Metabolic Fate and Cardiometabolic Effects of Phenolic Compounds from Red-Fleshed Apple in Hypercholesterolemic Rats: A Comparative Study with Common White-Fleshed Apple. The AppleCOR Study
Abstract
The present study aims to investigate the metabolic fate and the cardiometabolic effects of phenolic compounds provided by a red-fleshed apple variety biofortified in anthocyanins (ACN). Wistar rats are fed with high-fat diet (HFD) to induce hypercholesterolemia and supplemented with red-fleshed apple (HFD+R), white-fleshed apple (HFD+W), or an ACN-rich infusion from aronia fruit (HFD+A) providing matched content and profile of ACN. Plasma biochemical parameters, histological analysis, and phenol biological metabolites are determined. Plasma, urine, and feces show a significant increase of ACN metabolites after HFD+R and HFD+A, while flavan-3-ols are significantly increased after HFD+W and dihydrochalcones derivatives increased after both apples supplementation. A cardioprotective effect is observed after both apples and aronia infusion supplementation in the reduction of aortic thickness. The kidney function is improved after all supplementations and a decrease in insulin plasma concentration after both apples supplementation (HFD+R and HFD+W) is also observed. The findings support that ACN without apple matrix can induce cardioprotective effects. ACN or flavan-3-ols, together with dihydrochalcones, compose a phenolic phytocomplex in red- and white-fleshed apples, respectively, which can act synergistically in the attenuation of cardiovascular outcomes in hypercholesterolemic rats.
1 Introduction
Phenolic compounds (PC) are among the most abundant phytochemicals present in the human diet, and increasing evidence highlights their important health-promoting effects.[1, 2] At present, PC garner much attention due to their protection against cardiovascular diseases (CVD),[3] which are the first cause of death globally (17.9 million people died from CVD in 2016).[4] Understanding the bioavailability of PC, their metabolism, and tissue distribution is critical since their physiological impact depend on their delivery to target tissues.[5, 6] Once the PC are absorbed in the upper part of the gastrointestinal tract, they are mainly metabolized in the small intestine and liver by phase-II enzymes to their sulfate, methyl, and glucuronide conjugates. However, it is estimated that only 5–10% of the total polyphenol intake is absorbed in the small intestine. The remaining 90–95% may accumulate in the large intestinal lumen where they are subjected to the enzymatic activities of the gut microbial community.[7] All these metabolites are those that reach circulation and target tissues and may be responsible for the health effects derived from the consumption of PC-rich foods.
Another fact to consider in the study of PC bioavailability and their health effects is the food matrix in which these compounds are found, since in most fruits and vegetables, many of these compounds are linked to carbohydrates, fiber, proteins, and cell walls as well as to other PC by covalent bonds, hydrogen bonding, and hydrophobic and hydrophilic interactions.[8] During gastrointestinal digestion, the binding of PC to these molecules affects their release in the gut as well as the efficacy by which they are transported across the mucosal epithelium and this may lead to a decrease in the PC bioaccessibility,[9, 10] and in consequence in their bioactivity.
Anthocyanins (ACN) have been extensively reported to manifest therapeutic properties against hyperlipidemia and CVD, among others.[11-13] Some authors reported that after oral intake short-term experiments in animals, intact ACN were mainly detected in tissues like heart, liver, kidney, lung[14] or brain.[15] In the last few years, there has been an increasing interest in potential crops for coloring food naturally, such as red-fleshed apples cultivars. Due to their enhanced content of ACN, different studies have shown that the total phenolic content and antioxidant capacity of red-fleshed apples were significantly higher compared to common white-fleshed apple cultivars, which indicates that they could have presumably added healthy properties.[16] Indeed, red-fleshed apple supplementation in rats has already shown protective effects against colon carcinogenesis retarding/diminishing the appearance of the precancerous markers and the expression of genes related to this cancer.[17]
The emerging potential of red-fleshed apples as a novel ACN-rich fruit along with the differences reported in the PC bioavailability and bioactivity depending on the food matrix, substantiates the present research focused on the possible health benefits of red-fleshed apple. The main objective of the present study was to investigate the cardiometabolic effects of the diet supplementations with red-fleshed apple and compared to common white-fleshed apple in hypercholesterolemic rats through the analysis of histological parameters in target tissues (liver, heart, kidney, and aorta). To study the apple matrix effect, rats were also supplemented with an ACN-rich infusion from aronia fruit (Aronia melanocarpa). To fully understand the observed effects, we also performed a comprehensive analysis of the metabolic fate and metabolic pathways of ACN and other PC from apple in rat plasma, urine, and feces.
