The Effect of Free and Protein-Bound Maillard Reaction Products N-ε-Carboxymethyllysine, N-ε-Fructosyllysine, and Pyrraline on Nrf2 and NFκB in HCT 116 Cells
Abstract
Scope
Maillard reaction products (MRPs) are believed to interact with the receptor for advanced glycation endproducts (RAGE) and lead to a pro-inflammatory cellular response. The structural basis for this interaction is scarcely understood. This study investigates the effect of individual lysine modifications in free form or bound to casein on human colon cancer cells.
Methods and results
Selectively glycated casein containing either protein-bound N-ε-carboxymethyllysine (CML), N-ε-fructosyllysine (FL), or pyrraline is prepared and up to 94%, 97%, and 61% of lysine modification could be attributed to CML, FL, or pyrraline, respectively. HCT 116 cells are treated with free CML, pyrraline, FL, or modified casein for 24 h. Native casein is used as control. Intracellular MRP content is analyzed by UPLC-MS/MS. Microscopic analysis of the transcription factors shows no activation of NFκB by free or protein-bound FL or CML, whereas casein containing protein-bound pyrraline activates Nrf2. RAGE expression is not influenced by free or casein-bound MRPs. Activation of Nrf2 by pyrraline-modified casein is confirmed by analyzing Nrf2 target proteins NAD(P)H dehydrogenase (quinone 1) (NQO1) and heme oxygenase-1 (HO-1).
Conclusion
Studies on the biological effects of glycated proteins require an individual consideration of defined structures. General statements on the effect of “AGEs” in biological systems are scientifically unsound.
1 Introduction
The Maillard reaction (MR) describes the non-enzymatic reaction between reducing sugars and amino compounds. Unlike other name reactions, the Maillard reaction does not refer to one specific reaction, but describes a complex reaction cascade leading to a plethora of structurally diverse compounds.[1, 2] Based on the work of Hodge,[3] it is generally accepted that the reaction is divided into three stages: the early MR occurs when aldoses or ketoses react with amino groups to produce Amadori and Heyns products, respectively. During the late stage MR, Amadori and Heyns compounds either directly form so-called advanced glycation endproducts (AGEs) or degrade to 1,2-dicarbonyl compounds, which react further with amino components to form AGEs. The final stage of the MR describes the formation of complex browning products, so-called melanoidins. Maillard reaction products (MRPs) can either occur as single amino acid modifications (free form) or bound to a protein backbone (protein-bound form). The occurrence of glycation compounds in food and their contribution to food color and aroma was studied extensively during recent times.[4-6] The highest levels were found in high-heat processed nut or grain products, canned meats and milk products, whereas fruits, vegetables, butter, and coffee had the lowest content of glycation products. The main dietary glycation compounds are N-ε-fructosyllysine (FL) as a representative of Amadori products, and N-ε-carboxymethyllysine (CML) and pyrraline as representative AGEs.[7] Approximately 500–1200 mg fructosyllysine and 25–75 mg of protein-bound AGEs, mainly CML and pyrraline, are ingested with the daily diet rich in bakery products, pasta and coffee.[7] Therefore, the occurrence of MRPs in the human digestive tract and their contact with human tissues and cells appears to be inevitable.
In earlier studies, the presence of glycation compounds in human tissues originating from the endogenous reaction of glucose and proteins was linked to the development of age-related diseases or metabolic disorders, such as Alzheimer's or diabetes.[8, 9] Glycation compounds in vivo may lead to the production of promoters for oxidative stress and inflammatory processes.[10-12] Therefore, the presence of high amounts of circulating glycation compounds is often regarded as potentially harmful. The fate of dietary glycation compounds is discussed controversially in the scientific community. According to studies performed with human subjects, dietary advanced glycation compounds accumulate in vivo, and thus contribute to the endogenous “AGE pool”. There are several studies which show that concentrations of glycation compounds seem to correlate with their dietary intake.[13-16] Based on these results, dietary glycation compounds are discussed as possible risk factors for the development of diseases such as diabetic nephropathy and inflammation.[17, 18]
The main mechanism by which glycation compounds mediate inflammation is believed to be the binding of the compounds to the receptor for advanced glycation endproducts (RAGE), resulting in the activation of inflammatory responses.[19] This type I cell-surface receptor belongs to the immunoglobulin superfamily and has been described as a pattern recognition receptor.[20, 21] RAGE is expressed on several cell types (monocytes/macrophages,[22] T-lymphocytes,[23] endothelial cells,[24] dendritic cells,[25] fibroblasts,[26] neuronal cells, glia cells, chondrocytes, and[27] keratinocytes[28]) and recognizes a large number of different ligands such as amyloid β peptide, S100/calgranulin protein, high mobility group box 1 protein (HMGB1), and lipopolysaccharide (LPS). Moreover, it was shown that RAGE is expressed in intestinal epithelial cells as well as human colon tissue samples.[29] The interaction of ligands with RAGE promotes NADPH-oxidase activity, resulting in the activation of the transcription factor NFκB, followed by expression of pro-inflammatory genes such as interleukin 6 (IL-6) and tumor necrosis factor (TNF-α).
A counterpart of the RAGE-mediated NFκB signaling is the Nrf2 pathway, which is a major mechanism in cellular defense against oxidative and electrophilic stress. It functions through the activation of the Nrf2-antioxidant response-signaling pathway. This pathway controls gene expression of proteins involved in deactivation and metabolism of oxidants and electrophilic agents, such as glutathione reductase, glutathione S-transferase, glutathione peroxidases, glyoxalase 1, aldehyde reductases, and aldehyde dehydrogenases.[30, 31] In regard to the MR, it was shown for melanoidins to increase the nuclear translocation of Nrf2 and thereby leading to the transcription of cytoprotective genes.[32]
When MRPs are tested for their effects on cells, glycated model proteins without profound chemical characterization are commonly employed. In most cases albumin is used which is incubated with reducing sugars for several weeks at 37 °C in a phosphate buffer. This results in multiple modifications of mostly lysine and arginine residues and the formation of a variety of structurally diverse MRPs. As a result, the effects observed by such a protein containing multiple modifications cannot be directly linked to one specific MRP, but rather to the more general process of glycation. Therefore, the aim of the present study was to synthesize specific MRPs, namely CML, FL and pyrraline, at the lysine side chains of the model protein casein. The specifically glycated caseins were applied to human colon cancer cells HCT 116 and the effects on NFκB and Nrf2 and their target proteins were studied. Thus, our results contribute to the structure-effect relationship of MRPs and subsequent cellular reactions.
