Porphyra haitanensis Polysaccharides Attenuates Blood Lipid via Gut-Liver Axis in Diet-Induced High-Fat Mesocricetus auratus through Multiple Integrated Omics
Abstract
Scope
Hyperlipidemia is currently a global public health problem severely affecting people's physical and mental health, as well as their quality of life.
Methods and results
The present study is aimed at revealing the mechanism of Porphyra haitanensis polysaccharide (PHP) in decreasing blood lipids by acting through gut-liver axis in Mesocricetus auratus fed a high-fat diet. PHP significantly prevented increases in serum total cholesterol, triglycerides and low-density lipoprotein cholesterol, and alleviated damage to liver cells induced by a high-fat diet M. auratus, in a dose-dependent manner. PHP promotes proliferation of Muribaculaceae and Faecalibaculum, thereby enhancing the production of butyric acid both in the colon and liver, particularly high-dose PHP (HPHP). Low-dose PHP (LPHP) promotes the expression of phosphatidylcholine metabolites and fatty acid transport genes, and inhibits the expression of genes involved in fat degradation (Abhd5), adipogenesis (Me1), fatty acid synthesis (Fasn and Pnpla3), and fatty acid chain elongation (Elovl6) in the liver. However, HPHP inhibits the expression of triglyceride metabolites and promotes the expression of fatty acid transporter (CD36), fatty acid oxidation (Acacb), and peroxisome proliferator-activated receptor gamma (PPARg) genes in the liver.
Conclusion
PHP regulates lipid metabolism through the gut microbiota, and the gut-liver axis plays an important role in its hypolipidemic effects.
1 Introduction
In recent years, consumers’ dietary habits have markedly changed. High-energy fast food, often containing high fat and sugar content, is increasingly being consumed. Consequently, the number of people with hyperlipidemia has increased. Hyperlipidemia is characterized by elevated total cholesterol (TC), triglycerides (TG) and low-density lipoprotein cholesterol (LDLC), and low levels of high-density lipoprotein cholesterol (HDLC).[1] Hyperlipidemia is currently a global public health problem severely affecting people's physical and mental health, as well as their quality of life. Therefore, effective strategies are urgently needed to prevent the occurrence and development of hyperlipidemia. A major area of research involves finding methods to prevent diet-associated lipid metabolism disorders through food nutritional components.
The gut microbiota, which is considered a hidden organ or an organ within an organ, has crucial roles in physiological functions, such as digestion, metabolism, and vitamin synthesis.[2] The balance of the gut microbiota affects the host's nutrient acquisition and energy regulation.[3] The dynamic ecological balance between the gut microbiota and the host has important effects on human health and normal physiological metabolism.[4] Dietary fat and fatty acid composition regulate the gut microbiota in vivo.[5, 6] The liver and gut affect each other, thereby forming the gut-liver axis. The gut microbiota affects lipid metabolism in the liver through its metabolites, and polysaccharides with varying structures also affect the structure and metabolites of the gut microbiota. The carbohydrates not digested in the gastrointestinal tract enter the colon and serve as an important energy source for the colonic microbiota, which ferment them and produce short-chain fatty acids (SCFAs).[7, 8] SCFAs, the main metabolites of the gut microbiota, are constituents of lipids that provide energy. They might be beneficial in situations in which extra energy is required (e.g., a diet rich in fiber but low in fat or energy) but harmful in cases of obesity.[9, 10] Butyric acid is particularly important as an energy substrate for colonic epithelial cell metabolism, in which adenosine 5’-monophosphate-activated protein kinase (AMPK) is activated.[11] Peroxisome proliferator-activated receptor (PPAR) is a key target gene associated with the beneficial effects of SCFAs in liver metabolic syndrome, and SCFAs might function as a regulatory factor of PPAR.[12]
Porphyra haitanensis, also known as laver, belongs to the family of red algae. It originated from Pingtan Island in Fujian Province, China and is widely distributed in the coastal areas of Zhejiang and Fujian.[13] The proportions of protein, carbohydrate, fat, and ash in P. haitanensis are 35–37%, 47–49%, 1.7–2.1%, and 8.7–9.9%, respectively.[14] Polysaccharide is the main component of P. haitanensis. In our previous study, we isolated two fractions from P. haitanensis polysaccharide (PHP)[15] and characterized them as sulfated galactan[16] and sulfated glucogalactan.[17] Owing to its structure, high sulfate content and other factors, PHP has a variety of biological activities, such as anti-oxidation,[18] immune regulation,[19, 20] anti-tumor,[21, 22] intestinal flora regulation,[23, 24] and hypolipidemic[25] effects. Although previous studies have shown that PHP has hypolipidemic activity,[25] the relationship between the gut microbiota in high-fat diet (HFD)-fed animals and hypolipidemic activity has not been investigated.
Therefore, the hypolipidemic effects of PHP in Mesocricetus auratus fed an HFD were investigated with multiomics technology (including 16S rRNA high-throughput sequencing, lipomics, and transcriptomics), to reveal the mechanism through which PHP decreases blood lipids via the gut-liver axis.
2 Results
2.1 PHP Ameliorates Hyperlipidemia and Pathological Changes in HFD Fed M. auratus
The body weight and organ indexes of M. auratus after 28 days of the experiment are shown in Table S1, Supporting Information. The body weights in the HPHP group were significantly lower (p < 0.05) than that in the MC group. The liver index in the HPHP group was significantly lower (p < 0.05) than that in the MC group, and the kidney indexes in the LPHP and HPHP groups were significantly lower (p < 0.05) than that in the MC group. No significant differences were observed (p > 0.05) in body weight and kidney index between the HPHP and NC groups. The effects of PHP on the blood lipids in HFD fed M. auratus are shown in Figure 1A. The serum TG contents in the LPHP and HPHP groups were significantly lower (p < 0.05) than that in the MC group, and no significant differences were observed (p > 0.05) in serum TG content among the NC, LPHP, and HPHP groups. The serum TC content in the HPHP group was significantly lower (p < 0.05) than that in the MC group, and no significant differences were observed (p > 0.05) in serum TC content between the NC and HPHP groups, as well as between the LPHP and HPHP groups. No significant difference was observed (p > 0.05) in serum HDLC content among the MC, LPHP, and HPHP groups. The serum LDLC content in the HPHP group was significantly lower (p < 0.05) than that in the MC group.