2 Experimental Section
2.1 Chemicals and Reagents
Cyanidin-3-O-galactoside, eriodictyol, quercetin-3-O-glucoside, quercetin-3-O-rhamnoside, procyanidin dimer B2, phloretin-2’-O-glucoside, p-coumaric acid, and caffeic acid were purchased from Extrasynthese (Genay, France). p-Hydroxybenzoic acid, 3,4-dihydroxybenzoic acid (aka protocatechuic acid), hippuric acid, 3-(4’-hydroxyphenyl)acetic acid, 3-(3’,4’-dihydroxyphenyl)acetic acid, 3-(3’-hydroxyphenyl)propionic acid, 3-(3’,4’-dihydroxyphenyl)propionic acid (aka dihydrocaffeic acid), 3-(3’-hydroxy-4’-methoxyphenyl)propionic acid (aka dihydroferulic acid), epicatechin, and 5-O-caffeoylquinic acid (aka chlorogenic acid) were from Sigma-Aldrich (St. Louis, MO, USA). Vanillic acid and ferulic acid were from Fluka (Buchs, Switzerland). Vanillic acid-4-O-sulfate, catechol-4-O-sulfate, and 4-methyl catechol sulfate were synthesized according to ref. [18], and were kindly supplied by Dr. Claudia N. Santos (University of Lisbon, Portugal).
Methanol (HPLC grade), acetonitrile (HPLC grade), acetic acid, and hydrochloric acid were purchased from Scharlab Chemie (Sentmenat, Catalonia, Spain). The water used was Milli-Q quality (Millipore Corp, Bedford, MA, USA).
Stock solutions of standard compounds were prepared by dissolving each compound in methanol at a concentration of 1000 mg L−1, and stored in a dark flask at −30 °C.
2.2 Red- and White-Fleshed Apples and Aronia Fruit Infusion
To study and compare the cardioprotective effects, two different apple varieties were selected: i) the red-fleshed “Redlove” apple variety, a new genotype naturally biofortified in ACN, and ii) the common white-fleshed Granny Smith apple variety (both provided by NUFRI SAT, Mollerussa, Lleida, Spain) without ACN. Additionally, and to study the effect of ACN without the possible interaction of the apple matrix, an ACN-rich infusion from aronia fruit was selected.
To minimize changes in the bioactive compounds of the apples, a freeze-dried format was selected to prepare the supplemented diets. Briefly, the apple core was removed and the whole apple (with peel) was cut into 1 cm-sized cubes. The apple cubes were immediately frozen in liquid nitrogen and then lyophilized on a Lyobeta 15 TELSTAR Lyophilizer (Terrassa, Spain). The freeze-dried apple cubes were immediately transferred to airtight plastic containers and stored in refrigeration (2 °C) until the analysis of their phenolic composition, and the preparation of the supplemented diets. To obtain the ACN-rich extract, a cold water infusion of A. melanocarpa fruit powder (Aronia Pulver, BIOJOY, Nuremberg, Germany) was prepared, which was equivalent in dose and type of ACN to the red-fleshed apple. Briefly, distilled water was added to aronia fruit powder (in proportion 1:1), and the mixture was homogenized (Kinematica Polytron, Polytron Corporation, Montreal, Canada) for 60 s. The resulting mixture was centrifuged (5403 × g for 5 min), and the supernatant was analyzed and added to the drinking water of the rats. The phenolic composition of the freeze-dried apples and the aronia infusion is shown in Table 1.
