered arrangement of monomers. As such, the presence of lipids or sterols, which control the molecular ordering in the DA membranes, could lead to precise control over the PDA sensitivity.
The membrane fluidity is a quantitative indicator of the lipid ordering in a membrane, both in artificial (e.g., liposome)[30-32] and natural (e.g., exosome)[33, 34] systems. Considering the structural similarity between the bilayers of a DA vesicle and a biological membrane, it is expected that the ordered state of DA monomers in a vesicle could be controlled by lipid composition and temperature. Seemingly, most of studies focus on mimicking the fluidity of natural cell membranes;[27, 29] however, the added lipids might influence the molecular arrangement of DA in membranes, resulting in inhibition of PDA polymerization in membranes.
The aim of this study is to control the sensitivity of PDA without any chemical modification of DA monomers. Focusing on the membrane in which PDA is embedded, we investigated the factors that influence the PDA's sensitivity. PCDA, one of the most-widely used PDA precursors, was employed, and the PCDA vesicles were modified by incorporating PCs with different structures of hydrocarbon chains (Figure S1, Supporting Information). Because the polymerization temperature is one of the governing parameters to control the membrane fluidity, photopolymerization of lipid/DA vesicles was performed at different temperatures. The thermal sensitivity of PDAs was investigated by analyzing the colorimetric response (CR), and the key factors to control the sensitivity of PDA are discussed.
2 Results and Discussion
2.1 Photopolymerization of PCDA in Vesicle
PCDA vesicles were prepared by an ultrasound homogenization method,[7, 35, 36] and were polymerized by irradiating with UV light (λ = 254 nm). The hydrodynamic diameter of unpolymerized vesicles was 160–170 nm, and the vesicle size slightly shrank with irradiation time (Figure 2a), similar to the polymerization behavior of TCDA vesicles.[37] It is suggested that the influence of polymerization on the whole structure of a planar lipid bilayer or vesicle would be negligible, although photopolymerization of PCDA could induce a twisted structure of polymer chains.[38] Differential scanning calorimetry (DSC) analysis was performed for unpolymerized PCDA vesicles and polymerized ones (Figure 2b). For unpolymerized PCDA vesicles, the endothermic peak was observed at 58.1 °C. The phase transition temperature (Tm) of PCDA monomers is reported as 67–68 °C,[39, 40] which is slightly higher than the endothermic peak temperature observed in PCDA vesicles. The endothermic peak intensities decreased by UV irradiation, indicating the conversion of PCDA to PDA. The UV absorbance peak intensity at ca. 650 nm, derived from PDA in the blue phase, increased by UV irradiation (Figure 2c). PDA was quickly generated at the beginning (0–5 min), and the polymerization gradually continued until 120 min (Figure 2d).
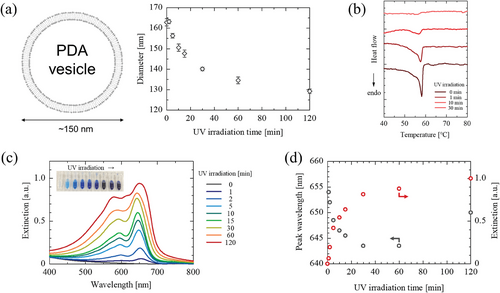
Interlamellar d-spacing of the bilayers of PCDA monomers and of poly(PCDA) are reported as 4.72 and 4.50 nm, respectively.[41] This reveals that the polymerization of PCDA proceeds in the lipid bilayer, where the bilayer structure can be maintained. The zeta potential of PCDA vesicles and poly(PCDA) vesicles were −43.4 ± 1.0 and −39.6 ± 0.6 mV,[42] respectively, suggesting that the vesicle membranes were composed of ionized (─COO−) and protonated (─COOH) species. DSC results revealed the existence of solid-state PCDA before polymerization, and its ratio decreased with the duration of UV irradiation. A good correlation was found between the UV absorbance peak at ca. 650 nm and the conversion ratio (Figure S2, Supporting Information), where the conversion ratio after 30 min polymerization (25 °C) was estimated as 13%. The PDA fabricated from fatty acid-type DAs is a 1D straight polymer, which takes a fibrous structure on the solid support.[43, 44] On the contrary in vesicle systems, the polymerization of PCDA could be restricted due to the size limitation of vesicle. This could be a possible reason for the limited conversion degree of DA monomers. Form these, it is suggested that most of the PCDA molecules in a vesicle acted as scaffolds. In addition, the absorbance peaks of PDA blue-shifted with increasing UV irradiation time, which could be due to the twisting of PDA chains.[38]