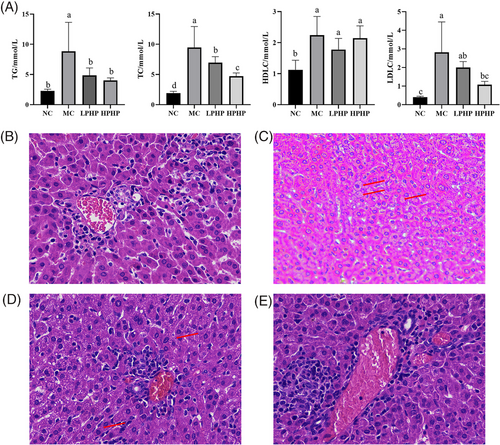
The histopathological analysis of the liver is shown in Figure 1B–E. The liver tissue structure in the NC group was clear and complete, and the boundaries of hepatocytes were clear (Figure 1B). The hepatocytes in the MC group showed severe pathological changes, including extreme cell swelling, blurred nuclei, blurred cell boundaries, many fat droplets, and many vesicles in the liver tissue (Figure 1C, red arrow). LPHP and HPHP partially attenuated these pathological characteristics (Figure 1D,E). Several fat vesicles were observed in the liver tissues in the LPHP group (Figure 1D, red arrow). The structure of the liver tissues in the HPHP group was clear and complete, and the boundaries of hepatocytes were clear (Figure 1E); results similar to those in the NC group.
2.2 Alterations in the Gut Microbiota and SCFAs in HFD Fed M. auratus
The α-diversity of the colonic contents at the amplicon sequence variant (ASV) level was investigated with the ACE, Chao, and Simpson indexes. The ACE and Chao indexes of the LPHP and HPHP groups were higher than those of the MC and NC groups (Figure S1A,B, Supporting Information). The Simpson indexes of the LPHP and HPHP groups were lower than those of the MC and NC groups (Figure S1C, Supporting Information). The β-diversity of the colonic contents at the ASV level was investigated with principal coordinate analysis. The NC group was distinct from the MC, LPHP, and HPHP groups (PERMANOVA, p = 0.001) (Figure 2A), thus suggesting that the HFD modulated the colonic microbiota. The numbers of common and unique microbiota at the ASV level in various samples are displayed in a Venn diagram (Figure S1D, Supporting Information). A total of 491 ASVs were shared by the NC, MC, LPHP, and HPHP groups, whereas 221, 193, 164, and 422 ASVs, respectively, were unique.
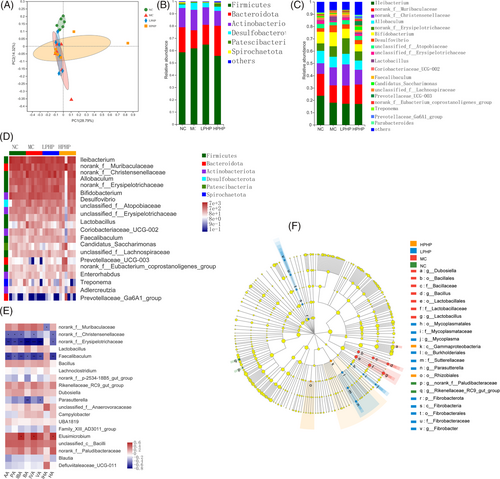
The compositions of the colonic microbiota at various classification levels are shown with a community bar diagram (Figure 2B,C). The microbiota at the phylum level contained mainly Firmicutes, Bacteroidota, Actinobacteriota, and Desulfobacterota (Figure 2B). Compared with that in the MC group, the relative abundance of Firmicutes and Bacteroidota was higher, whereas that of Actinobacteriota and Desulfobacterota was lower in the LPHP group; however, the relative abundance of Firmicutes and Actinobacteriota was lower, whereas that of Bacteroidota was higher in the HPHP group. Compared with that in the NC group, the relative abundance of Firmicutes was higher, and that of Bacteroidota was lower in the MC group. The colonic microbiota at the genus level is shown in Figure 2C. Compared with than that in the MC group, the relative abundance of Ileibacterium, Lactobacillus, Bifidobacterium, and Desulfovibrio was lower, whereas that of norank_f_Muribaculaceae, norank_f_Christensenellaceae, and norank_f_Erysipelotrichaceae was higher in the LPHP group; however, in the HPHP group, the relative abundance of Ileibacterium, Lactobacillus, norank_f_Christensenellaceae, Bifidobacterium, and Desulfovibrio was lower, whereas that of norank_f_Muribaculaceae was higher. Compared with that in the NC group, the relative abundance of Ileibacterium, norank_f_Muribaculaceae, and Allobaculum was lower, whereas that of norank_f_Christensenellaceae, Bifidobacterium, norank_f_Erysipelotrichaceae, Desulfovibrio, and Lactobacillus was higher in the MC group. We further analyzed the correlations among the top 20 genera, and the results are shown in Figure 2D. Compared with that in the MC group, the relative abundance of norank_f_Muribaculaceae was higher in the LPHP and HPHP groups. The relative abundance of Lactobacillus, Bifidobacterium, and Desulfovibrio was decreased in the LPHP and HPHP groups. Compared with that in the MC group, the relative abundance of Faecalibaculum and Prevotellaceae_UCG-003 was higher in the LPHP and HPHP groups. The linear discriminant analysis effect size indicated that bacteria belonging to the Fibrobacterota phylum, Fibrobacteria class, Fibrobacterales order, Fibrobacteraceae family, and Fibrobacter genus were differentially enriched in the LPHP group, whereas the Gammaproteobacteria class and Rhizobiales order were differentially enriched in the HPHP group.
The effect of PHP on SCFA content is shown in Table 1. Acetic acid, propionic acid, and butyric acid were the main SCFAs. The acetic acid and propionic acid content in the LPHP group was lowest, the butyric acid content in the HPHP group was significantly higher than that in the LPHP group (p < 0.05), and the n-pentanoic acid content in the MC and LPHP groups was lowest. The correlation between SCFAs and the microbiota was further analyzed (Figure 2F). Acetic acid, propionic acid, isobutyric acid, isovaleric acid, and hexanoic acid were significantly negatively correlated with norank_f_Christensenellaceae, norank_f_Erysipelotrichaceae, and Faecalibaculum (p < 0.05). Isobutyric acid, isovaleric acid, and hexanoic acid were significantly positively correlated with Elusmicrobium (p < 0.05). Butyric acid and valeric acid were significantly negatively correlated with norank_f_Erysipelotrichaceae, Elusmicrobium, and Parasutterella (p < 0.05). Isohexanoic acid was significantly negatively correlated with norank_f_Muribaculaceae (p < 0.05).
| NC | MC | LPHP | HPHP | |
|---|---|---|---|---|
| Acetic acid | 100.66 ± 12.39a | 73.16 ± 2.43b | 56.07 ± 3.6c | 72.18 ± 7.03b |
| Propanoic acid | 45.10 ± 8.06a | 34.27 ± 1.89a | 19.88 ± 2.38b | 30.88 ± 5.37b |
| Isobutyric acid | 6.13 ± 1.36a | 4.46 ± 0.33a | 2.58 ± 0.41a | 3.83 ± 0.90a |
| Butyric acid | 19.07 ± 4.90ab | 21.62 ± 2.83ab | 9.48 ± 2.13b | 24.30 ± 6.60a |
| Isovaleric acid | 6.54 ± 1.47a | 4.88 ± 0.44a | 2.78 ± 0.54a | 4.73 ± 1.18a |
| Valeric acid | 8.47 ± 1.99a | 7.07 ± 0.71a | 3.60 ± 0.69a | 6.22 ± 1.43a |
| Isohexanoic acid | 0.13 ± 0.03a | 0.11 ± 0.01a | 0.09 ± 0.01a | 0.15 ± 0.02a |
| Hexanoic acid | 2.00 ± 0.30a | 1.76 ± 0.28a | 1.25 ± 0.14a | 1.52 ± 0.33a |
- Note: There is significant difference between different lowercase letters in the same row (p < 0.05).
