Long-term culture of undifferentiated spermatogonia isolated from immature and adult bovine testes
Abstract
Undifferentiated spermatogonia eventually differentiate in the testis to produce haploid sperm. Within this cell population, there is a small number of spermatogonial stem cells (SSCs). SSCs are rare cells in the testis, and their cellular characteristics are poorly understood. Establishment of undifferentiated cell line would provide an indispensable tool for studying their biological nature and spermiogenesis/spermatogenesis in vitro. However, there have been few reports on the long-term culture of undifferentiated spermatogonia in species other than rodents. Here, we report the derivation and long-term in vitro culture of undifferentiated spermatogonia cell lines from immature and adult bovine testes. Cell lines from immature testes were maintained in serum-free culture conditions in the presence of glial-cell-line-derived neurotropic factor (GDNF) and bovine leukemia inhibitory factor (bLIF). These cell lines have embryonic stem (ES)-like cell morphology, express pluripotent-stem-cell-specific and germ-cell-specific markers at the protein and mRNA levels, and contributed to the inner cell mass (ICM) of embryos in the blastocyst stage. Meanwhile, cell lines established from adult testes were maintained in low-serum media in the presence of 6-bromoindirubin-3′-oxime (BIO). These cell lines have characteristics resembling those of previously reported male mouse germ cell lines as confirmed by their botryoidally aggregated morphology, as well as the expression of germ-cell-specific markers and pluripotent stem cell markers. These findings could be useful for the development of long-term culture of undifferentiated spermatogonia, which could aid in conservation of species and improvement of livestock production through genome editing technology.
Abbreviations
-
- BIO
-
- 6-bromoindirubin-3′-oxime
-
- bLIF
-
- bovine leukemia inhibitory factor
-
- DBA
-
- Dolichos biflorus agglutinin
-
- ES like cells
-
- embryonic stem like cells
-
- GS cells
-
- germline stem cells
-
- GDNF
-
- glial-cell-line-derived neurotropic factor
-
- GFRα-1
-
- GDNF family receptor α-1
-
- GSK3
-
- glycogen synthase kinase-3α
-
- KLF4
-
- kruppel-like factor 4
-
- MAPK
-
- mitogen-activated protein kinase
-
- OCT4
-
- octamer binding transcription factor 4
-
- SOX2
-
- (sex determining region Y)-box 2
-
- SSCs
-
- spermatogonial stem cells
-
- UCHL-1
-
- ubiquitin carboxyl-terminal hydrolase isozyme L1
1 INTRODUCTION
Spermatogenesis is achieved by a complex process, in which diploid SSCs proceed over time through a series of differentiation steps to produce haploid spermatozoa (de Rooij, 2001). Since SSCs are rare cells in immature and adult testes (Tegelenbosch & de Rooij, 1993), it is hard to isolate and study them and to distinguish them from their undifferentiated progeny. Currently, several markers are used to identify SSCs in mice: GDNF family receptor α-1 (GFRα-1) (Naughton, Jain, Strickland, Gupta, & Milbrandt, 2006), Thy-1 membrane glycoprotein (Thy-1) (Kubota, Avarbock, & Brinster, 2003), and inhibitor of DNA binding 4 (Id4) (Sun, Xu, Zhao, & Chen, 2015).
In domestic species, gonocytes, SSCs, and SSC progenitors are identified by expressions of Dolichos biflorus agglutinin (DBA) (Fujihara, Kim, Minami, Yamada, & Imai, 2011; Goel et al., 2007, 2010; Izadyar, Spierenberg, Creemers, den Ouden, & de Rooij, 2002), ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL-1) (Fujihara et al., 2011; Goel et al., 2010; Heidari et al., 2012; Herrid, Davey, & Hill, 2007; Reding, Stepnoski, Cloninger, & Oatley, 2010), and GFRα-1 (Costa et al., 2012; Lee et al., 2013; Oatley, de Avila, Reeves, & McLean, 2004). However, the expression of biomolecular markers in germ cells from domestic species is heterogeneous. They expressed in most of undifferentiated germ cells in the testis (Zheng et al., 2014). This makes difficult to characterize SSCs from domestic animals. Another method used to recognize SSCs is to identify their localization in the testis; SSCs are located close to the basal membrane in the seminiferous tubules.