| Phenolic compoundsa) | White-fleshed apple [µg/5 g/day] | Red-fleshed apple [µg/5 g/day] | Aronia [µg/20 mL infusion/day] |
|---|---|---|---|
| Cyanidin arabinoside | 6.88 ± 6.12 | 167 ± 6.00 | 480 ± 18.1 |
| Cyanidin galactoside | 21.8 ± 18.1 | 1690 ± 36.0 | 1426 ± 48.0 |
| Total anthocyanins | 28.6 ± 24.2 | 1857 ± 42.0 | 1906 ± 67.0 |
| Protocatechuic acid | n.d. | 108 ± 67.3 | 462 ± 65.2 |
| Coumaric acid hexoside | 48.7 ± 7.03 | 48.7 ± 7.03 | 2.00 ± 0.03 |
| Ferulic acid hexoside | 67.3 ± 7.44 | 134 ± 16.4 | 5.00 ± 0.02 |
| Vanillic acid | n.d. | n.d. | 14.0 ± 1.01 |
| Vanillic acid hexoside | 58.2 ± 4.05 | 271 ± 7.24 | 138 ± 22.0 |
| 5-O-caffeoylquinic acid | 1386 ± 28.08 | 5004 ± 174 | 814 ± 434 |
| 3-O-caffeoylquinic acid | n.d. | n.d. | 362 ± 97.0 |
| Gallic acid | n.d. | n.d. | 36.0 ± 5.03 |
| Gallic acid hexoside | n.d. | n.d. | 25.0 ± 6.02 |
| Caffeic acid | n.d. | n.d. | 32.0 ± 1.04 |
| Homogentisic acid | n.d. | n.d. | 21.0 ± 7.12 |
| Total phenolic acids | 1837 ± 56.2 | 5566 ± 211 | 1911 ± 638 |
| Catechin | 377 ± 25.3 | n.d. | n.d. |
| Epicatechin | 1867 ± 27.1 | 353 ± 49.3 | 12.0 ± 1.12 |
| Dimer | 3663 ± 121 | 438 ± 18.1 | 32.0 ± 7.15 |
| Trimer | 350 ± 51.6 | 82.0 ± 7.34 | 8.00 ± 1.06 |
| Total flavan-3-ols | 6259 ± 226 | 875 ± 74.9 | 52.0 ± 9.00 |
| Quercetin arabinoside | 166 ± 24.3 | 232 ± 28.7 | 5.00 ± 1.07 |
| Quercetin rhamnoside | 230 ± 26.6 | 587 ± 59.7 | n.d. |
| Quercetin glucoside | 541 ± 81.0 | 279 ± 35.9 | 133 ± 11.2 |
| Quercetin rutinoside | n.d. | n.d. | 87.0 ± 12.3 |
| Total flavonols | 938 ± 132 | 1098 ± 125 | 225 ± 24.0 |
| Eriodictyol hexoside | n.d. | 26.4 ± 1.42 | 7.00 ± 2.05 |
| Naringenin | n.d. | n.d. | n.d. |
| Total flavanones | n.d. | 26.4 ± 1.42 | 7.00 ± 2.00 |
| Phloretin glucoside | 247 ± 34.5 | 1371 ± 160 | n.d. |
| Phloretin xylosyl glucoside | 276 ± 17.6 | 739 ± 32.7 | n.d. |
| Hydroxyphloretin xylosyl glucoside | 14.2 ± 1.01 | 20.5 ± 1.52 | n.d. |
| Total dihydrochalcones | 536 ± 53.1 | 2130 ± 195 | n.d. |
| Total phenolics | 9598 ± 487 | 11 552 ± 1420 | 4101 ± 740 |
- a) The number of replicates was three (n = 3). n.d., not detected.
2.3 Animals and Experimental Procedure
Thirty Wistar rats weighting between 300 and 350 g were purchased from Charles River Laboratories (Barcelona, Spain). They were divided into five groups of six animals each one (three males and three females) as follows: Group 1: standard chow diet (SCD) (Teklad 2014, rodent maintenance diet, Envigo, Huntingdon, Cambridgeshire, UK); Group 2: high-fat diet (HFD) (Atherogenic Rodent Diet TD. 02028, Envigo, Huntingdon, Cambridgeshire, UK) to induce hypercholesterolemia; Group 3: HFD supplemented with white-fleshed apple (HFD+W); Group 4: HFD supplemented with red-fleshed apple (HFD+R); and Group 5: HFD supplemented with ACN extract from aronia (HFD+A). Male and female rats were included to observe possible different biological effects according to gender.