2.3 Alterations in Liver Lipid Metabolites in HFD Fed M. auratus
Orthogonal partial least squares discriminant analysis (OPLS-Da) of liver positive and negative lipid metabolites between the LPHP and MC groups is shown in Figure S2A,B, Supporting Information, respectively. LPHP was distinct from the MC group, thus suggesting that the positive and negative lipid metabolites differed. S-plot diagrams based on OPLS-DA of liver positive and negative lipid metabolites between the LPHP and MC groups are shown in Figure S2C,D, Supporting Information, respectively: t-tests revealed 68 metabolites that were significantly up- and down-regulated (p < 0.05) (Table S2, Supporting Information), including 56 metabolites significantly up-regulated (p < 0.05) and 12 metabolites significantly down-regulated (p < 0.05). OPLS-DA of liver positive and negative lipid metabolites between the HPHP and MC groups are shown in Figure S2E,F Supporting Information respectively. HPHP was distinct from the MC group, thereby suggesting that the positive and negative lipid metabolites differed. S-plot diagrams based on OPLS-DA of liver positive and negative lipid metabolites between the HPHP and MC groups are shown in Figure S2G,H, Supporting Information, respectively; t-tests revealed 58 metabolites that were significantly up- and down-regulated (p < 0.05) (Table S3, Supporting Information), including 18 significantly up-regulated metabolites (p < 0.05) and 40 significantly down-regulated metabolites (p < 0.05).
The lipid metabolites in the liver are shown in Figure 3A. Three categories of lipid metabolites were identified: sphingolipids, glycerides, and glycerophospholipids. To investigate the effects of PHP on lipid metabolites, we determined the correlations between PHP and the top 30 lipid metabolites (Figure 3B). Compared with that in the NC group, the relative expression of the lipid metabolite PC (18:1e/22:5) in the MC group was down-regulated. Compared with that in the MC group, the expression of the lipid metabolites PC (8:0e/18:2), PC (22:6/21:0), PC (18:2e/22:6), PC (16:1e/18:2), and PC (11:0/20:2) in the LPHP group was up-regulated. In the HPHP group, the expression of the lipid metabolites TG (16:1/18:2/22:6), TG (18:4/15:0/18:2), and TG (18:0/14:2/22:6) was down-regulated, whereas PC (22:6/21:0) was up-regulated.
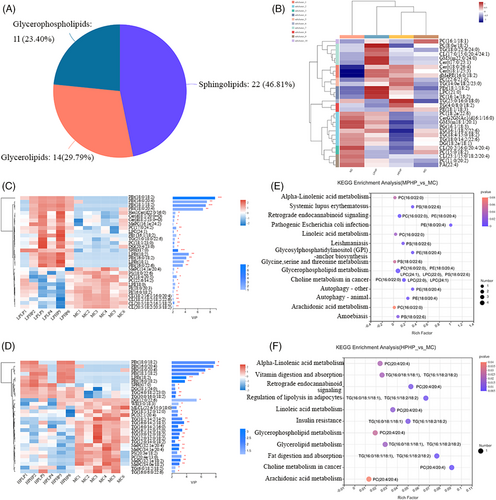
On the basis of OPLS-DA, the top 30 differential lipid metabolites between the LPHP and MC groups were analyzed (variable influence on projection (VIP) value > 1), and the results are shown in Figure 3C. Compared with that in the MC group, in the LPHP group, the expression of PC (17:0/24:2) and PC (18:1/23:0) was significantly up-regulated (p < 0.05). The differences in the top 30 lipid metabolites between the HPHP and MC groups are shown in Figure 3D. Compared with that in the MC group, the expression of TG (18:1/12:0/12:0), TG (18:2/14:2/14:2), TG (16:0/14:2/18:1), TG (16:0/14:2/16:0), TG (16:1/12:0/12:0), TG (10:0/12:0/18:2), TG (12:0/12:0/18:2), TG (12:0/14:0/18:2), TG (6:0/18:2/18:2), and TG (16:0/6:0/22:6) was significantly down-regulated (p < 0.05) in the HPHP group.
A kyoto Encyclopedia of genes and genomes (KEGG) enrichment analysis of differentially present metabolites between LPHP and MC is shown in Figure 3E. A total of 14 pathways were identified, including glycerophospholipid metabolism, alpha-linolenic acid metabolism, linoleic acid metabolism, glycosylphosphatidylinositol-anchor biosynthesis, and arachidonic acid metabolism, which are associated with lipid metabolism. PC (16:0/22:0), PE (18:0/20:4), LPC (24:1), LPC (22:0), and PS (18:0/22:6) were enriched in the glycerophospholipid metabolism pathway; PC (16:0/22:0) was enriched in alpha-linolenic acid metabolism, linoleic acid metabolism, and arachidonic acid metabolism pathways; and PE (18:0/20:4) was enriched in the glycosylphosphatidylinositol-anchor biosynthesis pathway. A KEGG enrichment analysis of differentially present metabolites between HPHP and MC is shown in Figure 3F. A total of 11 pathways were identified, including fat digestion and absorption, glycerolipid metabolism, glycerophospholipid metabolism, regulation of lipolysis in adipocytes, alpha-linolenic acid metabolism, linoleic acid metabolism, and arachidonic acid metabolism, which were associated with lipid metabolism. TG (16:0/18:1/18:1) and TG (16:1/18:2/18:2) were enriched in fat digestion and absorption, glycerolipid metabolism, and regulation of lipolysis in adipocytes, and PC (20:4/20:4) was enriched in glycerophospholipid metabolism, alpha-linolenic acid metabolism, linoleic acid metabolism, and arachidonic acid metabolism.