2.2 Effect of Photopolymerization Temperature on PDA Sensitivity
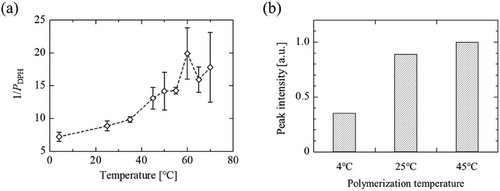
Biological membranes are dynamic systems, wherein lipids are laterally diffusing, depending on the surrounding temperatures. To uncover the dynamic properties of PCDA assembly, the membrane fluidity of vesicles was determined based on fluorescence anisotropy analysis using DPH. Herein, 1/PDPH values were employed as an indicator of membrane fluidity.[30, 33, 45] The 1/PDPH values of PCDA vesicles dose-dependently increased in proportion to the surrounding temperatures (Figure 3a). The 1/PDPH values at temperatures below 25 °C was low (<10). Herein, DPPC (saturated PC) and DOPC (unsaturated PC) vesicles are employed as the models of low-fluidity and high-fluidity vesicles, respectively (Figure S3, Supporting Information). Considering the fludities of model vesicles (DPPC and DOPC), the 1/PDPH values of PCDA vesicles observed at lower temperatures revealed that the membranes of PCDA vesicles at 4 °C was rigid as well as those of the saturated PC vesicles in gel phase (Lβ). In phospholipid vesicles (PC liposomes), it was reported that the 1/PDPH values at temperatures above Tm become a plateau.[30] Such behavior was also observed in PCDA vesicles. Considering the difference in 1/PDPH values at lower temperatures (<40 °C), the molecular ordering of PCDA in a vesicle could be variable. This would change the sensitivity of generated PDA depending on the polymerization temperature, because the sensitivity of PDA is influenced by the molecular ordering of DA molecules.
Polymerization of PDA at different temperatures was compared (Figure 3b). A decrease in temperature suppressed the polymerization of PCDA monomers in the vesicle, which could be due to the difference in the membrane fluidity. Basically, the molecules in vesicular self-assembly are dynamic, even in low-fluidity membranes. According to the result in Figure 3a, the membrane fluidity became higher by increasing temperature, suggesting that the lateral diffusion of DA molecules became faster. The polymerization of PDA would proceed by the collision of unpolymerized DA to PDA, thus the vesicles with higher membrane fluidity could be advantageous for polymerization. PDA was obtained from PCDA vesicles at polymerization temperatures below 50 °C, but not at 65 °C. Kolusheva et al. reported that the addition of phospholipid at the molar ratio of lipid:DA = 3:2 inhibited the generation of PDA.[29] This could be due to the miscibility of phospholipid and DA, which could disrupt the solid domain of DA molecules. At higher temperatures, a lateral diffusion rate of DA molecules becomes faster, which would promote the addition reaction of DA monomers. Takajo et al. reported that the distribution of polymer chain length was dependent on polymerization temperature,[43] wherein the ratio of longer PDA chains increased by increasing temperature. Namely, it was indicated that the polymerization temperature influenced not only the PDA generation ratio but also the structure of PDA.
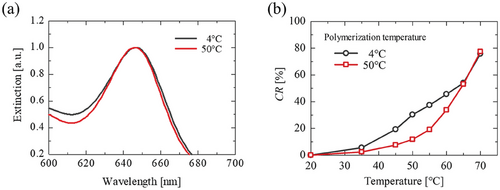
Subsequently, the effects of polymerization temperature were examined. The polymerization temperature hardly influenced on the absorbance peak wavelength (A650 = ca. 1.0 for 1 mM PCDA) (Figure 4a). The response of PDA with regard to temperature was determined based on the colorimetric response (CR). It was found that the PDA polymerized at 4 °C showed color transitions at lower temperatures than the PDA polymerized at 50 °C (Figure 4b), suggesting that the polymerization temperature should be decreased to enhance the sensitivity of PDA. The thermal sensitivity of PDA was also improved by shortening the photopolymerization time (Figure S4, Supporting Information). It was reported that temperature could influence on the degree of polymerization,[43] suggesting the possibility that the PDA generated at 4 °C is consistent with shorter polymer chains. The membrane fluidity of PCDA vesicles remained lower at 4 °C, which meant the rigid membrane restricted ongoing polymerization. Consequently, the sensitivity of PDA could be improved by keeping the membrane in a rigid state.