2 Experimental Section
2.1 Chemicals
Carboxymethyllysine, d4-carboxymethyllysine, pyrraline, and d4-pyrraline were purchased from Iris Biotech (Marktredwitz, Germany). Sodium borohydride, sodium hydroxide, acetone, trichloroacetic acid, and glucose were obtained from Carl Roth (Karlsruhe, Germany). Perfluoropentanoic acid (NFPA) was purchased from Alfa Aesar (Karlsruhe, Germany). Borate buffer (0.4 n, pH 10.2) was obtained from Agilent (Boeblingen, Germany) and ε-fructosyllysine dihydrochloride as well as the isotope labeled ε-fructosyllysine dihydrochloride (l-lysine 13C6,15N2) were from SynInnova (Edmonton, Canada). McCoy's 5A (modified) medium, penicillin streptomycin solution, hygromycin B solution, GlutaMAX supplement, and sulfuric acid were from Thermo Fisher Scientific (Schwerte, Germany) and fetal bovine serum was from BioSell (Feucht, Germany). HCT 116 pTRAF cells were kindly gifted by A. Kipp (Friedrich-Schiller University Jena, Germany). The detergent-compatible protein assay kit was purchased from Biorad (Feldkirchen, Germany). Dihydrorhodamine 123 was from Biotium (Fremont, USA). Auranofin was obtained from Enzo Life Sciences (Lörrach, Germany) and recombinant human TNF-α was from PeproTech (Hamburg, Germany). Hoechst staining solution was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). LC-MS grade water was from VWR (Darmstadt, Germany) and benzaldehyde, sodium acetate, sodium cyanoborohydride, selenium reagent mixture, pronase E from Streptomyces griseus and LC-MS grade acetonitrile was from Merck (Darmstadt, Germany). Sodium caseinate was from Meggle (Wasserburg, Germany). Glyoxylic acid, p-toluidine, reduced glutathione, pepsin from porcine gastric mucosa, prolidase from porcine kidney as well as dialysis tubing membrane (MWCO 14 kDa) were from Sigma–Aldrich (Darmstadt, Germany). Sodium dihydrogenphosphate dihydrate, disodium hydrogenphosphate, magnesium nitrate, sodium hydroxide, and boric acid were from Grüssing (Filsum, Germany). Benzhydrazide was from abcr (Karlsruhe, Germany) and Diethylether was from Honeywell (Seelze, Germany). Serdolit MB-2 was purchased from Serva (Heidelberg, Germany). Native porcine leucine aminopeptidase was from Creative Enzymes (Shirley, USA). For all experiments except LC-MS/MS, ultra-pure water was used prepared by a Milli-Q Advantage A10 water system from Merck (Darmstadt, Germany).
2.2 Synthesis of Modified Caseins
FL- and CML-enriched casein was prepared according to the literature[33] with slight modifications. Incubations of FL-enriched casein were scaled up: 6 g of sodium caseinate and 1.1 g of glucose were suspended in 50 mL of water. After lyophilization and storing in a desiccator over a saturated solution of magnesium nitrate (aw = 0.52) for 5 days, the mixture was heated in a drying oven for 4 h at 60 °C and then suspended and dialyzed against distilled water for 2 days, the distilled water was changed three times daily. The retentates were freeze-dried and stored at −18 °C.
CML-enriched casein was synthesized by incubation of 2 g of sodium caseinate and 60 mg of glyoxylic acid in 100 mL of 0.2 m sodium phosphate buffer (pH 7.4) after the addition of 1.1 g NaBH3CN for 20 h at 40 °C in an oil bath. A dialysis against distilled water followed, until cyanide was no longer detectable with Quantofix Cyanid (Macherey-Nagel, Düren, Germany). The retentates were freeze-dried and stored at −18 °C.
Pyrraline-enriched casein[34] and 3-DG[35] were prepared according to the literature with slight modifications: Sodium caseinate (8 g) was dissolved in 1 L 0.1 m sodium acetate buffer (pH 5.5). The pH was adjusted to 5.5 with sodium hydroxide, and 2.8 g of crude 3-DG (purity approximately 50%) was added. The solution was freeze-dried and heated for 10 min at 70 °C in a sand bath. After the incubation, pyrraline-enriched casein was suspended in water and dialyzed as described for FL-enriched casein. The retentates were freeze-dried and stored at −18 °C.
Synthesis blanks were prepared by the same procedure stated above but without the addition of glucose (FL-enriched casein), glyoxylic acid (CML-enriched casein), or 3-DG (pyrraline-enriched casein).
2.3 Characterization of Native and Modified Casein
2.3.1 Enzymatic Hydrolysis
Enzymatic hydrolysis was performed according to literature[36]: In brief, a sample containing 2–4 mg protein was dissolved in 1 mL 0.02 m HCl and 50 µL pepsin solution was added (containing 100 µg, i.e., 320 U pepsin/50 µL 0.02 m HCl). Twenty microliter internal standard mixture A (containing 12 µg mL−1 d4-CML, d4-pyrraline, 13C6,15N2-FL) and 20 µL internal standard mixture B (containing 20 µg mL−1 13C3-CEL, 13C6-MG-H1) or 40 µL internal standard mixture C (containing 7.5 nmol mL−1 13C6-3-DG-H) were added. After incubation for 24 h at 37 °C, 250 µL 2 m Tris-HCl buffer (pH 8.2) and 50 µL pronase E solution (containing 100 µg pronase E [400 PU]/50 µL 2 m Tris-HCl buffer pH 8.2) were added. After another incubation for 24 h at 37 °C, 2.5 µL prolidase (0.1 U µL−1) and 0.3 U leucine-aminopeptidase were added and the solution was subsequently incubated another 24 h at 37 °C.
After centrifugation at 10 000 g for 10 min, the supernatant was treated with solid phase extraction (SPE) after addition of 50 µL water and 100 µL NFPA according to literature.[37] SPE cartridges (Chromabond HLB, Macherey-Nagel, Düren, Germany) were prepared by flushing them with 2 mL of methanol, 2 mL of methanol/10 mm NFPA (50 + 50, v + v), and 4 mL of 10 mm NFPA. After application of the prepared supernatant, the SPE cartridge was washed with 4 mL of methanol/10 mm NFPA (5 + 95, v + v) and the analytes were eluted with 5 mL of methanol/10 mm NFPA (90 + 10, v + v). The eluate was evaporated and redissolved in 100 µL mixture of eluent A and B (86 + 14) for HPLC-MS/MS or in 50 µL 10 mm NFPA in water for analysis of FL, CML, and pyrraline from modified caseins.