2.4 Alterations in Liver Gene Expression and SCFAs in HFD Fed M. auratus
The differentially expressed genes between the MC and LPHP groups are shown in Table S4, Supporting Information. In total, 15 genes were significantly up-regulated and 28 genes were significantly down-regulated. The volcano plot of the differentially expressed differential genes between the MC and LPHP groups is shown in Figure 4A. The genes, including abhydrolase domain containing 5 (Abhd5), ELOVL fatty acid elongase 6 (Elovl6), fatty acid binding protein 5 (Fabp5), fatty acid synthase (Fasn), malic enzyme 1 (Me1), pyruvate kinase, liver and RBC (Pklr), and patatin like phospholipase domain containing 3 (Pnpla3), which are associated with lipid metabolism, were significantly down-regulated. The differentially expressed genes between the MC and HPHP groups are shown in Table S5, Supporting Information. In total, nine genes were significantly up-regulated and 16 genes were significantly down-regulated. The volcano plot of the differentially expressed genes between the MC and HPHP groups is shown in Figure 4B. Genes including Abhd5 and lipoprotein lipase (Lpl) were significantly down-regulated, whereas phosphoenolpyruvate carboxykinase 1 (Pck1), which are associated with lipid metabolism, were significantly up-regulated. The correlations among the 43 differentially expressed genes between the MC and LPHP groups are shown in Figure 4C. The samples in the MC and LPHP groups clustered into one group, thus indicating that LPHP affected the expression of liver genes. Compared with those in the MC group, the expression levels of Fasn, Elovl6, Pklr, Me1, Pnpla3, Abhd5, and Fabp5 were down-regulated in the LPHP group. The correlations among the 25 differentially expressed genes between the MC and HPHP group are shown in Figure 4D. The samples in the MC and HPHP groups clustered into one group, thereby indicating that LPHP affected the expression of liver genes. Compared with that in the MC group, the expression of Pck1 was up-regulated in the HPHP group, and the expression of Abhd5 and Lpl was down-regulated.
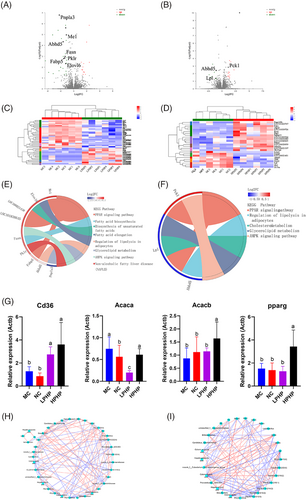
A KEGG enrichment analysis of 43 differentially expressed genes between LPHP and MC is shown in Table S6, Supporting Information. In total, 43 genes were enriched in 86 KEGG pathways, 32 of which were significant (p < 0.05). Eight pathways were associated with lipid metabolism, including fatty acid biosynthesis, the PPAR signaling pathway, the AMPK signaling pathway, fatty acid elongation, biosynthesis of unsaturated fatty acids, lipolysis regulation in adipocytes, glycerolipid metabolism, and non-alcoholic fatty liver disease. A KEGG enrichment chordal diagram between the MC and HPHP groups is shown in Figure 4E. Abhd5 was enriched in the regulation of lipolysis of adipocytes, Pnpla3 was enriched in glycerolipid metabolism, Pklr was enriched in non-alcoholic fatty liver disease, Elovl6 was enriched in fatty acid elongation and biosynthesis of unsaturated fatty acids, Me1 and Fabp5 were enriched in the PPAR signaling pathway, and Fasn was enriched in fatty acid biosynthesis and the AMPK signaling pathway. A KEGG enrichment analysis of 25 differentially expressed genes between HPHP and MC is shown in Table S7, Supporting Information. A total of 47 KEGG pathways were enriched, five of which were significant (p < 0.05). Five pathways were associated with lipid metabolism, glycerolipid metabolism, the PPAR signaling pathway, the AMPK signaling pathway, cholesterol metabolism, and regulation of lipolysis in adipocytes. A KEGG enrichment chordal diagram between the MC and HPHP groups is shown in Figure 4F. Abhd5 was enriched in the regulation of lipolysis of adipocytes, Lpl was enriched in glycerolipid metabolism, the PPAR signaling pathway and cholesterol metabolism; and Pck1 was enriched in the PPAR and AMPK signaling pathways. The mRNA expression of key genes in lipid metabolism, determined by qRT-PCR, indicated that the expression of fatty acid translocase, cluster of differentiation 36 (Cd36) in the LPHP and HPHP groups was significantly up-regulated with respect to that in the MC group (Figure 4G). The expression of acetyl-CoA carboxylase alpha (Acaca) in the LPHP group was significantly down-regulated with respect to that in the MC group (Figure 4G). The expression of acetyl-CoA carboxylase beta (Acacb) in the HPHP group was significantly up-regulated with respect to that in the MC group (Figure 4G). The expression of ppar gamma (pparg) in the HPHP group was significantly up-regulated with respect to that in the MC group (Figure 4G).
The effects of PHP on the SCFA content of the liver are shown in Table 2. The acetic acid, propionic acid, and isovaleric acid content in the NC group was significantly higher (p < 0.05) than that in the MC, LPHP, and HPHP groups. The content of isobutyric acid in the NC and HPHP groups was significantly higher (p < 0.05) than that in the MC and LPHP groups. The butanoic acid content in the HPHP group was significantly higher than that in the NC and MC groups (p < 0.05).
| NC | MC | LPHP | HPHP | |
|---|---|---|---|---|
| Acetic acid | 58 459.39 ± 4135.88a | 41 648.99 ± 2453.33b | 42 651.39 ± 2620.76b | 43 132.68 ± 3360.53b |
| Propanoic acid | 6947.15 ± 531.39a | 4917.68 ± 637.11b | 3977.17 ± 308.07b | 4213.09 ± 408.39b |
| Isobutyric acid | 123.81 ± 7.97a | 71.25 ± 5.82b | 83.99 ± 5.54b | 119.42 ± 20.60a |
| Butanoic acid | 384.37 ± 29.50b | 405.36 ± 51.63b | 480.19 ± 31.37ab | 640.79 ± 56.82a |
| Isovaleric acid | 200.37 ± 26.97a | 64.31 ± 3.26b | 57.93 ± 4.74b | 67.82 ± 9.31b |
| Valeric acid | 146.56 ± 30.32a | 110.09 ± 22.03a | 139.97 ± 13.14a | 165.41 ± 37.66a |
| Isohexanoic acid | 12.42 ± 1.85a | 7.47 ± 1.10b | 8.37 ± 0.44a | 7.56 ± 1.08b |
| Hexanoic acid | 138.76 ± 18.75a | 111.33 ± 7.40a | 104.14 ± 4.69a | 111.84 ± 10.30a |
- Note: There is significant difference between different lowercase letters in the same row (p < 0.05).
2.5 The Gut Microbiome's Influence on Serum Lipid and Liver Lipid Metabolites
The correlation between serum lipids and the gut microbiota is shown in Figure 5A. TG was significantly positively correlated with norank_f_Erysipelotrichaceae, Lactobacillus and Faecalibaculum (p < 0.05), but significantly negatively correlated with Rikenellaceae_RC9_gut_group, Elusimicrobium, and norank_f_Paludibacteraceae (p < 0.05). TC was significantly positively correlated with norank_f_Erysipelotrichaceae, Lactobacillus, and Faecalibaculum (p < 0.05), but significantly negatively correlated with Rikenellaceae_RC9_gut_group, Elusimicrobium, unclassified_c_Bacilli, and norank_f_Paludibacteraceae (p < 0.05). HDLC was significantly positively correlated with Lactobacillus (p < 0.05), but significantly negatively correlated with Dubosiella and Parasutterella (p < 0.05). LDLC was significantly positively correlated with norank_f_Erysipelotrichaceae, Lactobacillus, Faecalibaculum, and Family_XIII_AD3011_group (p < 0.05), but significantly negatively correlated with Rikenellaceae_RC9_gut_group, Elusimicrobium, unclassified_c_Bacilli, and norank_f_Paludibacteraceae (p < 0.05).