It has been shown that in vitro culture of neonatal mouse SSCs allows to male germline stem (GS) cells (Kanatsu-Shinohara et al., 2003) and ES-like cells (Kanatsu-Shinohara et al., 2004) to be produced. Moreover, in vitro culture of adult mouse SSCs generates GS cells and multipotent male germline stem (MGS) cells (Guan et al., 2006; Seandel et al., 2007). GS cells form botryoidally aggregated colonies, retain their germ cell characteristics, and contribute to the germ cell lineage (Kanatsu-Shinohara et al., 2003). By contrast, ES-like cells form tightly packed 3-dimensional colonies, express pluripotent stem cell markers, and contribute to chimeras (Kanatsu-Shinohara et al., 2004; Ko et al., 2009). MGS cells have tightly packed 3-dimensional colonies, express pluripotent stem cell markers, and develop into cardiac tissue in vitro and functional blood vessels in vivo (Seandel et al., 2007). Moreover, genetically modified cultured SSCs produced transgenic animals (Kanatsu-Shinohara et al., 2008). This suggests that cultured SSCs can be a useful tool for making genetic modifications through gene targeting and genome editing technologies.
Undifferentiated spermatogonia including SSCs from neonatal and pre-pubertal stage of domesticated animal such as pig (Zhang et al., 2017; Zheng et al., 2013) and goat (Pramod & Mitra, 2014) can also be cultured in vitro. In cattle, although gonocytes and SSCs isolated from neonatal and immature bovine testes can be maintained in culture (Aponte, Soda, van de Kant, & de Rooij, 2006; Fujihara et al., 2011; Izadyar et al., 2002; Oatley, Kaucher, Yang, Waqas, & Oatley, 2016; Sahare et al., 2016), long-term culture systems for SSCs from adult testes have been established only in mice.
Several growth factors including GDNF are employed to derive germ cell lines from immature bovine testes (Oatley et al., 2016; Sahare et al., 2015). GDNF is produced by Sertoli cells and supports mouse SSC proliferation in vivo (Meng et al., 2000) and in vitro (Hasegawa, Namekawa, & Saga, 2013). In cattle, the addition of GDNF to culture medium activates the mitogen-activated protein kinase (MAPK) signaling pathway and supports long-term proliferation of gonocyte isolated from neonatal and immature testes (Sahare et al., 2015). However, when the neonatal culture system was applied to culture adult bovine spermatogonia, they could not be maintained for a long time (Fujihara et al., 2011). Hence, experimental evidence suggests that the establishment of male germ cell lines seems to be dependent on the age of the animal from which the cells are derived. The application of male germ cell lines in domestic species will be more comprehensive when it have been established from both immature and adult animals.
In this study, we developed different culture systems for bovine undifferentiated spermatogonia isolated from both immature and adult testes and characterized the cells. This finding demonstrates the feasibility of male germ cell culture in domestic species, which could facilitate progress in research related to transgenic animal production, genome editing technology for improvement of livestock production or conservation of endangered species.
2 RESULTS
2.1 Differential gene expression in undifferentiated spermatogonia of immature and adult testes
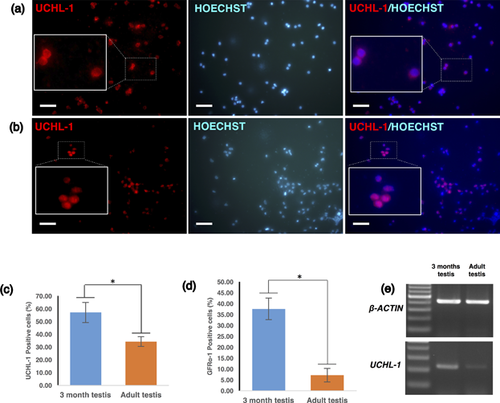
The expression patterns of undifferentiated spermatogonia markers from immature and adult testes was compared. Differential expression patterns of both GFRα-1-positive and UCHL-1-positive cells were observed (Figure 1a–e). The number of UCHL-1-positive cells was higher in immature testes (57.11 ± 7.93%) than in adult testes (34.25 ± 3.80%) (n = 6, Figure 1c). A similar result was observed for another undifferentiated spermatogonia marker, GFRα-1, in immature testes (37.61 ± 4.94%) and in adult testes (7.14 ± 3.1%) (Figure 1d). These results were also confirmed by RT-PCR (Figure 1e).