2.3.2 LC-MS/MS
HPLC-MS/MS analysis was performed according to literature[38] with an Agilent 1200 series chromatographic system coupled to an Agilent 6410 mass spectrometer with electrospray ion source (all from Agilent, Waldbronn, Germany). An Intrada Amino Acid column (50 mm × 3 mm) (Imtakt, Kyoto, Japan) was used for chromatographic separation at a column temperature of 35 °C. The elution buffers were 0.1% formic acid in acetonitrile (eluent A) and 100 mm ammoniumformiate in water (eluent B). The elution program (flow rate: 0.4 mL min−1) started with 86% A until 3 min, which then reduced to 0% at 10 min. From 10 to 12 min eluent A rose to 86% and the column was equilibrated for 4 min until 16 min at initial conditions. The injection volume was 10 µL. Gas temperature was set to 350 °C, gas flow to 11 L min−1, nebulizer pressure to 35 psi and capillary voltage to 4000 V. For quantitative analysis, analytes were measured in positive MRM mode with the following transitions and collision energies (CEs) and fragmentor voltages (FVs). CML: 205 → 84 (q, FV 100 V, CE 20 V), 205 → 130 (Q, FV 100 V, CE 10 V), d4-CML: 209 → 88 (q, FV 90 V, CE 20 V), 209 → 134 (q, FV 90 V, CE 5 V), CEL: 219 → 84 (q, FV 100 V, CE 20 V), 219 → 130 (Q, FV 100 V, CE 10 V), 13C3-CEL: 222 → 84 (q, FV 90 V, CE 20 V), 222 → 130 (q, FV 90 V, CE 10 V), pyrraline: 255 → 175 (q, FV 80 V, CE 5 V), 255 → 148 (Q, FV 80 V, CE 17 V), d4-pyrraline: 259 → 179 (q, FV 95 V, CE 5 V), 259 → 152 (Q, FV 95 V, CE 17 V), MG-H1: 229 → 114 (q, FV 75 V, CE 10 V), 229 → 166 (Q, FV 75 V, CE 10 V), 13C6-MG-H1: 235 → 115 (q, FV 120 V, CE 10 V), 235 → 171 (Q, FV 120 V, CE 10 V), FL: 309 → 84 (q, FV 125 V, CE 36 V), 309 → 225 (Q, FV 125 V, CE 13 V), 13C6,15N2-FL: 317 → 233 (q, FV 70 V, CE 13 V), 317 → 129 (Q, FV 70 V, CE 15 V), 3-DG-H: 319 → 204 (Q, FV 135 V, CE 17), 319 → 116 (q, FV 135 V, CE 22), 13C6-3-DG-H: 325 → 210 (Q, FV 135 V, CE 17), 325 → 116 (q, FV 135 V, CE 20). Transitions used for quantification are labeled with q and transitions used for the confirmation of the presence of the analyte are labeled with Q. For data acquisition and peak integration, Masshunter software (Agilent, Waldbronn, Germany) was used.
Analysis of FL, CML, and pyrraline content from modified caseins was performed with an Acquity Ultra Performance LC system coupled to a Waters TQ-XS mass spectrometer (both Waters Corporation, Milford, MA, USA). An Acquity UPLC BEH C18 column, (1.7 µm, 2.1 mm × 50 mm, Waters Corporation) was used for chromatographic separation at a column temperature of 40 °C. The elution buffers were 10 mm NFPA in water (eluent A) and 10 mm NFPA in acetonitrile (eluent B). The elution program (0 min, 5% B; 8 min, 29% B; 8.1 min, 85% B; 13 min, 85% B; 13.1 min, 5% B; 15 min, 5% B) was performed at a flow rate of 0.25 mL min−1. The injection volume was 10 µL. The ESI source was operated in positive mode and nebulizer nitrogen gas flow was set to 650 L h−1 and a gas temperature of 350 °C. The capillary voltage was set to 2.4 Kv and the source temperature was 150 °C. Analytes were measured in MRM mode with the following transitions and optimized CEs and cone voltages (CVs). CML: 205 → 84 (q, CV 38, CE 18 V), 205 → 142 (Q, CV 38, CE 14 V), d4-CML: 209 → 88 (q, CV 82, CE 16 V), 209 → 145.9 (Q, CV 82, CE 12 V) pyrraline: 255 → 175 (q, CV 3, CE 12 V), 255 →148 (Q, CV 3, CE 19 V), d4-pyrraline: 259.1 → 179.1 (q, CV 24, CE 10 V), 259.1 → 152 (Q, CV 24, CE 16 V), FL: 308.9 → 84 (q, CV 30, CE 31 V), 308.9 → 225 (Q, CV 30, CE 16 V), 13C6,15N2-FL: 317.1 → 90 (q, CV 2, CE 30 V), 317.1 → 233 (Q, CV 2, CE 14 V). Transitions used for quantification are labeled with q and transitions used for the confirmation of the presence of the analyte are labeled with Q. Data was acquired and evaluated with the MassLynx Software (Waters, version 4.1).
2.3.3 Size Exclusion Chromatography
SEC analysis was performed with an Azura GPC-System consisting of Autosampler AS 6.1L and ASM 2.1L with UV-detector UVD 2.1S and pump P 2.1S (all from Knauer, Berlin, Germany). For separation a Shodex Protein KW-803 column (300 mm × 8.0 mm, 5 µm) (Showa Denko, Gersthofen, Germany) was used. Elution was performed at room temperature at a flow rate of 0.3 mL min−1 with elution buffer[39] 0.1 m sodium phosphate buffer pH 6.8, containing 6 m urea, 0.1 m sodium chloride and 0.1% 3-[(3Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). Samples were dissolved at a concentration of 1 mg mL−1 in the elution buffer containing 1% dithiothreitol (DTT). After reduction at room temperature for 2 h, samples were kept at 4 °C. After membrane filtration (0.45 µm), 100 µL samples were injected and analyzed at 280 nm. For data acquisition and peak integration ClarityChrom software (Knauer, Berlin, Germany) was used.
2.3.4 Protein Content
Nitrogen content was determined with Kjeldahl method. The protein content was calculated by multiplication of the nitrogen content with the correction factor 6.38 for milk proteins.
2.3.5 Amino Acid Analysis
Amino acid analysis was performed with an amino acid analyzer consisting of amino acid reaction module S4300, amino acid reagent organizer S7130, and sample injector S5200 (all from Sykam, Fürstenfeldbruck, Germany). For separation a cation separation column LCA K07 (250 mm × 4.6 mm) (Sykam, Fürstenfeldbruck, Germany) was used. Separation buffers where purchased from Sykam (Fürstenfeldbruck, Germany). After post-column derivatization with ninhydrin, detection was performed photometrically at 440 and 570 nm. For external calibration, amino acid standard solution AAS-18 (Sigma–Aldrich, Darmstadt, Germany) was used.
2.4 Cell Culture
HCT 116 pTRAF cells were cultured in McCoy's 5A Medium with 5% fetal bovine serum, 1% GlutaMAX, and 1% penicillin streptomycin solution. Cells were passaged twice a week at a density of 400 000 cells in T75 flasks, adding 40 µL of hygromycin B 50 mg mL−1 stock solution. All cell culture experiments were performed in triplicate using three independent passages between passage number 10 and 20. The neutral red uptake assay, which is based on the ability of viable cells to incorporate and bind the supravital dye neutral red in the lysosomes, was used to evaluate cell viability.
2.5 Analysis of Transcription Factors Nrf2 and NFκB
Cells were seeded at a density of 80 000 cells in 35 mm glas bottom dishes (MatTek, Ashland, USA) followed by growth for 24 h. Cells were subsequently stimulated for 24 h with media containing auranofin (2 µm) or TNF-α (10 ng mL−1) as positive controls for Nrf2 and NFκB, respectively. To test the effect of MRPs on expression of transcription factors, cells were incubated with CML (1.2 mm), FL (500 µm), pyrraline (197 µm), native casein, CML-casein, pyrraline-casein, FL-casein, or casein synthesis blanks (each at 10 mg mL−1). Medium without addition of test substance was used as negative control. Nuclei were stained with 1 mL Hoechst 33442 staining solution (1 µg mL−1 in PBS) and washed twice with PBS followed by the addition of 1 mL medium to the cells. Fluorescence of the reporter proteins mCherry (Nrf2), TFP (NFκB), and stained nuclei was analyzed using a Zeiss LSM 780 confocal microscope (Zeiss, Jena, Germany) with a Plan-Apochromat 63x/1.40 Oil DIC M27 lens. Of each sample, five pictures consisting of 3 × 3 tiles with approximately 200 cells each were taken. ImageJ software and a macro developed in our group (T. Jung) was used to extract fluorescence area by counting colored pixels of mCherry and TFP expression and of blue stained nuclei in accumulated pictures. Quantification was achieved by calculating fluorescence areas and normalizing them to cell number based on nuclei staining. Nrf2 and NFκB activation was analyzed by calculating the ratio of the area of mCherry or TFP fluorescence to nuclei fluorescence and normalization to the negative control.