The design of the study is shown in Figure S1, Supporting Information. Group 1 was fed with chow diet for 9 weeks. The other groups were fed during 3 weeks with an HFD and the following 6 weeks with the HFD supplemented with the different products. For HFD+R and HFD+W (Groups 3 and 4), HFD pellets were crushed in a mill along with the freeze-dried apples. For HFD+A (Group 5), the A. melanocarpa extract was dissolved daily in the drinking water and was administered to the rats in bottles protected from light. Moreover, diets from Group 2 (HFD) and Group 5 (HFD+A) were modified by adding 25% of chow diet in the same proportion as apples, so that all groups except Group 1, would take the same proportion of HFD during the supplementation period.
To prepare the supplemented diets, HFD pellets and lyophilized apples (red- or white-fleshed) were crushed in a mill (MC300132, Moulinex, Alençon, France) until a homogeneous powder was obtained. Then, distilled water (10%) was added, the mixture was homogenized, and new apple-enriched pellets were prepared and dried in an oven (JA Selecta, Barcelona, Spain) at 25 °C for 3 days (at darkness).
During the 9 weeks of the experiment, the rats were housed in cages on a 12 h light-12 h dark schedule at controlled temperature (21 ± 1 °C), and humidity (55 ± 10%). Food and water were available ad libitum. The body weight, food and water intakes were recorded every 3 days. Table S1, Supporting Information, summarized this data according to the different diets.
The day before sacrifice, the rats were caged in metabolic cages for 24 h to collect urine and feces. The rats were sacrificed by an intracardiac puncture after isoflurane anesthesia (IsoFlo, Veterinarian Esteve, Bologna, Italy). Blood was collected in EDTA tubes, and plasma samples were obtained by centrifugation (3000 × g, 10 min at 4 °C) and stored at −80 °C until chromatographic analysis of phenolic metabolites. After blood collection, the rats were perfused with an isotonic solution of sodium chloride (0.9%) to remove the remaining blood in tissues. The heart, aorta, liver, and kidneys were excised and immediately frozen in liquid nitrogen. A part of the tissue samples was stored at −80 °C until analysis and the other part was fixed in 10% (v/v) formalin for a minimum of 24 h.
The animal procedures were conducted in accordance with the guidelines of the European Communities Directive 2010/63/EU regulating animal research. The protocols were approved by the Animal Ethical Committee of the University of Lleida (CEEA 01-10/17), and performed under a Generalitat de Catalunya Project License (10038). The study complies with the ARRIVE guidelines developed by the NC3Rs.[19]
2.4 Dosage Information
The supplementation of ACN through red-fleshed apple or aronia was based on the human equivalent dose of 70 mg day−1 of ACN, according to ref. [20]. So, the quantity of red-fleshed apple administered and the aronia fruit extract in water was adjusted to a dose of 1.8 and 1.9 mg/day/rat of ACN, respectively (Table 1). The quantity of white-fleshed apple to prepare HFD+W was equivalent to HFD+R.
2.5 Plasma, Urine, and Feces Analysis of ACN and Phenolic Metabolites by Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry (UPLC-MS/MS)
2.5.1 Pre-Treatment of Plasma Samples
Before the chromatographic analysis, the rat plasma samples were pre-treated by micro-Elution solid-phase extraction (μSPE) using OASIS HLB (2 mg, Waters, Milford, MA, USA) micro-cartridges. The methodology used was reported in the previous study.[21] Briefly, the micro-cartridges were conditioned sequentially with 250 µL of methanol and 250 µL of 0.2% acetic acid. Then, 350 µL of 4% phosphoric acid solution was added to 350 µL of the rat plasma sample, and then this solution was loaded into the micro-cartridges. The loaded micro-cartridges were cleaned-up with 200 µL of Milli-Q water, and 200 µL of 0.2% acetic acid. Afterwards, the retained PC were eluted with 2 × 50 µL of methanol. Each sample was prepared in duplicate.