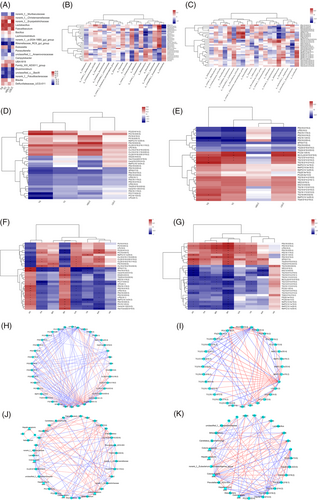
The correlations between liver lipid metabolites and the gut microbiota in the MC and LPHP groups are shown in Figure 5B. norank_f_Christensenellaceae was significantly positively correlated with TG (25:0/18:0/22:6) (p < 0.05). Bifidobacterium was significantly negatively correlated with PC (17:0/24:2) (p < 0.05). unclassified_f_Erysipelotrichaceae was significantly positively correlated with PC (18:1/23:0) (p < 0.05).
The correlations between liver lipid metabolites and the gut microbiota in the MC and HPHP groups are shown in Figure 5C. norank_f_Eubacterium_coprostanoligenes_group was significantly negatively correlated with TG (16:1/12:0/12:0) (p < 0.05). Bifidobacterium was significantly positively correlated with TG (16:1/12:0/12:0) and TG (18:2/14:2/14:2) (p < 0.05), and significantly negatively correlated with TG (30:0/16:0/18:2) and TG (24:0/18:2/23:0) (p < 0.05). Unclassified_f_Erysipelotrichaceae was significantly negatively correlated with TG (16:0/14:2/18:1) (p < 0.05).
2.6 Serum Lipids are Associated with Hepatic Lipid Metabolites
The correlations between serum lipids and the top 30 hepatic lipid metabolites (VIP value) in the MC and LPHP groups are shown in Figure 5D. Serum TG was significantly negatively correlated with PC (17:0/24:2) (p < 0.05). The correlations between serum lipids and the top 30 hepatic lipid metabolites (VIP value) in the MC and HPHP groups are shown in Figure 5E. Serum TG was significantly positively correlated with TG (12:0/14:0/18:2), TG (12:0/12:0/18:2), TG (18:2/14:2/14:2), TG (16:0/6:0/22:6), and TG (6:0/18:2/18:2) (p < 0.05). Serum TC was significantly positively correlated with PC (32:1/20:4), TG (12:0/14:0/18:2), and TG (12:0/12:0/18:2) (p < 0.05). Serum LDLC was significantly positively correlated with PC (32:1/20:4) (p < 0.05).
2.7 Colonic SCFAs are Associated with Hepatic Lipid Metabolites
The correlations between colonic SCFAs and differentially present lipid metabolites in the LPHP and MC groups are shown in Figure 5F. Colonic acetic acid was significantly negatively correlated with PC (18:4/20:5). Colonic propanoic acid was significantly negatively correlated with PC (17:0/24:2) (p < 0.05). Colonic butyric acid was significantly positively correlated with PC (17:0/24:2) and PC (18:1/23:0) (p < 0.05).
The correlations between colonic SCFAs and differentially present lipid metabolites in the HPHP and MC groups are shown in Figure 5G. Colonic butyric acid was significantly negatively correlated with TG (16:0/6:0/22:6), TG (16:0/14:2/16:0), TG (16:0/14:2/18:1), TG (16:1/12:0/12:0), TG (12:0/14:0/18:2), TG (12:0/12:0/18:2), TG (18:1/12:0/12:0), TG (10:0/12:0/18:2), TG (18:2/14:2/14:2), and TG (6:0/18:2/18:2) (p < 0.05).
2.8 Hepatic Lipid Metabolites are Associated with Hepatic Gene Expression
The correlations between hepatic differentially expressed genes and metabolites in the LPHP and MC groups are shown in Figure 5H. Abhd5 was significantly negatively correlated with PC (18:1/23:0) (p < 0.05). Elovl6 was significantly negatively correlated with PC (17:0/24:2) (p < 0.05). Fabp5 was significantly negatively correlated with PC (17:0/24:2) (p < 0.05). Me1 was significantly negatively correlated with PC (17:0/24:2) and PC (18:1/23:0) (p < 0.05). Pnpla3 was negatively correlated with PC (17:0/24:2) and PC (18:1/23:0) (p < 0.05). Acaca was significantly negatively correlated with PC (17:0/24:2) and PC (18:1/23:0) (p < 0.05).
The correlations between hepatic differentially expressed genes and metabolites in the HPHP and MC groups are shown in Figure 5I. Abhd5 was significantly negatively correlated with TG (24:0/18:2/23:0) and TG (30:0/16:0/18:2) (p < 0.05). Lpl was significantly positively correlated with TG (18:1/12:0/12:0), TG (18:2/14:2/14:2), TG (16:0/14:2/18:1), TG (16:0/14:2/16:0), TG (16:1/12:0/12:0), TG (10:0/12:0/18:2), TG (12:0/12:0/18:2), and TG (12:0/14:0/18:2) (p < 0.05). Pck1 was significantly negatively correlated with TG (16:0/6:0/22:6), TG (18:1/12:0/12:0), TG (16:0/14:2/18:1), TG (16:0/14:2/16:0), TG (10:0/12:0/18:2), TG (12:0/12:0/18:2), and TG (12:0/14:0/18:2) (p < 0.05). Pparg was significantly negatively correlated with TG (16:1/12:0/12:0), TG (10:0/12:0/18:2), and TG (12:0/14:0/18:2) (p < 0.05). Acacb was significantly negatively correlated with TG (10:0/12:0/18:2) (p < 0.05). CD36 was significantly negatively correlated with TG (16:1/12:0/12:0), TG (10:0/12:0/18:2), and TG (12:0/14:0/18:2) (p < 0.05).