2.6 Quantification of Intracellular MRPs
Cells were cultured in 6 well plates at a density of 80.000 cells. After 24 h, culture medium was replaced by medium containing CML (1.2 mm), FL (500 µm), pyrraline (197 µm), native casein, CML-casein, pyrraline-casein, or FL-casein (each at 10 mg mL−1). Medium without addition of test substance was used as a control. After another 24 h, cells were washed two times with 3 mL of PBS and harvested by scratching with 200 µL phosphate-buffered saline solution (PBS). Cells were lysed by sonication (10 shocks at 70% power, ultra-sonic device UP50H, Hielscher Ultrasonics GmbH) followed by centrifugation (10 min, 10 000 rpm at 4 °C). A 5 µL aliquot of the supernatant was used for protein determination based on Lowry method following manufacturer's instructions (Biorad).
An aliquot of 100 µL of cell lysate was mixed with 200 µL borate buffer (0.4 n, pH 10.2) and 200 µL sodium borohydride (1 m in 0.1 n NaOH) and incubated at room temperature for 2 h. Proteins were precipitated by adding 100 µL of acetone and 1 mL of TCA (20%) followed by centrifugation (10 min, 10 000 rpm at 4 °C). An aliquot of 1500 µL of the supernatant was dried in a vacuum concentrator and resolubilized in 250 µL of 10 mm NFPA in LC-MS grade water containing internal standards (1.2 µm d4-CML, 1.0 µm d4-pyrraline, and 0.6 µm 13C6,15N2-FL). UPLC analysis was performed with an Acquity Ultra Performance LC system coupled to a Waters TQ-XS mass spectrometer (both Waters Corporation, Milford, MA, USA). For chromatographic separation, an Acquity UPLC BEH C18 column (1.7 µm, 2.1 mm × 50 mm, Waters Corporation) at a column temperature of 30 °C was used. Solvent A was 10 mm NFPA in LC-MS grade water and solvent B was 10 mm NFPA in ACN. The solvents were pumped at a flow rate of 0.6 mL min−1 in gradient mode (0 min, 1% B; 1 min, 1% B; 6 min, 40% B; 8 min, 80% B; 9 min, 80% B; 9.1 min, 1% B; 12 min, 1% B). The injection volume was 5 µL. The ESI source was operated in positive mode and nitrogen was utilized as the nebulizing gas with a gas flow of 650 L h−1 and gas temperature of 350 °C. The capillary voltage was set to 2.4 kV and the source temperature was 150 °C. Analytes were measured in MRM mode with the following transitions and optimized CEs and CVs. CML: 204.9 → 84.2 (q, CV 24, CE 18 V), 204.9 → 130.2 (Q, CV 24, CE 12 V), d4-CML: 209.2 → 88.1 (q, CV 24, CE 20 V), 209.2 → 134.1 (Q, CV 24, CE 12 V) pyrraline: 255.2 → 175.2 (q, CV 14, CE 12 V), 255.2 → 148.1 (Q, CV 14, CE 18 V), d4-pyrraline: 259.2 → 179.2 (q, CV 16, CE 12 V), 259.2 → 152.1 (Q, CV 16, CE 18 V), FL: 308.1→84.1 (q, CV 24, CE 38 V), 308.1 → 225.1 (Q, CV 24, CE 16 V), 13C6,15N2-FL: 317.1 → 90 (q, CV 2, CE 30 V), 317.1 → 233 (Q, CV 2, CE 14 V). Transitions used for quantification are labeled with q and transitions used for the confirmation of the presence of the analyte are labeled with Q. Data were acquired and evaluated with the MassLynx Software (Waters, version 4.1).
2.7 Gel Electrophoresis and Western Blotting
For detection of RAGE and heme oxygenase-1 (HO-1), 80.000 cells were seeded in a six-well plate with 3 mL of cell culture medium. After 24 h, the medium was removed and test substances as mentioned for analysis of transcription factors were added to the wells. After 24 h of treatment, cells were washed with PBS, lysed with 200 µL of homogenization buffer (100 mm Tris-HCl, 300 mm KCl, 0.01% Triton X-100) containing 2 µL of protease inhibitor cocktail III (Calbiochem, Bad Soden, Germany) and scratched from the surface. Afterwards, lysates were sonicated (10 shocks at 70% power, ultra-sonic device UP50H, Hielscher Ultrasonics GmbH) and centrifuged at 20 000 × g for 10 min at 4 °C. Protein concentrations in the supernatants were obtained by the Lowry protein assay (manufacturer instructions). Reducing Laemmli buffer (0.25 m Tris, pH 6.8, 8% SDS, 40% glycerol, 0.03% orange G) was added to 15 µg protein of each sample and proteins were denatured at 95 °C for 5 min. Samples were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Blocking was performed using Odyssey Blocking Buffer (LICOR; 927-40000) 1:5 diluted in PBS. Primary and secondary antibodies were also diluted in Odyssey Blocking Buffer/PBS, containing 0.1% Tween-20. Anti-RAGE (SantaCruz, sc-365154, 1:500) and anti-HO-1 (Cell signaling, 43966S, 1:1000) were used as primary antibodies. Protein expressions obtained were normalized to GAPDH using anti-GAPDH mouse (Abcam, ab8245, 1:20 000). The experiments were carried out in triplicate.
2.8 NQO1 Activity
Activity of the Nrf2 target NAD(P)H dehydrogenase (quinone 1) (NQO1) was measured according to ref. [40]. In brief, NQO1 reduced menadione to menadiol, which reduced 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to insoluble formazan. After solubilization of formazan, its formation was followed by reading its absorption at 590 nm. An aliquot of 10 µL of cell lysate obtained for gel electrophoresis and western blotting was used for the analysis. Cell lysate was mixed with 190 µL of the reaction buffer, containing 25 mm Tris-HCl, pH 7.4, 0.665 mg L−1 BSA, 0.01% Tween 20, 5 µm FAD, 1 mm glucose-6-phosphate, 30 µm NADP, 0.72 mm MTT, 0.3 U mL−1 glucose-6-phosphate dehydrogenase, 50 µm menadione, and 50 µL of water or stop solution, containing 0.3 mm dicoumarol, 0.5% DMSO, and 5 mm potassium phosphate at a pH 7.4. The absorption of the samples containing reaction buffer and stop solution represented NQO1-independent formazan formation and was subtracted from the absorption values of the samples containing reaction buffer and water. All measurements were conducted in triplicates on a 96-well plate using a microplate reader (Synergy2, BioTek, Bad Friedrichshall, Germany).