2.2 Enrichment for undifferentiated spermatogonia isolated from adult testes
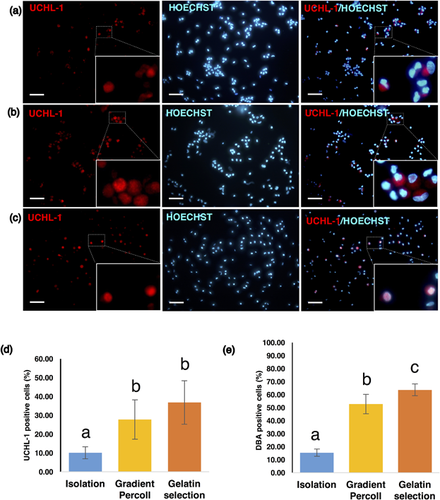
In adult testicular cell suspensions, two fractions that appeared at approximately 20% and 40% Percoll gradients were collected after gradient centrifugation. Cell suspensions were seeded in pregelatinized dishes for 2 hr to allow somatic cells to attach to the dishes, and the floating cell suspensions were collected. DBA-positive cells were identified in both the 20% and 40% Percoll fractions. In the preliminary experiments, the isolated fractions were evaluated for colony formation by short-term culture. Cells that were collected from the 20% fraction formed colonies and could be passaged for further expanded culture. In contrast, cells from the 40% fraction did not form colonies and could not proliferate after primary culture (Figures S1a and S1b). Therefore, only the cell suspension from the 20% fractions was used for further studies. The number of SSCs in culture was estimated by UCHL-1 and DBA staining (Figures 2a and 2b). The percentage of UCHL-1-positive cells in the freshly isolated cell suspensions was 10.03 ± 3.22% and significantly increased to 27.71 ± 10.46% after Percoll gradient centrifugation, then further increased to 36.81 ± 11.52% after negative selection on gelatin-coated dishes (Figures 2c and 2d). Similar results were obtained by using DBA staining (before Percoll: 15.31 ± 2.87%; after Percoll: 52.70 ± 7.50%; after plating on gelatin-coated dishes: 63.62 ± 4.60%) (Figure 2e).
2.3 Derivation of cell lines from undifferentiated spermatogonia of immature bovine testes
Enriched populations of undifferentiated spermatogonia from immature bovine testes were isolated from 3-month-old calves. Isolated cells were cultured in medium containing both 15% KSR and 1% FBS and supplemented with GDNF for the initial culture. After 5–7 days in primary culture, the cultured cells formed botryoidally aggregated multicellular colonies. After the first passage, the culture medium was changed to DMEM/F12 medium supplemented with 20% KSR, and the cells were passaged every 5 days. In the later passages (passages 7 and 8), the cultures were passaged every 2–3 days owing to robust proliferation of undifferentiated spermatogonia. The cell lines formed botryoidally aggregated colonies in primary culture (Figure 3a, white arrow) and gradually changed their morphology to become dome-shaped ES cell-like colonies after several passages (Figure 3a, black arrow).

The effects of different growth factors on the proliferation of undifferentiated spermatogonia were also tested. We compared the effect of KSR media supplemented with GDNF only, bLIF only, and a combination of GDNF and bLIF on the number of colonies formed (Figure 3b) and the total number of cells (Figure 3c). The results suggested that there were no significant differences among different culture conditions (Figures 3b and 3c). However, RT-PCR results revealed that the colonies cultured in the presence of bLIF or GDNF and bLIF strongly expressed UCHL-1 after long-term culture (Figure 3d).
2.4 Characterization of cell lines from undifferentiated spermatogonia of immature bovine testes
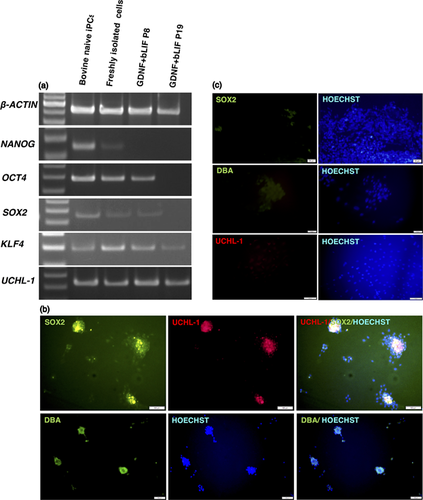
RT-PCR analysis was performed on freshly isolated undifferentiated spermatogonia and 4 cultured cell lines at passage 8 and passage 19 (Figure 4a). Freshly isolated undifferentiated spermatogonia express pluripotency-associated genes (NANOG, OCT4, SOX2, and KLF4), as well as the germ cell marker UCHL-1. Cell lines also expressed OCT4, SOX2, and KLF4 and UCHL-1 at passage 8. At the passage 19, however, only KLF4 and UCHL-1 were detected (Figure 4a). Immunofluorescent analysis was performed on the cell lines and detected a pluripotent marker (SOX2) and germ cell markers (UCHL-1 and DBA) (Figure 4b).
2.5 Derivation of cell lines from undifferentiated spermatogonia of adult bovine testes
Isolated undifferentiated spermatogonia were cultured in one of four different media: (i) the basic medium (DMEM/F12 containing 15% KSR and 1% FBS); (ii) the basic medium supplemented with GDNF only; (iii) the basic medium supplemented with BIO only; or (iv) the basic medium supplemented with both GDNF and BIO. After 7 days of primary culture, colonies were observed (Figure 5a). There were no differences in the total number of cells in the primary cultures (P0) among the different culture conditions (Figure 5b). RT-PCR analysis showed that P0 colonies expressed the germ cell marker gene UCHL-1 (Figure 5c). In the basic medium or the basic medium with GDNF only, however, enhanced proliferation of somatic cells was observed, and those cells overwhelmed the growth of germ cell colonies at the first passages (Figure 5a). On the other hand, the basic medium with BIO or the basic medium with BIO and GDNF similarly allowed the grow of germ cell colonies (Figure 5a); therefore, only the basic medium with BIO was used as a culture medium for further experiments.