2.3 Sensitivity Control by Incorporating PCs into Vesicles
The physicochemical properties of biological membranes are in principle controlled by the hydrocarbon chain length of the phospholipid, the degree of unsaturation in the hydrocarbon chain, and the amount of sterol. Inspired by natural systems, it is expected that the properties, particularly fluidity, of the membrane including DA molecules can be controlled by phospholipids. Employing phosphocholine lipids (PCs, Figure S1, Supporting Information), lipid/PCDA vesicles were successfully prepared (Table 1). By employing the ultrasonication method, which is the method to provide small-sized vesicles,[46] the sizes of generated vesicles become smaller. DOPC and POPC are unsaturated phospholipids that have a fusogenic nature,[47] which might induce the fusion between the generated vesicles. The PDI values of lipid/PCDA vesicles were below 0.3, indicating good dispersibility of vesicles in water.[48] The molar ratio of lipid:PCDA was 1:1, and the polymerization was performed at 4 °C. All the lipid/PCDA vesicle suspensions turned to blue, hence it was found that PCs did not inhibit polymerization at a lipid:PCDA ratio of 1:1. However, the polymerization efficiency was different within vesicles (Figure S5, Supporting Information). The vesicles modified by incorporating the lipids with lower Tm (i.e., DMPC and POPC) required shorter UV irradiation time.
| Tm [°C] | Vesicle size dDLS [nm] | PDI [-] | |
|---|---|---|---|
| PCDA | 58 | 162 | 0.153 |
| DMPC | 23 | 256a) | 0.285a) |
| DPPC | 41 | 194a) | 0.300a) |
| DSPC | 60 | 168a) | 0.246a) |
| DBPC | 75 | 193a) | 0.265a) |
| POPC | 4 | 419a) | 0.186a) |
| DOPC | −18 | 440a) | 0.199a) |
- a) vesicles were prepared with an equimolar mixture of lipid and PCDA.
Thermal sensitivity of PDAs was varied by the incorporation of PCs into vesicles (Figure 5). Given that no interaction occurred between PC and PCDA in a vesicle, all lipid/PDA vesicles would behave like the poly(PCDA) vesicles polymerized at 4 °C. The vesicles modified by incorporating the PCs possessing higher Tm (e.g., DSPC) started color transition at ≈45 °C. Within saturated PCs (DMPC, DPPC, DSPC, DBPC), DSPC was most effectively enhanced thermal sensitivity. Because of a similar Tm of PCDA and DSPC, thermal phase transition of DSPC and PCDA (PDA) domains might influence each other. On the other hand, the thermal response of the vesicles modified by incorporating POPC became insensitive (Figure 5a). By comparing CR values of lipid/PDA vesicles, it was found that the vesicles modified by incorporating the PCs possessing lower Tm (e.g., DOPC, POPC, DMPC) decreased the sensitivity, like the poly(PCDA) polymerized at 50 °C.
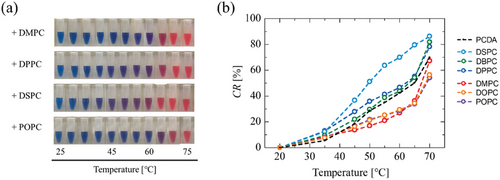
For an effective polymerization, it is required to maintain a phase separation of PCDA and PC within the vesicle membrane. Because of the PCDA's Tm (58 °C in vesicle), the addition of PCs with lower Tm could promote phase separation, resulting in enhanced polymerization. On the other hand, the polymerization seems to be inhibited in the vesicles modified by incorporating the PCs with higher Tm. The PDA vesicles exhibiting lower polymerization efficiencies showed more sensitive color transitions than those with higher polymerization efficiencies. Notably, the polymerization efficiency was promoted by storing lipid/PCDA vesicles for 1 week at 4 °C (Figure S5, Supporting Information). It is speculated that the phase separation of lipid and PCDA could be promoted via an aging process. These results suggest that the color change of PDAs is related to the polymerization process, that is, the color transition property of PDA can be controlled by the phase behavior of lipids in vesicles.
The phase states of phospholipid membranes are roughly classified as a liquid crystalline phase (Lα), ripple gel phase (Pβ), and gel phase (Lβ), illustrated in Figure S6 (Supporting Information). Heating induces a phase transition: from an ordered state (gel) to a disordered state (liquid crystalline), depending on temperature.[49] In this study, only PCDA is polymerizable, thus the PDA must be generated from the solid domain (gel phase) of PCDA in vesicle membrane. A supposed mechanism of PDA photopolymerization in lipid/PCDA vesicles is drawn in Figure 6: i) the homogeneous mixture of PC and PCDA forms a bilayer; ii) by cooling to 4 °C, phase separation spontaneously occurs; iii) an aging process could promote phase separation, herein high-fluidity lipids could enlarge the PCDA domain while low-fluidity membrane maintain small PCDA domain; iv) photopolymerization (i.e., topochemical polymerization) proceeds within the PCDA domain formed in vesicle.