2.9 Analysis of Glutathione (GSH)
HCT 116 pTRAF cells were cultured as mentioned above and incubated with casein-bound MRPs or native casein (10 mg mL−1) for 24 h. Cells were washed with PBS two times and harvested by scratching in 200 µL PBS. Cells were lysed by the addition of 100 µL meta-phosphoric acid (5%) followed by sonication and centrifugation (10 min, 10 000 rpm at 4 °C). The supernatant was used for HPLC-UV analysis according to ref. [41]. Briefly, GSH was separated on a C18 column (Dr. Maisch, 5 µm, 4.6 mm × 25 cm with pre-column) at 30 °C, solvent A was 10 mm potassium phosphate at pH 3.0 and solvent B was HPLC-grade methanol. Separation was achieved isocratically with 3% solvent B in 15 min at a flow rate of 1 mL min−1. Injection volume was 25 µL and GSH was detected at 200 nm. An exemplatory GSH chromatogram is shown in the figure SI 2, Supporting Information.
2.10 Analysis of Reactive Oxygen Species (ROS)
For detection of ROS, cells were seeded at a density of 40.000 cells per well in a 96 well plate for 24 h in culture medium. Cells were washed with HANK's buffer two times followed by incubation with Dihydrorhodamine 123 (DHR123, 20 µm in HANK's buffer) for 45 min at culturing conditions. Afterwards, cells were washed with HANK's buffer and incubated with medium containing auranofin (2 µm), TNF-α (10 ng mL−1), CML (1.2 mm), FL (500 µm), pyrraline (197 µm), native casein, CML-casein, pyrraline-casein, or FL-casein (each at 10 mg mL−1). Medium was used as a negative control and tert-butyl hydroperoxide (TBHP, 200 µm in medium) was used as a positive control. DHR123 was oxidized to fluorescent R123 within cells in the presence of ROS and it localizes in mitochondria. After 60 min of incubation at culturing conditions, cells were washed with HANK's buffer. For fluorescence measurement, 100 µL of HANK's buffer were added and fluorescence was recorded at excitation wavelength 485 nm and emission wavelength 530 nm using a multimode reader Tecan Infinite M200 (Mainz, Germany). A neutral red uptake assay was performed subsequently in the same plate to normalize fluorescence measurement to viable cells.
2.11 Statistical Analysis
Statistical analysis was performed by using Graph Pad Prism version 9.2.0 (San Diego, USA). Shapiro−Wilk normality test was used to assess normal distribution. Group comparisons were performed by Student's t-test analysis. All data were presented as mean values ± SD. Statistically significant differences were considered if p < 0.05 and marked with * for p values ≤ 0.05, with ** for p values ≤ 0.01 and *** for p values ≤ 0.001.
3 Results and Discussion
3.1 Characterization of Modified Caseins
In the present study, specific lysine modifications, namely CML, FL, and pyrraline were introduced into cow milk casein. Casein was used as a model protein because it lacks well-defined secondary and tertiary structures due to large amount of prolyl residues. Tertiary structural elements can interfere with RAGE or other receptors. By choosing a model protein with a rather disordered structure, we aimed to minimize the basal interaction not related to glycation with RAGE. Moreover, casein is a protein highly abundant in animal-product-based nutrition, making it one of the most important dietary proteins.
Native casein as well as CML-, pyrraline-, and FL-modified caseins were analyzed for their lysine content as well as CML, FL, and pyrraline and CEL, MG-H1, and 3-DG-H content after enzymatic hydrolysis. The degree of hydrolysis was determined by comparing the release of isoleucine and leucine after acid hydrolysis with the release after enzymatic hydrolysis. All caseins showed a degree of hydrolysis above 75% after enzymatic hydrolysis. Lysine modification, determined by comparing the quotient of the lysine to isoleucine ratios of the modified with the native casein, was 20% for CML-casein, 55% for FL-casein, and 11% for pyrraline-casein. Arginine modification, determined by comparing the quotient of the arginine to isoleucine ratios of the modified with the native casein, was 0.5%, 1%, and 3%, respectively. The concentration of CML was 13.7 mg g−1 CML-casein, of pyrraline was 6.2 mg g−1 pyrraline-casein, and of FL was 60.8 mg g−1 FL-casein. Up to 94%, 97%, and 61 % of lysine modification could be attributed to CML, FL, and pyrraline in the respective modified casein. A summary of the modified casein characterization is shown in Table 1. The modified caseins contain mainly the desired modification and only minor concentrations of the respective other modifications were found.
| Sodium caseinate | FL-casein | CML-casein | Pyrraline-casein | |
|---|---|---|---|---|
| FL | 0.01 ± 0.001 | 60.75 ± 5.08 | 0.57 ± 0.37 | 0.52 ± 0.26 |
| CML | 0.01 ± 0.0005 | 1.39 ± 0.79 | 13.75 ± 1.77 | 1.12 ± 0.57 |
| Pyrraline | < 0.01 | 0.23 ± 0.04 | 0.03 ± 0.02 | 6.25 ± 1.25 |
| CEL | < 0.01 | < 0.01 | 0.06 ± 0.001 | < 0.01 |
| MG-H1 | 0.02 ± 0.002 | 0.03 ± 0.004 | 0.02 ± 0.0005 | 0.04 ± 0.004 |
| 3-DG-H | < 0.01 | 0.04 ± 0.001 | < 0.01 | 0.24 ± 0.01 |
- MRPs were analyzed via HPLC-MS/MS after enzymatic hydrolysis. Data are mean ± SD, n = 3. CML, N-ε-carboxymethyllysine; FL, N-ε-fructosyllysine; CEL, N-ε-carboxyethyllysine; MG-H1, methylglyoxal-derived hydroimidazolone 1; 3-DG-H, 3-deoxyglucosone-derived hydroimidazolones; MRP, Maillard reaction product.
Oligomerization of native casein as well as modified caseins was studied with SEC. The oligomerization degree was calculated from the peak area of all casein oligomers divided by the total protein peak area (see Figure SI 1, Supporting Information for an exemplatory SEC chromatogram). The average oligomerization degree of native casein was 6% and did not change in the synthesis blanks. The synthesis blanks were obtained by following the same treatment of caseins as during synthesis of glycated caseins but with omission of glyoxylic acid, glucose, or 3-DG. Glycation of casein with glucose led to an average oligomerization degree of 9% and treatment of casein with glyoxylic acid to 11% oligomerization. The most pronounced increase of oligomerization was seen for the treatment of casein with 3-DG to produce pyrraline-casein, resulting in an oligomerization degree of 25%. Although mild conditions such as dry heating and short incubation time of 10 min were applied, oligomerization could not be completely prevented. This is mainly due to the high reactivity of 3-DG resulting not only in intramolecular side chain modifications but also intermolecular cross-linking reactions. The protein content determined by Kjeldahl method was 88% for unmodified casein, 81% for CML-Casein, 83% for FL-casein, and 85% for pyrraline-casein. The color of the caseins was white for CML-casein, off-white for unmodified and FL-casein, and light-yellow for pyrraline-casein.