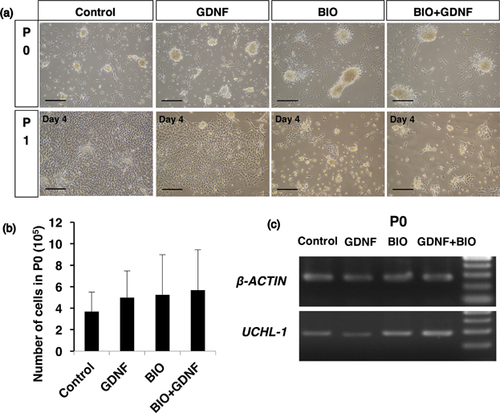
2.6 Characterization of cell lines from undifferentiated spermatogonia of adult bovine testes
Typical germ cell colonies were maintained in the presence of BIO (Figure 6a). RT-PCR analysis performed on the colonies at passages 3, 8, and 10 showed that the germ-cell-specific marker UCHL-1 was stably expressed, and the stem-cell specific markers OCT4 and KLF4 were also detected with a slightly different expression pattern than SOX2 (Figure 6b). Moreover, immunofluorescence analysis confirmed the expression of DBA and GFRα-1 (Figure 6c). The same culture conditions were used to culture enriched cell suspensions from a 17-year-old bull. Colonies appeared in the presence of BIO and were maintained for at least three passages (Figure S3a). The expression of UCHL-1 was shown by RT-PCR at passage 2 (Figure S3b).
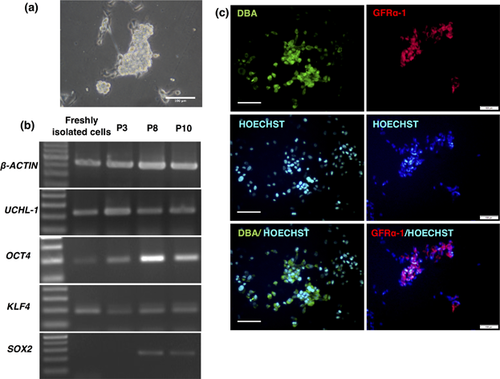
2.7 Karyotype analysis of cell lines from undifferentiated spermatogonia of immature and adult bovine testes
Karyotype analysis also showed normal metaphase spreads and chromosome number (2n = 60) in SSC cell lines from immature testes (93,3%, 28 out of 30 spreads) and adult testes (96.7%, 29 out of 30 spreads), while 6.67% had 59 chromosomes in cell lines from immature testes and 3.33% had 59 chromosomes in cell lines from adult testes (Figures 7a and 7b).
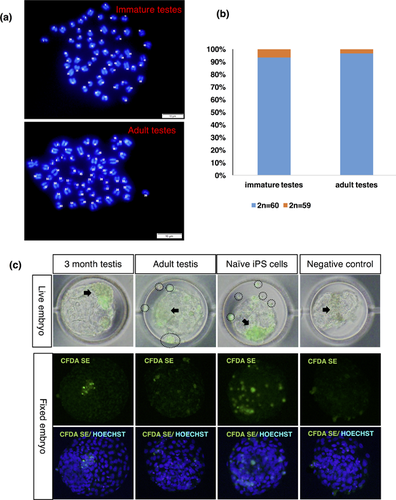
2.8 Stem cell potential of established cell lines
To examine the stem cell potential of cell lines from both immature and adult bovine testes, we tagged the cells with a long-term fluorescent tracer and allowed them to aggregate into embryos in the 8- to 16-cell stages (Figure 7c). Cell lines from immature testes were incorporated into the ICM of blastocysts (26.67%, Table 1 ), whereas cell lines from adult testes only scattered around embryos or formed cell clumps outside of embryos (Figure 7c), and a few of them incorporated specifically into the TE region of embryos (15.38%, Table 1). In contrast, neither freshly isolated cells nor bovine embryonic fibroblasts (BEFs) were incorporated into the embryos. Bovine naïve-type iPS cells, which were previously established (Kawaguchi et al., 2015), were used as positive control. These cells were highly incorporated into the embryos (14.29% ICM only and 85.71% ICM and TE, Table 1).