2.9 Relationships among Serum Lipids, the Colonic Microbiota, Hepatic Lipid Metabolites, Hepatic SCFAs, and Hepatic Genes
The correlations among serum lipid, the colonic microbiota, hepatic lipid metabolites, hepatic SCFAs, and hepatic genes in the LPHP and MC groups are shown in Figure 5J. Colonic butyric acid was significantly positively correlated with unclassified_f_Erysipelotrichaceae, hepatic butyric acid, PC (18:1/23:0), and PC (17:0/24:2) (p < 0.05). PC (18:1/23:0) was significantly negatively correlated with Me1, Pnpla3, Acaca, and Cd36 (p < 0.05), and was significantly positively correlated with unclassified_f_Erysipelotrichaceae and hepatic butyric acid (p < 0.05). PC (17:0/24:2) was significantly negatively correlated with Abhd5, Elovl6, Fabp5, Me1, Pnpla3, Acaca, Bifidobacterium, and TG (p < 0.05). TG was significantly negatively correlated with norank_f_Muribaculaceae and was significantly positively correlated with Bifidobacterium and Me1 (p < 0.05).
The correlations among serum lipid, colonic microbiota, hepatic lipid metabolites, hepatic SCFAs, and hepatic genes in the HPHP and MC groups are shown in Figure 5K. Colonic butyric acid was significantly negatively correlated with PC (20:4e/13:0), TG (16:1/12:0/12:0), TG (10:0/12:0/18:2), TG (18:2/14:2/14:2), TG (16:0/14:2/18:1), TG, TC, Bifidobacterium, Abhd5, and Lpl (p < 0.05), and was significantly positively correlated with hepatic butyric acid, Pck1, pparg, Acacb, and Cd36 (p < 0.05). Hepatic butyric acid was significantly negatively correlated with TG (16:1/12:0/12:0) and TG (10:0/12:0/18:2) (p < 0.05), and was significantly positively correlated with pparg and Cd36 (p < 0.05). TG was significantly positively correlated with TG (18:2/14:2/14:2) and Bifidobacterium (p < 0.05). TC was significantly positively correlated with Bifidobacterium (p < 0.05).
3 Discussion
HFD consumption may cause liver enlargement and an increase in the liver index reflecting the existence of hyperlipidemia.[26] LDLC is the most important transporter of TC, and redundant LDLC easily accumulates and oxidizes in the blood vessels, thus accelerating the formation of foam cells and plaques.[27, 28] The increases in TC and LDLC are important risk factors leading to hyperlipidemia, and decreases in TC and LDLC can delay or reverse hyperlipidemia.[29, 30] In this study, the content of TG, TC, and LDLC in the NC group was significantly lower than that in the MC group, thus indicating that the model of hyperlipidemia, M. auratus, induced by an HFD, was formed successfully. HPHP inhibited TG, TC, and LDLC, thus indicating that HPHP prevents the occurrence of hyperlipidemia. This finding was consistent with the results of Wang et al.[25] in which PHP has been found to effectively decrease the levels of TG, TC, and LDLC in HFD fed mice.
The gut microbiota is a micro-ecosystem composed of microorganisms, which has important roles in physiological functions, such as digestion, metabolism, and vitamin synthesis.[2] The Bacteroidetes, Firmicutes, and Actinobacteria phyla might be responsible for the degradation of complex non-digestible polysaccharides.[31] Bacteroidota produce SCFAs through a series of glycoside hydrolases and carbohydrate metabolism pathways, which are highly important in macromolecular metabolism.[32, 33] In the present study, compared with that in the MC group, the abundance of Bacteroidota after PHP intervention increased, thus indicating that PHP might be degraded by Bacteroidota. Muribaculaceae have been found to inhibit the effects of an HFD in mice.[34] Lactobacillus and Bifidobacterium play important roles in decreasing blood lipid levels, because they decompose bound bile acids into free bile acids by secreting bile acid hydrolases.[35, 36] In the present study, compared with that in the MC group, the abundance of Muribaculaceae was higher in the LPHP and HPHP groups, thus indicating that their effects in decreasing blood lipids might be associated with Muribaculaceae rather than the bile acid pathway. After exposure to PHP, the abundance of Bifidobacterium in the colon decreased, in agreement with the results of Shang et al.[7] This study revealed that the abundance of Bifidobacterium in the intestines in mice decreases after intragastric administration of brown algae fucoidan. SCFAs, the products of carbohydrate fermentation by the gut microbiota, provide energy for intestinal microorganisms and intestinal cells, which are highly important for human health.[7, 8] Faecalibaculum degrades polysaccharides and fibers, thus producing butyric acid.[37, 38] Butyric acid not only provides the main energy supply for colonic epithelial growth but also decreases the inflammatory response by activating its target G protein-coupled receptor and histone deacetylase inhibitor activity, thus potentially protecting intestinal permeability.[39] Butyric acid may enter the peripheral circulation through intestinal cells.[40] The intake of an HFD might decrease the concentration of butyric acid, which serves as an energy source for mitochondria and consequently prevents AMPK-induced autophagy. These findings have indicated that the hypolipidemic effects of LPHP and HPHP are associated with the increase in the colonic n-butyric acid content. PHP promotes the production of butyric acid in the colon, and this effect might be associated with the increased abundance of Faecalibaculum in the colon.
In the liver, phosphatidylcholine (PC) emulsifies TG, promotes TG metabolism, and decreases hepatic deposition of TG.[41, 42] Thus, the accumulation of lipids in the liver in the MC group might have been associated with the down-regulation of PC expression. TG accumulation in non-adipose tissues and the formation of lipid droplets are a sign of lipotoxicity.[43] A decrease in fat vacuoles was observed in the liver histopathology and might have been associated with the up-regulation of PC expression in the LPHP group. The expression of TG metabolites in the liver in the HPHP group was down-regulated, thus confirming the results of liver histopathology. In the HPHP group, in contrast to the LPHP group, the expression of TG metabolites was significantly down-regulated, thus potentially explaining the observation of fat vacuoles in the liver histopathology of the LPHP group but not the HPHP group.