3.2 Intracellular CML, Pyrraline, and FL in HCT 116 Cells
To assess whether and in which amounts glycated amino acids can enter the cells, CML, FL, and pyrraline were applied as free amino acids as well as in protein-bound form to HCT 116 pTRAF cells. The colon cancer cell line HCT 116 was used as a mammal model cell line, which originated in the gastrointestinal tract and is conveniently culturable. HCT 116 are able to express RAGE[42] comparable to the human intestinal epithelium,[29] which makes them a suitable model to study the interaction of glycation compounds with RAGE.
The concentration of 10 mg of casein per mL of cell culture medium was chosen to mimic a realistic concentration in which proteins can interact with human cells. Based on the assumption that a meal contains ≈30 g of protein and is diluted in 3 L of stomach fluid, this equals a concentration of 10 mg mL−1. Since the modification degree of lysine is not the same for the three glycated caseins, different concentrations of protein-bound MRPs resulted: a concentration of 10 mg mL−1 equals 0.7 mm CML, 0.2 mm pyrraline, and 2.0 mm FL in the respective caseins. The concentrations used for the incubation with free MRPs (CML 1.2 mm, pyrraline 0.2 mm, and FL 0.5 mm) roughly equal the amount of MRPs which are present in the casein incubations. The average daily intake of CML with a human diet rich in MRPs was estimated between 6 to 20 mg day−1.[43-45] Assumed that the complete daily dose reaches the stomach in one portion, this would equal a maximum concentration of 10–33 µm CML in 3 L of stomach fluid. For FL, the average daily intake was estimated to range between 500 and 1200 mg[7] which equals a maximum stomach concentration of 0.5–1.3 mm. For pyrraline, an uptake of ≈25 mg day−1 is assumed,[7] which translates to a stomach concentration of 33 µm. Compared to the concentrations used in our study, FL is applied in realistic concentrations also present after consumption of glycated food proteins. For CML and pyrraline, the concentrations used in the study exceed the concentration of dietary glycation compounds in the intestinal tract by factor 20 for CML and 6 for pyrraline and thereby present rather “worst-case” scenarios.
No effect of free MRPs as well as glycated and native casein on cell viability was observed in the concentrations used for the incubation experiments when analyzed via the neutral red uptake assay. When cells were incubated with free CML, pyrraline, or FL, an amount of 117.3, 1.5, and 5.1 nmol mg−1 protein was recovered in the cell lysate, respectively (Figure 1, left side). For CML and pyrraline, the recovered amount was significantly higher compared to the basal concentration of the cells in the absence of exogenous MRPs. For FL, no increase compared to the basal level was found, when exogenous FL was applied to the cells. When HCT 116 cells were incubated with casein-bound MRPs, an increase of free CML to a level of 15.3 nmol mg−1 protein after incubation with CML-casein was detected, but no increase of free pyrraline or FL after incubation with the respective caseins (Figure 1, right side).
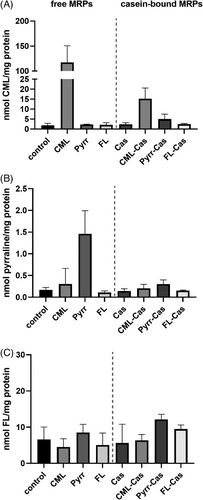
It was shown in previous studies, that MRPs are mainly transported across the cell membrane in dipeptide form with the help of the peptide transporter PepT1.[46, 47] A limited amount of free glycated amino acids is assumed to pass the cell membrane via passive diffusion. In the current study, the basal concentrations of free glycated amino acids were ≈1.8 nmol CML mg−1 protein, 0.2 nmol pyrraline mg−1 protein, and 6.6 nmol FL mg−1 protein. The Amadori product FL showing the highest basal concentrations might be explained by the high abundance of the reaction educts lysine and glucose in the cell culture medium (0.2 and 16.6 mm, respectively, according to the formulation provided by the supplier). FL was analyzed in the undoped cell culture medium in a concentration of 4 µm, whereas pyrraline and CML were not detectable prior to the addition of MRPs. Pyrraline and CML are products of the advanced stage of the Maillard reaction and are mainly formed under more severe reaction conditions, such as longer incubation time or higher temperatures. Since the cell culture medium did not experience heat treatment prior to its use and cells were only incubated for 48 h in the medium at 37 °C, the formation of advanced stage MRPs might occur in lower extents compared to Amadori products. Exogenous application of the respective free MRPs led to a 65-fold increase of intracellular CML and a 7.5-fold increase of pyrraline. For free CML and pyrraline the recovered amounts equal an uptake of 0.01% and 0.001%, respectively. Intracellular FL did not change compared to the basal level when extracellular FL was applied. The limited recovery of free MRPs in cells after exogenous incubation confirm previous results.[47] A limitation of the study is the separation of free MRPs which were added to the cell culture medium and free MRPs in cells. Theoretically, it is possible that intracellular MRPs are overestimated due to residual free MRPs originating from the fortified cell culture medium. The carryover of free MRPs from the cell culture medium was tried to be minimized by washing the cells two times with PBS before cell lysis. An indication, that the carryover was successfully prevented is the lack of increased FL concentrations in the cells after incubation with exogenous FL.
When protein-bound MRPs were applied to the cells, an increase of CML could be shown when CML-casein was present in the cell culture medium. Compared to the incubation of cells with free CML, the availability of protein-bound CML is approximately lower by the factor 7. The intracellular increase of CML after the incubation with protein-bound CML could be explained by the partial degradation of CML-casein in the medium, e.g. by membrane-bound proteases, followed by the uptake of the degradation products. Matrix metalloproteinases (MMPs) are overexpressed in cancer cells and correlate with the cells invasive and metastatic potential.[48] Originally, MMPs are responsible for degrading extracellular matrix. Membrane-bound MMPs are believed to play a crucial role in tumor growth.[49] A broad array of endogenous substrates for membrane-type (MT-)MMPs has been revealed.[50] Whether MT-MMPs are able to cleave glycated proteins, and whether cleavage favors acidic residues, such as present in CML-modified casein, is unknown.
Moreover, peptides with molecular weight above 1 kDa can be absorbed intactly via transcytosis.[51] Once the CML-modified casein entered the cell, intracellular proteases could degrade the glycated protein and thereby contribute to the CML increase. In the current study, no increase of free pyrraline or FL was detected after the application of pyrraline- and FL-modified casein. This could be due to the limited release of free or peptide-bound pyrraline and FL in the extracellular space, which would then be available for absorption. However, if the intact modified proteins are taken up by the cells, this could also be due to a limited intracellular degradability of the pyrraline- and FL-modified caseins. In conclusion, the results show that HCT 116 cells take up free CML and pyrraline in a low extent, but not FL. The incubation with casein-bound MRPs only affected intracellular CML.
3.3 Activation of Nrf2 and NFκB and Analysis of Cellular Redox Status
Although intracellular MRP concentrations might be of importance regarding transport and effects of MRPs, the currently accepted mode of action of MRPs involves cell-surface receptors, such as RAGE. Hence, MRPs might have an effect on cells without prior internalization. For the activation of the transcription factor Nrf2, mainly intracellular redox-regulated mechanisms were described. Thus, the intracellular increase of MRPs following incubation with the respective compounds might affect RAGE-mediated NFκB activation and Nrf2 activation differently. The pattern-recognition receptor RAGE is believed to bind Maillard-modified proteins followed by the activation of the transcription factor NFκB, which results in a pro-inflammatory gene activation. Therefore, the activation of the transcription factor NFκB after 24 h incubation with test substances was studied with HCT 116 cells transfected with the plasmid pTRAF, which enabled time- and cell-resolved activity monitoring of the redox-regulated transcription factors Nrf2 and NF-κB.