| Chimeric blastocysts | |||||
|---|---|---|---|---|---|
| Donor cells | No. of aggregated embryos | No. of blastocysts developed | No. integrated to ICM (%) | No. integrated to both ICM and TE (%) | No. integrated to TE (%) |
| 3 month testes | 37 | 15 (40.54) | 4 (26.67) | 0 (0.00) | 0 (0.00) |
| Adult testes | 35 | 13 (37.14) | 0 (0.00) | 0 (0.00) | 2 (15.38) |
| Freshly isolated cells | 13 | 5 (38.46) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| BEF | 19 | 8 (42.11) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Naïve-type bovine iPSCs | 14 | 7 (50.00) | 1 (14.29) | 6 (85.71) | 0 (0.00) |
| Embryo only | 19 | 10 (52.63) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
3 DISCUSSION
Cell suspensions from immature and adult bovine testes were purified by Percoll gradient centrifugation. The proportions of UCHL-1-positive and GFRa-1 positive cells in the cell suspensions from immature testes were higher and sufficient to enrich undifferentiated spermatogonia by Percoll gradient centrifugation (Figures 1c and 1d). However, the population of undifferentiated spermatogonia in adult testicular cell suspension was significantly lower than that from immature testes (Figure 1c–e). Differential plating on gelatin-coated dishes after Percoll gradient centrifugation was necessary to enrich undifferentiated spermatogonia from adult testes.
Undifferentiated spermatogonia from immature testes formed colonies in culture in the presence of GDNF and FBS; however, testicular somatic cells also proliferated and overgrew in the cultures after a few passages (Figure S4). By contrast, when undifferentiated spermatogonia were cultured under serum-free conditions in the presence of KSR medium containing both GDNF and bLIF, undifferentiated spermatogonia colonies were formed and maintained for a long period.
The colonies derived from immature testes undifferentiated spermatogonia were identified by their botryoidally aggregated cell morphology (Figure 3a) and then transformed to tightly packed 3-dimensional colonies, similar to mouse ES cells, after a month of culture (Figure 3a). These ES cell-like colonies were maintained for only 2 months (11 passages); however, the botryoidally aggregated colonies were more stable and could be maintained for more than 3 months under serum-free culture conditions. SSCs isolated from neonatal mouse testes start to form ES-like colonies within 4–7 weeks after initial culture (Kanatsu-Shinohara et al., 2004) and can subsequently be cultured under ES cell culture conditions while maintaining their ES-like properties (Kanatsu-Shinohara et al., 2004). ES-like bovine undifferentiated spermatogonia colonies were cultured on STO feeder cells, which is the same culture condition used for previously established bovine naïve-type iPS cells (Kawaguchi et al., 2015); however, they could not be maintained under this condition.
The established cell lines from immature testes had mouse ES cell-like morphology in serum-free medium supplemented with GDNF and bLIF, and they expressed both germ-cell-specific (UCHL-1 and DBA) and pluripotent stem cell markers (OCT4, SOX2, KLF4). Consistent with our results, other studies have reported that in vitro culture of gonocytes from neonatal bovine testes yields ES cell-like colonies (Sahare et al., 2015) that express cell surface markers of ES cells (Li et al., 2016). In contrast, isolated undifferentiated spermatogonia from adult testes could be maintained in the medium only in the presence of BIO and formed colonies with botryoidally aggregated cell morphology, similar to typical mouse GS cells. All four established cell lines expressed the germ-cell-specific markers UCHL-1, DBA, and GFRα-1 and the pluripotent stem cell markers OCT4, SOX2, and KLF4. BIO is an inhibitor of glycogen synthase kinase-3α (GSK3) (Sato, Meijer, Skaltsounis, Greengard, & Brivanlou, 2004). Inhibition of GSK3 leads to the activation of the Wnt/β-catenin signaling pathway. As recently reported in tree shrew (Li et al., 2017), Wnt/β-catenin signaling pathway is involved in the maintenance of undifferentiated spermatogonia from adult testes during the early stage of in vitro culture. Moreover, Wnt signaling also supports regulation of the character as stem cells of mouse spermatogonial stem/progenitor cells (Golestaneh et al., 2009) and promotes the proliferation of SSC progenitor cells (Takase & Nusse, 2016).