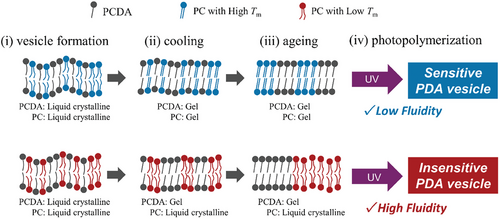
It was reported that left-over monomers (unpolymerized DAs) play an important role in the blue-to-red color transition of PDA.[50] Gel phase lipids (e.g., DPPC) would have an affinity to solid-state PCDA (and poly(PCDA)), in which gel-phase lipid domains could be inserted between PDA domains in vesicle. The pre-phase transition of DPPC (Lβ-to-Pβ) occurs at 35 °C.[49] At around this temperature, a phase segregation between PCDA (and poly(PCDA)) and DPPC would occur, and subsequently disturb the PDA structure. This is the reason why the highest sensitivity was obtained by the addition of DPPC. For precise control of PDA sensitivity, it is concluded that the photopolymerization of DAs with a lower membrane fluidity leads to highly sensitive PDA, while the photopolymerization of DAs with higher membrane fluidity induces insensitive PDA with robustness toward stimuli.
3 Conclusion
We demonstrated control over the sensitivity of PDA vesicles via the polymerization temperature and the vesicle modification by incorporating PC lipids. Highly sensitive PDAs were generated by decreasing the polymerization temperature, and a similar effect was confirmed in cases of the vesicles modified by incorporating the PCs with a higher Tm. Such PC molecules could induce a lower membrane fluidity, resulting in an ordered arrangement of PCDA molecules in the vesicle. On the contrary, the sensitivity of PDA was decreased by the polymerization at high temperatures (50 °C). The membrane fluidity of PCDA vesicles increased by a temperature rise, which could make a longer polymer chain with a twisted structure.[38] Shorter chain PC (such as DMPC) and unsaturated PCs (DOPC, POPC) could increase membrane fluidity. Consequently, the photopolymerization of PCDA performed in a high-fluidity membrane resulted in an insensitive PDA. At the same time, such PDAs have a stimulus tolerance, which enables sensitivity control of PDAs and is important to develop PDA nanocomposite sensors.
4 Experimental Section
Materials
PCDA (purity >97%, TCI Co., Ltd.) was dissolved in chloroform and was purified by passing through a PTFE filter (Millex-LG, pore size: 0.22 µm, Merck KGaA) to remove any polymerized impurities. 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC, C18:1), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC, C16:0/C18:1), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC, C14:0), 1,2-palmitoyl-sn-glycero-3-phosphocholine (DPPC, C16:0), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC, C18:0), and 1,2-dibehenoyl-sn-glycero-3-phosphocholine (DBPC, C22:0) were purchased from Avanti Polar Lipids, Inc. and used as received. 1,6-Diphenyl-1,3,5-hexatriene (DPH) was purchased from Sigma-Aldrich and used as received. Water was deionized in advance (>18.2 mΩ cm).
Vesicle Preparation
PCDA vesicles were prepared based on the literature.[35] An aliquot amount of purified PCDA was dissolved in chloroform, and the solvent was removed by evaporation. The obtained lipid thin film was dried for at least 3 h under a high vacuum and subsequently, ultrapure water was added. The film was heated to ≈70 °C, and then hydrated by ultrasonication (20 kHz, 40 W, 5–10 min), using a probe-type ultrasonicator UD 211 (TOMY SEIKO Co., Ltd.). The obtained vesicle suspensions were kept at least 12 h in the dark at 4 °C. PCDA vesicles were prepared at a total lipid concentration of 2.0 mm.
PDA was obtained from PCDA or lipid/PCDA vesicles. Briefly, vesicle suspensions were transferred into a polypropylene micro tube, and then irradiated using a UV illuminator (λ = 254 nm, 6 W, UVGL-58 (Tokyo Garasu Kikai Co., Ltd.). UV irradiation was performed for 1–120 min at an arbitrary temperature, a distance of 3 cm. After UV irradiation, the vesicle suspension turned blue, which reveals successful PDA synthesis. Polymerization was continued until the absorbance peak intensity at 650 nm reached to 1.0. Lipid/PCDA vesicles were prepared by the same procedure.
DLS Measurements
DSC Measurements
Thermograms of unpolymerized PCDA vesicles and PDA vesicles were obtained using DSC (DSC7020, Hitachi High Tech). The total concentration of DA sources was 16–20 mM. Ten microliter of vesicle suspension was put in an aluminum pan (ϕ : 6.8 mm, height: 2.6 mm), and the reference was pure water. Measurements were performed at a heating ramp of 0.5 °C min−1.
Membrane Fluidity Measurements
Thermal Sensitivity Evaluation Based on CR
Acknowledgements
The authors thank the technical support staff in the Department of Engineering, Tohoku University, for fluorescent spectroscopy measurements. This research was supported by the Ministry of Education, Culture, Sports, Science and Technology: JSPS KAKENHI grants (20K21097, 21H04628, 21K14491, 22H01843, 23H00242, 23K17839, 23K23111, 24K17550), Materials Processing Science project (“Materealize”) of MEXT (grant no. JPMXP0219192801), and Fuji Seal Foundation.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