The cells were incubated with test substances for 24 h followed by nuclei staining and confocal microscopy. Representative images of confocal microscopy can be seen in Figure 2. Neither in media control samples nor in samples incubated with native casein, mCherry or TFP fluorescence was detected, which translates to no activation of Nrf2 and NFκB, respectively.
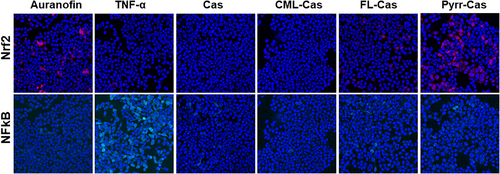
The known Nrf2 activator auranofin showed an increased mCherry fluorescence, whereas the NFκB activator TNF-α showed increased TFP expression. The quantitative results for Nrf2 and NFκB activation of three independent treatments are shown in Figure 3. Incubation of cells with auranofin led to four-fold increase of Nrf2 compared to the unstimulated medium control. The incubation of the cells with casein synthesis blanks did not lead to a pronounced activation of Nrf2 (Figure 3a). The synthesis blanks model the structural changes in the protein without the presence of glycation agents. Structural changes such as aggregation due to heat treatment can lead to interaction with RAGE.[52]
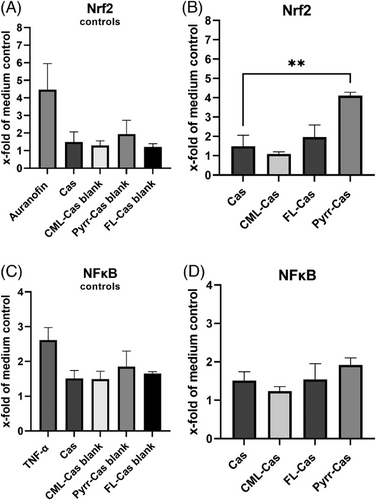
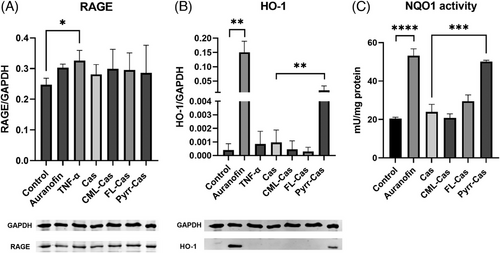
When cells were incubated with CML- and FL-modified caseins, the Nrf2 activation was not increased compared to the incubation with native casein. Pyrraline-casein showed a significant four-fold induction of Nrf2 (Figure 3B). The Nrf2-activating effect of pyrraline-casein was confirmed by analysis of the Nrf2 target proteins NQO1 and HO-1. The expression of HO-1 increased by factor 18 and the activity of NQO1 increased by factor 2 in the presence of pyrraline-casein compared to the incubation with native casein (Figure 4B,C) and were in a similar range compared to the Nrf2 positive control auranofin. Native casein and CML- and FL-modified casein did not show an effect on the antioxidant response of HCT116pTRAF cells.
Since Nrf2 also activates enzymes responsible for the biosynthesis of the natural antioxidant glutathione (GSH),[53] we checked whether Nrf2 activation by pyrraline-casein had an effect on intracellular GSH. Figure 5B shows that incubation with auranofin as well as pyrraline-modified casein significantly increased the amount of GSH in the cells. This clearly demonstrates that pyrraline-modified casein triggers a Nrf2-dependent antioxidative effect in HCT 116 cells and activates the expression of cytoprotective proteins.
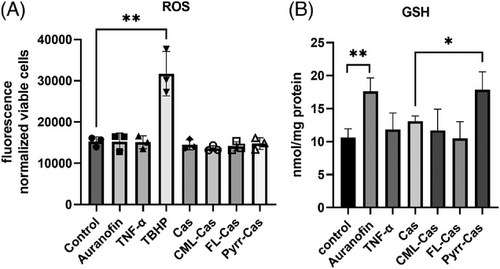
No activation of Nrf2 or its target proteins was seen when cells were incubated with free pyrraline. Apparently, the protein backbone of pyrraline-modified casein is of utmost importance for the interaction with the antioxidative transcription factors of HCT 116 cells. It is described that oxidants and electrophilic structures are activators of Nrf2, as they interact with cysteine residues of the sensor protein Keap1, thereby preventing the proteasomal degradation of the Keap1-Nrf2 complex. Subsequently, the Keap1 gate opens, which results in the nuclear accumulation of Nrf2 and antioxidative gene expression.[54] Although pyrraline exhibits an electrophilic formyl group, it is unlikely that this interacts with the Keap1-Nrf2 complex, since no activation of Nrf2 in the presence of free pyrraline was detected.
To study whether the production of intracellular ROS plays a role in the putative pyrraline-dependent Nrf2 activation, intracellular ROS was analyzed by applying the fluorescent dye DHR123 to the cells after treatment with free and protein-bound MRPs. In Figure 5A it is shown, that an increase of ROS could only be detected in the presence of the positive control TBHP but not protein-bound MRPs or free MRPs (data not shown for free MRPs).
Further mechanisms of Nrf2 activation include covalent modification of Nrf2 or disruption of Keap1-Nrf2 binding due to the binding of certain proteins such as p62.[55] The autophagy protein p62 is an important regulator in the degradation of modified and damaged proteins and activates Nrf2 by binding to Keap1. Autophagy is a cytoplasmic degradation mechanism for usually large proteins and organelles.[56] The basal level of autophagy plays an important role for quality control of macromolecules and energy homeostasis, but it can also be induced in response to cytotoxic stresses. If exogenous Maillard-modified proteins enter cells, e.g., via transcytosis or endocytosis, cellular defense in form of autophagy might be activated. Therefore, the ability of pyrraline-modified proteins to induces Nrf2 by increasing autophagy and p62 should be studied further. In this context it must be emphasized that the pyrraline-modified casein may also contain further, currently unknown modifications due to the preparation procedure. As mentioned above, the incubation of casein with 3-DG resulted in unavoidable protein oligomerization for which the underlying mechanism and the cross-linking amino acids are still unknown. Although these modifications are quantitatively far below the amino acid pyrraline, which is the main amino acid formed, an influence of potential by-products on the cellular response cannot be ruled out.
Regarding the activation of NFκB, only the positive control TNF-α showed a 2.6-fold increase compared to the medium control. Neither casein synthesis blanks, nor glycated caseins showed an effect on NFκB (Figure 3C,D). Glycated proteins are discussed to bind to the receptor of advanced glycation endproducts (RAGE) and thereby lead to the activation of an inflammatory signaling cascade. To test the effect of glycated caseins on RAGE, cells were lysed after 24 h incubation with test substances and RAGE was analyzed via Western Blotting with an anti-RAGE antibody. The results are shown in Figure 4A. The positive control TNF-α showed a significant increase of RAGE expression, whereas the native and glycated caseins did not show changes in RAGE expression.