In this study, undifferentiated spermatogonia isolated from immature testes could be maintained for long term in the basic medium with GDNF. Therefore, we hypothesized that once adult undifferentiated spermatogonia gained stem cell-like properties in the presence of BIO, BIO could be omitted from the culture and replaced by GDNF. After removal of BIO at 10 passages, colonies can be maintained in the basic medium with only GDNF (Figure S5). Moreover, colonies could not be maintained in culture by the removal of BIO before 10 passages. This result suggests that during the first 10 passages in the presence of BIO, a fraction of undifferentiated spermatogonia in heterogeneous differentiated states in the adult testis may transform to more undifferentiated states such as SSCs. In contrast, undifferentiated spermatogonia from immature testes could be maintained in the presence or absence of BIO with no significant differences (data not shown). To examine the stem cell potential of the established cell lines, we co-cultured three different cell lines with in vitro fertilized bovine embryos at 8–16 cell stage. Cell lines from immature testes were incorporated into the ICM region in 26.67% of blastocysts (Table 1 and Figure 7c); however, none of the freshly isolated undifferentiated spermatogonia could be incorporated into blastocysts. In contrast, the contribution of cell lines from adult testes was limited, consistent with a study showing that the contribution of mouse SSC-derived cell lines to chimeras is dependent on the age of the testes (Azizi et al., 2016). In our previous reports (Kawaguchi et al., 2015), established naïve-type bovine iPS cells were abundantly incorporated into both the ICM and TE regions of blastocysts after aggregation with 8- and 16-cell embryos. Taken together, the evidence shows that cells of cell lines derived from immature testes have partial stem cell potential, probably because of a heterogeneous stem cell population in the established cell lines or a cell population with both stem cell and germ cell characteristics, but those from adult testis have narrower limits on their potential as stem cells. However, further studies are necessary to examine the ability of the cell lines to contribute to chimeric fetuses and contribute to spermatogenesis through their germ cell potential in bovine testes.
To extend this culture system for aged animals or wild animals, we isolated undifferentiated spermatogonia from a 17-year-old bull that was a sire but was no longer used for semen collection. Isolated undifferentiated spermatogonia from that bull could be successfully cultured for three passages at most (Figure S3), and the population of undifferentiated spermatogonia in the testis was very limited (Figure S1). Procedures for enrichment of the undifferentiated spermatogonia population and stimulation of undifferentiated spermatogonia proliferation are necessary for aged and seasonally breeding animals.
4 MATERIALS AND METHODS
4.1 Ethical statement
All animal experiments were performed in accordance with the “Welfare Management of Livestock” standards of Gifu Prefectural Livestock Research Institute and the guidelines of the Kyoto University Livestock Farm and were approved by the Kyoto University Animal Care and Use Committee.
4.2 Isolation of undifferentiated spermatogonia from bovine testes
Undifferentiated spermatogonia were isolated by sequential enzymatic digestion methods as previously reported (Fujihara et al., 2011) with minor modifications. Briefly, the testes were collected from Japanese Black cattle of the following ages: 3 months old, 1–2 years old, and 17 years old. The collected testes were placed immediately into a tube and immersed in Dulbecco's modified Eagle's medium (DMEM)/F12 medium (GIBCO BRL Invitrogen, Carlsbad, CA) supplemented with 100 IU ml−1 penicillin (Sigma–Aldrich, St. Louis, MO), 50 mg ml−1 streptomycin (Sigma–Aldrich), 40 mg ml−1 gentamicin sulfate (Sigma–Aldrich) and 15 mM HEPES (Wako, Osaka, Japan). The testes were transported to the laboratory at 4–10 °C within 24 hr. To prepare cell suspensions, we minced the decapsulated testicular tissues and treated with a three-step enzymatic digestion method. The cells were incubated with the first collagenase solution for 50 min at 37 °C, then washed three times with DMEM/F12. This was followed by incubation with a second collagenase solution for 50 min at 37 °C, after which the cells were washed again three times with DMEM/F12. The obtained suspensions were centrifuged, and the resulting cell pellets were incubated with a third collagenase solution for only 10 min at 37 °C, washed three times and filtered through a 50-μm nylon mesh.
4.3 Enrichment of isolated undifferentiated spermatogonia from immature bovine testes
Percoll gradient centrifugation was used for enrichment of undifferentiated spermatogonia isolated from immature testes. We used a Percoll gradient of 60%, 40%, and 20% Percoll (GE Healthcare Life Sciences, Sweden) as previously reported (Fujihara et al., 2011) with minor modifications. Cell suspensions were loaded on top of the Percoll gradients and centrifuged at 3,000 rpm for 30 min. After centrifugation, different cell fractions were observed. The germ-cell fraction was collected from the 20–40% Percoll fraction. The purity of the fraction was assessed by immunofluorescence staining of cell suspensions for GFRα-1 and UCHL-1.