Fasn is a fat synthesis gene whose expression product, fatty acid synthase, is the central enzyme in the pathway of newborn fat formation, through catalyzing all steps of malonyl CoA conversion to palmitic acid.[44] Palmitate (16:0), the main product of Fasn, is elongated by Elovl6, and some products of Fasn are endogenous PPARα agonists, which maintain fatty acid metabolism.[45, 46] Palmitoleic acid ester is the main end product of de novo fatty acid synthesis in patients with Elovl6 deficiency. It effectively binds TGs and cholesteryl esters, and helps prevent insulin resistance.[47] LPHP inhibits fat synthesis in the liver and fatty acid chain elongation by down-regulating the expression of Fasn and Elovl6. Fabp5, a member of the Fabp family, plays important roles in binding free fatty acids, and regulating and transporting lipids.[48] The adipose tissue in mice without Fabp5 is resistant to the harmful effects of dietary lipid exposure.[49] Here, LPHP inhibited fat transport into cells by down-regulating the expression of Fabp5. The Me1 gene encodes malic enzyme, which is expressed in adipose tissue and participates in fatty acid metabolism.[50] In an animal model of diet-induced obesity, the expression of Me1 and other adipogenic genes in cells has been found to be elevated.[51, 52] Our results suggested that LPHP inhibits the production of fat and cholesterol in liver cells by down-regulating the expression of Me1. The Pnpla3 gene is a member of the Pnpla family. The down-regulation of Pnpla3 expression in the livers of rats fed an HFD has been found to decrease the accumulation of fat in the liver.[53] Here, LPHP inhibited hepatic TG production by down-regulating the expression of Pnpla3. Lpl plays a key role in lipid metabolism and transportation by catalyzing the rate limiting step of hydrolysis of TG components in circulating chyle particles and very low density lipoprotein.[54] LPL simultaneously binds lipoprotein and cell surface receptor/proteoglycan, thus performing non-catalytic bridging functions that result in lipoprotein accumulation and cell uptake.[55, 56] Transgenic mice overexpressing LPL in the liver show a twofold increase in liver TG content and develop tissue-specific insulin resistance.[57] The expression of Lpl mRNA is often considered an early marker of adipocyte differentiation.[58] Here, HPHP inhibited fatty acid transport and decreased liver fat production by down-regulating the expression of LPL. CD36 is a lipogenic gene in the PPAR signaling pathway. It encodes a membrane receptor that absorbs modified forms of LDL and fatty acids from the circulation.[59, 60] The up-regulation of CD36 indicated that LPHP and HPHP promote the transportation of fatty acids. Acetyl-CoA carboxylase catalyzes the carboxylation of acetyl CoA, thus producing malonyl CoA. This enzyme is encoded by two genes, Acaca and Acacb. Acaca plays a main role in the biosynthesis of long-chain fatty acids, whereas the malonyl-CoA product of acacb inhibits fatty acid oxidation.[61] The down-regulation of acaca indicated that LPHP inhibited the biosynthesis of long-chain fatty acids, whereas the up-regulation of acacb indicated that LPHP promoted fatty acid oxidation. Ppar is a key target gene associated with the beneficial effects of SCFAs in liver metabolic syndrome, and SCFAs might regulate ppar.[12] SCFAs (acetic acid, propionic acid, and butyric acid) stimulate the expression of pparg, prevent and reverse the abnormal lipid metabolism induced by an HFD in mice, and promote the oxidation of hepatic fatty acids.[62] The up-regulation of pparg in the HPHP group might have been associated with the increase in butyric acid.
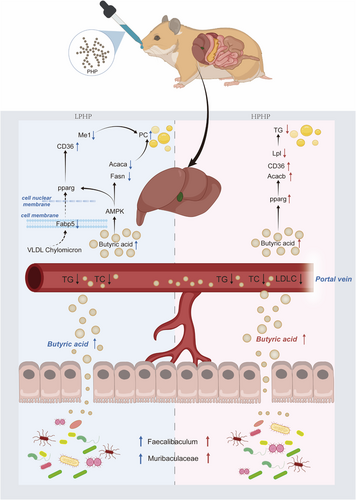
On the basis of the above results, we hypothesized a molecular mechanism underlying the hypolipidemic effect of LPHP on the gut-liver axis (Figure 6). First, LPHP is degraded by Muribaculaceae and Faecalibaculum, thereby promoting the production of butyric acid in the colon. Second, the butyric acid enters the liver through the peripheral circulation. The expression of Fabp5, Fasn, Me1 Acaca, and Elovl6 is down-regulated, and that of Cd36 is up-regulated. The levels of liver phosphatidylcholine metabolite production are up-regulated. Finally, the liver TG and blood lipids are down-regulated. In addition, a molecular mechanism underlying the hypolipidemic effect of HPHP on the gut-liver axis was also hypothesized (Figure 6). First, HPHP is used by the Muribaculaceae and Faecalibaculum, thereby promoting the production of butyric acid. Second, the butyric acid enters the liver through the peripheral circulation. Third, butyrate acid activates the hepatic pparg signaling pathway, inhibits the expression of a fatty acid transport gene (Lpl) and promotes the expression of a fatty acid oxidation gene (Acacb). The levels of liver TG metabolite production are down-regulated. Finally, the hepatic TG and blood lipids are down-regulated.
4 Conclusions
In the present study, we demonstrated that dietary intervention with PHP, particularly HPHP, prevented an increase in blood lipids and the accumulation of hepatic fat droplets by regulating the gut microbiota. HPHP promoted an increase in microbiota-derived butyric acid, which induced pparg signaling activation, thereby preventing the expression of fatty acid oxidation genes. These results indicated that PHP might be used as an efficacious functional food supplement.
5 Experimental Section
Material
P. haitanensis was purchase from Pingtan Island, Fujian Province, China. P. haitanensis polysaccharide was extracted according to the previous method.[15] M. auratus [license No. SCXK (Shanghai) 2017-0008] were purchased from the Songjian Experimental Animal Farm (Songjiang District, Shanghai, China).
Animals
All animal studies were performed in compliance with the Guidelines for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication 85-23, 1996), and the experimental study protocol was approved by the Animal Ethics Committee of Fujian Academy of Traditional Chinese Medicine (approval number FJATCM-IAEC2020014). M. auratus individuals were randomly divided into four groups (n = 6 per group): 1) the normal control (NC) group, fed regular chow (carbohydrate 72.3%, protein 14.9%, fat 12.8%), 2) the model control (MC) group, fed a diet containing high fat chow (coconut oil 5%, corn oil 5%, cholesterol 0.2%, and regular chow 89.8%), 3) the low-dose PHP (LPHP) group, fed a diet containing high-fat chow and intragastric PHP (100 mg (kg d)−1), and 4) the high-dose PHP (HPHP) group, fed a diet containing high-fat chow and intragastric PHP (200 mg (kg d)−1). During the feeding period, M. auratus animals ate and drank water freely under a 12-h light and dark cycle for 4 weeks.
Physiological Index and Histological Evaluation
After 4 weeks of treatment, M. auratus individuals were fasted for 12 h. After weighing, they were euthanized with pentobarbital sodium (WS20130112, product batch No. 20160111). Blood was collected from the abdominal aorta into vacuum blood collection vessels, and cervical dislocation was then performed. The liver, kidney, and spleen tissues were quickly removed and weighed. Serum TG, TC, HDLC, and LDLC were determined with an automatic biochemical analyzer (AU2700, Beckman Kurt Co., Ltd., Carlsbad, CA, USA). The organ indexes were the ratios of organ weight to body weight. Liver tissue was stored in 4% paraformaldehyde and embedded in paraffin. Liver tissue was sectioned (5 µm in thickness) and stained with hematoxylin and eosin. The stained samples were observed with a light microscope (Nikon Eclipse E10, Nikon Instrument Co., Ltd., Tokyo, Japan) with an imaging system (Nikon DS-U3, Nikon Instrument Co., Ltd., Tokyo, Japan).