2.5.2 Pre-Treatment of Urine Samples
The urine samples were also pre-treated by μSPE. The micro-cartridges and their conditioning and equilibration steps were the same as reported for plasma samples. In this case, 100 µL of phosphoric acid at 4% was added to 100 µL of the urine sample, and this solution was loaded into the micro-cartridge. The retained PC were then eluted with 2 × 50 µL of methanol. Each sample was prepared in duplicate.
2.5.3 Pre-Treatment of Feces Samples
The feces were pre-treated as it was reported in the previous study.[22] Briefly, 100 mg of lyophilized feces were mixed in 1 mL of the solution methanol/HCl/Milli-Q water (79.9/0.1/20, v/v/v), and shaken in a vortex (Multi vortex, VWR, Franklin, MA, USA) for 15 min. After that, the sample was centrifuged at 8784 × g for 10 min at 4 °C, and the supernatant was collected, and centrifuged under the same conditions. Finally, the supernatant was filtered with a 0.22 µm syringe Nylon filter and transferred into chromatographic vials until the chromatographic analysis.
2.5.4 Chromatographic Analysis (UPLC-MS/MS)
Liquid chromatography analyses were carried out on an AcQuity Ultra-Performance liquid chromatography and tandem mass spectrometry equipment from Waters (Milford, MA, USA). Two chromatographic methods were used for the analysis of 1) ACN and their metabolites, and 2) the rest of the PC and their metabolites. In both methods, the flow rate was 0.3 mL min−1, and the injection volume 2.5 µL. The UPLC-MS/MS conditions were the same used in the previous studies.[16, 21, 23] Tandem mass spectrometry analyses were carried out on a triple quadrupole detector mass spectrometer (Waters, Milford, MA, USA) equipped with a Z-spray electrospray interface.
Due to the lack of commercial phenolic standards and their generated metabolites, some of these compounds were tentatively quantified by using the calibration curve of their precursor or another PC with a similar structure. Therefore, the concentration of these metabolites is presented as putative values as the quantification with surrogate references may produce over and under estimates. Table S2, Supporting Information, shows the selected reaction monitoring conditions as well as its cone voltage and collision energy used for the quantification of these PC. This table also shows in which phenolic standard compound, these PC have been quantified.
2.6 Histological Analysis
To investigate the possible protective effects against the HFD of red-fleshed apple, white-fleshed apple, or aronia infusion, different histological stains of aorta, kidney, heart, and liver tissues were performed and different parameters were assessed.
2.6.1 Aorta and Kidney Hematoxylin-Eosin Staining
Aorta and kidney samples were fixed in 10% formaldehyde, dehydrated in a graded alcohol series, and cleared in xylene. Later, the samples were embedded in paraffin (Panreac Quimica Slu, Castellar del Vallès, Spain) and the different sections were cut using a microtome (Microm HM 340E, Barcelona, Spain). Each paraffin block was cut to 4 µm thickness. The sections were stained with hematoxylin and eosin for light microscopic examination according to standard procedures. Microscopic tissue images were taken in an Olimpus BX50 microscopic system (Olympus Corporation, Shinjuku, Tokyo, Japan) at 10× magnification.
To evaluate possible deposition of lipids in aorta, which can lead to the formation of atherosclerotic plaques, its thickness was measured in the stained sections.
To assess possible protective effects in kidney, the renal structure was evaluated through the analysis of the Bowman's space, which can be altered after the chronic administration of dietary lipids.
The aortic thickness and the Bowman's space were determined using the program CellSens Entry (Microscopy Imaging Software by Olympus Life Science, Olympus Corporation, Shinjuku, Tokyo, Japan). To evaluate the thickness of the aorta and the Bowman's space, 60 and 30 measurements were taken per animal, respectively.