4.4 Enrichment of isolated undifferentiated spermatogonia from adult bovine testes
Since undifferentiated spermatogonia are a rare cell population in the adult testis, the undifferentiated spermatogonia fraction of the filtered suspension of adult testicular cells was enriched by a two-step purification protocols. Percoll gradient centrifugation was performed as the first germ cell selection step, and the germ cells were collected from the 20–40% Percoll fraction. Subsequently, differential plating of the cell suspension on a gelatin-coated culture dish were used to remove any additional remaining somatic cells from the enriched Percoll fractions. The undifferentiated spermatogonia-rich fractions were plated on 0.1% pregelatinized dishes in DMEM/F12 media supplemented with 5% fetal bovine serum (FBS, Invitrogen) for 2 hr. Most of the somatic cells attached to the culture dish, while the undifferentiated spermatogonia floated. Floating cells were collected and used for further studies. The purity of the cell suspensions was assessed by immunofluorescence staining for DBA and UCHL-1.
4.5 Histological analysis of the testes
Isolated testes were fixed with Bouin's solution, embedded in paraffin using standard procedures, and sliced to a thickness of 6 μm. The paraffin sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin.
4.6 Culture of undifferentiated spermatogonia isolated from immature bovine testes
Collected undifferentiated spermatogonia were cultured at 5 × 104 cells/cm2 on poly-L-lysine (0.01%)-precoated wells in 24-well plates with basic media containing DMEM/F12, 100 μg/ml penicillin (Sigma–Aldrich), 50 μg/ml streptomycin (Sigma–Aldrich), 40 μg/ml gentamycin sulfate (Sigma–Aldrich), 10 μg/ml apotransferrin (Sigma–Aldrich), 10 μg/ml insulin (Sigma–Aldrich), 110 μg/ml sodium pyruvate (Sigma–Aldrich), 0.015% (v/v) sodium lactate (Sigma–Aldrich), 0.1% (v/v) non-essential amino acid solution (GIBCO BRL Invitrogen), and 0.01 mM β-mercaptoethanol (Wako). In the initial culture, the basic media were supplemented with 15% knockout serum replacement (KSR), 1% FBS and 20 ng/ml GDNF. After the first passage, the media were replaced with DMEM/F12 supplemented with 20% KSR and growth factors. Different growth factors—GDNF, bLIF, or both in conjunction—were added to find the optimal culture conditions for undifferentiated spermatogonia from immature testes. To establish cell lines, we employed a combination of GDNF and bLIF. The culture media were changed every 3 days.
4.7 Culture of undifferentiated spermatogonia isolated from adult bovine testes
Isolated and enriched undifferentiated spermatogonia from adult testes were cultured at a cell density of 5 × 104 cells/cm2 on poly-L-lysine (0.01%)-precoated 24-well plates (Nunc) using DMEM/F12 supplemented with 15% KSR (Invitrogen) and 1% FBS. Culture conditions for undifferentiated spermatogonia were studied using four different culture media: the basic medium only, the basic medium supplemented with 20 ng/ml GDNF (Peprotech, Rocky Hill, NJ), the basic medium supplemented with 2 μM of BIO, and the basic medium supplemented with both GDNF and BIO. After 6–7 days of primary culture, multicellular botryoidally aggregated colonies were observed in all cultures. The cells were passaged with 0.25% trypsin and 0.5 mM EDTA (Nacalai Tesque, Kyoto, Japan) and further cultured at 37 °C in a humidified atmosphere with 5% CO2. The culture media were changed every 3 days.
4.8 Immunocytochemical analysis of cultured undifferentiated spermatogonia
Immunocytochemical analysis on freshly isolated or cultured cells was performed as described previously (Kim et al., 2014). Briefly, the cells were fixed with 4% paraformaldehyde, permeabilized, blocked, and incubated with the appropriate primary and secondary antibodies indicated below.
The following antibodies and dilutions were used: anti-DBA conjugated with fluorescein isothiocyanate (FITC) (1:200; Vector Laboratories, Burlingame, CA), anti-GFRα-1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), anti-UCHL-1 (PGP 9.5; 1:200; Santa Cruz Biotechnology), anti-SOX2 (1:200; Santa Cruz Biotechnology), and anti-GATA4 (1:200, Santa Cruz Biotechnology). All primary antibodies were incubated with the specimens overnight at 4 °C. Then, the cells were washed three times with TBST (Sigma), incubated with the appropriate secondary antibodies—either Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) or Alexa Fluor 594 rabbit anti-goat (Invitrogen)—for 1 hr at room temperature, and washed three more times with TBST. The nuclei were counterstained with Hoechst 33342 (Sigma), and the specimens were mounted in 50% glycerol. Samples were observed under an immunofluorescence microscope (BX 50; Olympus, Tokyo, Japan).