Although with our experimental design we cannot exclude the interaction of caseins with RAGE, this does not translate into an activation of NFκB in the present study. When cells were incubated with free CML, pyrraline, or FL neither NFκB activation nor RAGE expression changed compared to the control (data not shown). The analyses were also performed after 6 and 48 h of incubation to exclude the possibility that NFκB activation only occurs already after very short or only after prolonged incubation. No NFκB activation with glycated caseins could be detected after 48 h incubation. After 6 h, the positive control TNF-α showed only a 1.2-fold activation of NFκB compared to the medium control. HCT 116 pTRAF cells produce the fluorescent reporter protein TFP upon activation of NFκB and concluded from our results this process takes longer than 6 h. The absence of NFκB activation and no increase in RAGE expression for free CML, pyrraline, and FL was expected, since it was reported previously that a polypeptide backbone is required for recognition by RAGE.[19] In the current study, the protein-bound MRPs also did not show an activation of NFκB or an increase of RAGE. The lack of RAGE-binding for protein-bound early glycation compounds, such as FL, was shown in a previous study using NMR spectroscopy and isolated RAGE.[57] The same study also described a strong affinity of CML-modified peptides and proteins, namely CML-BSA, toward RAGE. The authors showed that highly positively charged areas of the V domain of RAGE are involved in the CML-peptide binding and postulated that CML has to be embedded in strongly negatively charged areas of the modified protein. The authors also established that RAGE-binding occurs independent of the primary sequence of the CML-peptide as well as the location of CML within the folded protein. Whether interaction of CML and RAGE also leads to subsequent activation of RAGE was not analyzed in the respective study. Buetler et al.[58] used carboxymethylated human serum albumin and β-lactoglobulin as model proteins and did neither confirm RAGE-binding in a cell-free assay nor showed subsequent pro-inflammatory signaling in human lung epithelial cells.[58] The authors reached a similar degree of lysine to CML modification compared to our study with approximately 20%. The study by Buetler et al.[58] as well as our results are in contrast with previous literature, analyzing the effect of carboxymethylated ovalbumin on HUVEC.[19] The authors showed that CML-OVA binds to RAGE and subsequently activates NFκB and pro-inflammatory gene expression. The authors used a highly carboxymethylated ovalbumin containing 310 mmol CML mol−1 lysine, whereas in the present study carboxymethylation of casein was 111 mmol mol−1 lysine. Another reason for the different outcome might be the different cell models which were used. The current study worked with human colon cancer cells whereas Kislinger[19] worked with human umbilical vein endothelial cells. The primary endothelial cells might be more sensitive toward protein modifications compared to the immortalized colon cell line. In addition, colon cells might be more adapted to handle modified proteins since the colon is in close contact with a variety of food ingredients. However, this assumption is somewhat speculative and the different reactivity of different cells toward glycation products needs further attention. Dietary proteins passing through the colon are not desired to elicit inflammatory reactions on colonic cells. Therefore, the lack of an effect of individual free and protein-bound MRPs on NFκB activation might be relevant to improve the knowledge regarding metabolism and effects of dietary MRPs on the gastrointestinal system.
Besides RAGE, also other receptors such as AGER1 and members of the scavenger receptor family (e.g., SR-A, SR-B, and CD36) which can bind glycated proteins were identified. Whereas RAGE is assumed to rather promote oxidative stress in response to binding glycated proteins, AGER1 promotes AGE uptake and degradation and thereby is believed to reduce oxidative stress. That AGER1 expression can be influenced by a diet high in AGEs was shown by Poulsen et al.,[59] who fed a diet containing 20% of hydrolyzed heated or native milk powder to Sprague–Dawley rats for 2 weeks and observed an increase of AGER1 in whole blood of animals fed the intervention diet. Although the study also observed an increase of RAGE in whole blood of the animals, no increase of inflammatory markers was seen. Whether AGER1 functions as an antagonist of RAGE and thus prevents the inflammatory action of glycated proteins, and whether this mechanism plays a role for dietary glycated proteins should be further explored.
An aspect which was omitted in the current study design is the role of protein degradation in course of intestinal digestion and microbial fermentation of dietary MRPs in the human intestine. Dietary proteins are usually degraded by several gastric and intestinal proteases and the degradation products can interact with intestinal epithelial cells and might be absorbed. The impact of protein degradation in course of digestion on the inflammatory effect of protein-bound MRPs was not part of the present study but would certainly be an interesting experiment with physiological relevance. Undigested fractions of glycated peptides or proteins, which were not resorbed in the intestine, might be fermented by human intestinal bacteria, such as Oscillibacter, Cloacibacillus evryensis, and Escherischia coli, which were shown to degrade CML during anaerobic in vitro incubation.[60, 61] The microbial fermentation of CML resulted in the formation of carboxymethylated biogenic amines and carboxylic acids. The physiological effect of the resulting degradation products on intestinal cells is yet unknown.
Taken together, the presented study bears some limitations such as the use of one cell line, the application of one concentration instead of various doses, and the focus on inflammation as the sole output. The focus of our study was the specific structure–effect relationship of individual MRPs, which will lead to a better understanding of the biological effects of MRPs.
4 Concluding Remarks
In the presented study, the specific Maillard-modifications CML, FL, and pyrraline were introduced into cow's milk casein and applied to HCT 116 cells. For free CML and pyrraline, but not for free FL, a limited resorption into the cells was shown. Protein-bound CML, but not pyrraline and FL, led to a significant increase of free intracellular CML. The transfection of the cell line with pTRAF allowed the simultaneous detection of the transcription factors NFκB and Nrf2. In contrast to previous studies, we found no activation of NFκB or increased expression of RAGE in HCT 116 cells in the presence of glycated caseins after 24 h. Contrarily, we describe for the first time a Nrf2-dependent antioxidative effect of protein-bound but not free pyrraline. The mechanism behind the Nrf2 activation by pyrraline-modified casein is yet unknown, but an involvement of autophagy and protein degradation might be of relevance and needs further elucidation. Taken together, our results show that glycation and glycated proteins do not per se activate NFκB and lead to a pro-inflammatory response, but that an effect is strongly structure-dependent. Moreover, different cell models might give different results due to adaption reactions and variations in the sensitivity toward protein modifications.
Acknowledgements
The authors thank Stefanie Deubel for excellent technical assistance and Dr. Tobias Jung for development of the evaluation macro. Cells were kindly gifted by Prof. Anna Kipp, University of Jena. The cells were originated by Prof. Elias Arnér and colleagues (Karolinska Institutet, Stockholm, Department of Medical Biochemistry and Biophysics [MBB]). This work was supported by the Deutsche Forschungsgemeinschaft (RA 3524/2-1 to J.R. and T.H. and 491394008).
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interest
The authors declare no conflicts of interest.
Author Contributions
J.R., S.K.M., A.H., and T.H. designed the experiments. J.R., S.K.M., V.S., and S.F. performed the experiments. T.G., A.H., and T.H. gave advice and suggestions on this work. J.R. and S.K.M. prepared the manuscript, and S.F., V.S., A.H., T.H., and T.G. critically read the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