16S rRNA Sequencing of Colonic Microbiota
The total DNA extraction of the colonic microbiota was performed with a DNA extraction kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer's instructions. The total DNA concentration and purity were detected with an ultra-micro spectrophotometer (NanoDrop 2000, Thermo Fisher Technology Co., Ltd., Wilmington, DE, USA). The extraction quality of DNA was detected with 1% agarose gel electrophoresis, and the V3–V4 variable region was amplified with primers 338F (5´-ACTCCTACGGGAGGCAGCAG-3´) and 806R (5´-GGACTACHVGGGTWTCTAAT-3´). The amplification procedure included pre-denaturation (95 °C, 3 min), denaturation (95 °C, 30 s), annealing (55 °C, 30 s), and extension (72 °C, 30 s). After 27 cycles of denaturation, annealing, and extension, a final extension was performed at 72 °C for 10 min. A 2% agarose gel was used to recover the amplified PCR products. The recovered PCR products were purified with a DNA gel extraction kit (Axygen Biosciences, Union City, CA, USA), and the purified DNA was sequenced on the Miseq PE300 platform (Illumina, San Diego, CA, USA).
Determination of Colonic SCFAs
The colonic SCFAs were determined. Briefly, 100 mg of colonic contents was mixed with 1 mL of water (containing 0.5% phosphoric acid and 50 µg mL−1 2-ethylbutyric acid), ground for 3 min at −10 °C, sonicated in an ice water bath for 30 min and incubated for 30 min at 4 °C. Afterward, samples were centrifuged for 15 min (4 °C, 13 000 × g), and the supernatant was extracted with 500 µL ethyl acetate. After vortex mixing, they were placed in an ice water bath and subjected to ultrasound treatment for 10 min. Samples were then centrifuged for 10 min (4 °C, 13 000 × g), and the supernatant was obtained for GC-MS (Agilent 8890B-5977B, Agilent Technologies Inc., Santa Clara, CA, USA) detection. The chromatographic column was an HP FFAP capillary column (30 m × 0.25 mm × 0.25 µm, Agilent J&W Scientific, Folsom, CA, USA). The carrier gas was high purity helium at a flow rate of 1.0 mL min−1. The injection port temperature was 260 °C, and the injection volume was 1 µL. The split ratio was 10:1, and the solvent delay was 2.5 min. The initial temperature was 80 °C, and was increased to 120 °C at a heating rate of 40 °C min−1, increased to 200 °C at a heating rate of 10 °C min−1, and then run for 3 min at 230 °C. The ion source temperature was 230 °C, the four-stage rod temperature was 150 °C, the electron energy was 70 eV, and the scanning range was 25–300 m/z.
Determination of Hepatic Lipid Metabolites
Briefly, each 50 mg liver sample was mixed with 280 µL 40% methanol and 400 µL methyl tert-butyl ether, ground for 6 min at –10 °C, sonicated for 30 min at 5 °C, and maintained for 30 min at −20 °C. Samples were then centrifuged for 15 min (4 °C, 13000 × g). Then 350 µL supernatant was dried with nitrogen and redissolved with 100 µL isopropanol:acetonitrile (1:1) solution, then sonicated for 5 min at 5 °C and centrifuged for 10 min (4 °C, 13 000 × g). Each supernatant was analyzed with LC-MS on a UHPLC-Q Exactive HF-X system (Thermo Fisher Technology Co., Ltd, Wilmington, DE, USA). A 20 µL volume of supernatant per sample was mixed as a quality control sample. The chromatographic column was an Accucore C30 (3.0 mm × 150 mm × 2.6 µm, Thermo Scientific, Bellefonte, PA, USA), mobile phase A was 50% acetonitrile (containing 0.1% formic acid and 10 mmol L−1 ammonium acetate), and mobile phase B was acetonitrile:isopropanol:water (10:88:2) (containing 0.02% formic acid and 2 mmol L−1 ammonium acetate). The flow rate was 0.4 mL min−1, the injection volume was 2 µL, and the column temperature was 40 °C. The gradient elution procedure was as follows: 65% A and 35% B elution for 4 min, 40% A and 60% B elution for 8 min, 15% A and 85% B elution for 3 min, 100% B elution for 6 min, and 65% A and 35% B elution for 3 min. The scanning range was 200–2000 m/z, the ion source heating temperature was 370 °C, the ionization positive voltage was 3000 V, and the ionization negative voltage was 3000 V.
Determination of Hepatic Genes
Liver total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). An RNA-seq transcriptome library was constructed with a TruSeq RNA sample preparation Kit from Illumina. The RNA-seq sequencing library was sequenced with an Illumina HiSeq xten/NovaSeq 6000 sequencer (2 × 150-bp read length) by Shanghai Majorbio Biopharm Biotechnology (Shanghai, China).
Reverse transcription of RNA was performed in accordance with the instructions of the PrimeScript RT Master Kit (TaKaRa, Japan). ACTB was used as an endogenous control gene. Average Ct values from triplicate analyses were normalized to the average Ct values of ACTB. The target genes’ primer sequences were synthesized by Shanghai Majorbio Biopharm Biotechnology.
Determination of Hepatic SCFAs
The hepatic SCFAs were determined. Briefly, 50 mg of liver was mixed with 80% methanol, ground for 6 min at −10 °C, sonicated in an ice water bath for 30 min, and maintained at −20 °C for 30 min. Samples were then centrifuged for 15 min (4 °C, 13 000 × g). The supernatant was mixed with 20 µL 200 mM 3NPH.HCl and 20 µL 120 mM EDC.HCL and then reacted for 30 min at 40 °C. Subsequently, the samples were diluted to 1000 µL with 50% acetonitrile and detected with UHPLC-Qtrap LC-MS (QTRAP 6500+, AB SCIEX Pte. Ltd., Framingham, MA, USA). The chromatographic column was a Waters BEH C18 (150 × 2.1 mm, 1.7 µm), the column temperature 40 °C, and the injection volume 1 µL. Mobile phase A was 0.1% formic acid aqueous solution and mobile phase B was 0.1% formic acid acetonitrile. The flow rate was 0.35 mL min−1. The gradient program was as follows: 90% A + 10% B for 11 min, 45% A + 55% B for 1 min, 5% + 5% B for 1 min, and 90% A + 10% B for 3 min. The curtain gas was 35, the collision gas was medium, the ion spray voltage −4500, the temperature 450 °C, the ion source gas 1 40, and the ion source gas 2 40.
Statistical Analyses
All figures were constructed with GraphPad Prism 8.0.1 (GraphPad Software Inc., San Diego, CA, USA). One-way ANOVA and Tukey's tests were used to analyze repeated measures data in SPSS (version 13.0, Chicago, IL, USA).
Acknowledgements
This work was supported by the Program for Leading Talent in Fujian Provincial University (Grant Number 660160190).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The data of 16S r RNA sequence are available in SRA within the BioProject number PRJNA847401. Code for data analysis is available at h t t p s : / / submit.ncbi.nlm.nih.gov/subs/sra/SUB11580517/overview.