4.9 RT-PCR
RNA was isolated from either freshly isolated testicular cells or cultured cells using TRIzol reagent (Ambion, Austin, TX) according to the manufacturer's instructions. cDNA synthesis was performed using 1 μg of total RNA per 20 μl of PCR reaction mixture. Briefly, oligo(dT) primers were added to the isolated RNA and incubated for 5 min at 65 °C. ReverTra Ace (Toyobo, Tokyo, Japan) was added to the RNA mixture for the reverse transcriptase reaction. The samples were incubated for 60 min at 42 °C followed by 5 min at 99 °C. The collected cDNA was stored at −20 °C or immediately used for PCR. PCR amplification was performed using 1 μl of cDNA per 20 μl of PCR reaction mixture containing 2 mM MgCl2, 0.25 mM dNTPs, 1 × PCR buffer, 5 pmol of each primer and 1 U of Taq DNA polymerase (ExTaq; TaKaRa, Tokyo, Japan). The primer sequences used for the amplification of specific genes are listed in Table 2. The PCR products were separated and visualized on 2% (w/v) agarose gels containing ethidium bromide.
| Primer sequence (5′–3′) | ||
|---|---|---|
| Gene name | Forward | Reverse |
| UCHL-1 | ACCCCGAGATGCTGAACAAAG | CCCAATGGTCTGCTTCATGAA |
| OCT4 | AGAGAAAGCGGACGAGTAT | AGTACAGAGTAGTGAAGTGAGG |
| SOX2 | TTACCTCTTCTTCCCACTCC | TTCTTGCTGTCCTCCATTTC |
| KLF4 | CCCACACAGGTGAGAAACCT | ATGTGTAAGGCGAGGTGGTC |
| C-KIT | GACCTGGAGGACTTGCTGAG | AGGGGCTGCTTCCTAAAGAG |
| STRA8 | GATGGGAATGCAAACAGCTT | GTCCAGGAAACTTGCCACAT |
| SYCP3 | TGACTTTGTTCCAGCAGTGG | ACTTTCGGACACTTGCCATC |
| β-ACTIN | TCCCTGGAGAAGAGCTACGA | ACATCTGCTGGAAGGTGGAC |
4.10 Karyotype analysis
Karyotype analysis was performed using a previously described protocol (Campos, Sartore, Abdalla, & Rehen, 2009). Cultured cells were incubated in 0.1 μg/ml KaryoMAX Colcemid Solution (Invitrogen) for 3 hr. The cells were dissociated to a single-cell suspension using 0.25% trypsin-EDTA solution. Metaphase spreads were prepared by incubating single-cell suspensions in a prewarmed (37 °C) hypotonic solution (KCl 75 mM) for 15 min and fixed in 3:1 (methanol [Wako]:glacial acetic acid [Sigma]) fixative solutions overnight at 4 °C. Chromosomes were stained with VECTASHIELD mounting medium with DAPI (Vector) and observed under an immunofluorescence microscope (BX 50; Olympus). Thirty metaphase spreads from three independent cell lines were counted.
4.11 Aggregation of cell lines with bovine embryos
Bovine 1-cell embryos at 20 hr post-insemination (hpi) and produced in vitro as described elsewhere (Ikeda, Kawahara-Miki, Iwata, Sugimoto, & Kume, 2017) were treated with pronase solution (0.5% [w/v] in PBS) to remove the zona pellucida. The zona-free 1-cell embryos were individually allocated to a well-of-the-well system (Vajta et al., 2008) using the LinKID micro25 culture dish (Dai Nippon Printing, Tokyo, Japan) with 50 μl of modified synthetic oviduct fluid (mSOF) and cultured until 70 hpi. At 70 hpi, only the embryos that had developed to the 8-cell to 16-cell stages were left, and the culture medium was replaced with mSOF containing 5% (v/v) KSR, bLIF-conditioned medium (1:1,000 dilution), and 20 ng/ml GDNF.
Cell lines were tagged with 0.1 μl of Vybrant CFDA SE cell tracer dye (Invitrogen) according to the manufacturer's instructions. Subsequently, 10–20 cells from each of three independent cell lines were transferred to zona-free embryos at the 8- to 16-cell stages. After four to 5 days in culture, the embryos were observed under a fluorescence microscope. For subsequent examination, embryos were fixed with 4% PFA, prepared on slides, and counterstained with Hoechst 33342 (Sigma).
4.12 Statistical analysis
Pairwise comparisons of group means were conducted using Student's t-test. Multiple comparison analysis was carried out using ANOVA followed by the least significant difference test.
ACKNOWLEDGEMENTS
We thank Dr. K. Mukojima of the Gifu Prefectural Livestock Research Institute for providing adult testes, and we thank Dr. Y. Hoshino, Mr. H. Yoshioka, and Ms. E. Itoyama of the Kyoto University Livestock Farm for providing immature testes. We also thank Drs. Gabriela Durcova-Hills and Sandeep Goel for discussion and critical reading of the manuscript. This work was supported by the Grant-in-Aid for Scientific Research (B) (no. 26292168) from Japan Society for the Promotion of Science.