2.6.2 Liver Oil Red O Staining
A frozen preserved portion of the liver of each rat was subjected to cryostat (Microm HM 505N, Barcelona, Spain) section to a thickness of 8 µm. The stock solutions of Oil Red O were prepared by completely dissolving 0.5 g of Oil Red O (Merck, Darmstadt, Germany) with 100 mL 2-isopropanol 60% (Sigma-Aldrich, St. Louis, MO, USA) using a magnetic stirrer and later it was boiled and filtered using a Whatman No. 2 filter paper (GE Healthcare Life Sciences). The samples were stained following the procedure recommended by the manufacturer. Finally, the nuclei were lightly stained by dipping the slides into hematoxylin solution (Casa Álvarez, Madrid, Spain) for 5 min and rinsing with distilled water for examination using light microscopy (Olympus BX50 microscopic system).
2.6.3 Heart Masson's Trichrome Staining
To assess possible protective effects on heart fibrosis, heart samples were stained with Masson's trichrome stain to observe collagen deposition. Heart samples were embedded in paraffin and cut following the same procedure as kidney and aorta. The sections were stained with Masson's trichrome staining. Briefly, the paraffin samples were deparaffinized by submerging into xylol and rehydrated by submerging in ethanol 100%, 95%, and 70% in this order. The slides were submerged in Bouin's solution, in hematoxilin by Weigert, in trichromic solution, and in green light solution in this order (with distilled water washes between each step). After that, slides were washed with distillated water and put in ethanol 100%. Before observation, slides were dipped into xylol and xylol-eucalyptol and finally mounted with cover slip for examination using light microscopy (Olympus BX50 microscopic system).
2.7 Plasma Biochemical Parameters
Total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDLc), non-high-density lipoprotein cholesterol (non-HDLc), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and glucose were measured in the rat plasma by standardized methods using the Cobas Mira Plus autoanalyzer (Roche Diagnostics, Spain). Insulin was measured by Mercodia Rat Insulin Enzyme-linked Immuno Sorbent Assay (ELISA; reference 10-1250-10) from AD Bioinstruments S.L. (Barcelona, Spain). TC, TG, HDLc, non-HDLc, and glucose were expressed as mg dL−1, insulin was expressed as µg L−1, and ALT and AST were expressed as U L−1. Non-HDLc was calculated by subtracting the HDLc value from the TC value, for each case.
2.8 Statistical Analysis
The results were presented as mean values ± standard deviation (SD) for phenolics in SCD, HFD, HDF+W, HFD+R, and HFD+A. The concentration of the ACN and phenolic metabolites in plasma, urine, and feces samples were presented as mean values ± standard error of the mean (SEM). For the concentration of the phenolic metabolites, the values of males and females, and the sum of both were compared intra-groups and inter-day with one-way repeated measures analysis of variance General Linear Model and one-way ANOVA.
The results of the plasma biochemical parameters were presented as mean values ± SD, and were analyzed using Student's t-test comparing each treatment versus HFD.
The results of the histological analysis were presented as mean values ± SEM. The mean of Bowman's space and the mean of the aorta thickness were compared between different groups with analysis of variance General Linear Model and one-way ANOVA. These values were also compared intra-groups with Student's t-test (between males and females) and between the males of the different groups and between the females of different groups with analysis of variance General Linear Model and one-way ANOVA.
Differences were considered significant at p < 0.05. All data were analyzed with Minitab Statistical Software, version 17.2.1 (Minitab Inc., State College, PA, USA).
3 Results
3.1 Daily Dose of Phenolics Administered
A complete phenolic characterization and quantification of the administered products (red-fleshed apple, white-fleshed apple, and aronia infusion) were performed in order to study their metabolic fate after their supplementation in rats and to evaluate their possible biological activities depending on the product administered. Table 1 shows the daily dose of PC administered to the rats through the supplemented diets.
The daily dose of total PC administered through white-fleshed and red-fleshed apples was very similar, 9.60 and 11.6 mg/day/rat, respectively. Regarding the phenolic composition, both apples had a similar phenolic profile and content of phenolic acids, flavonols, flavanones, and dihydrochalcones, being the daily dose of ACN the main difference between them. Both apples were also different in the flavan-3-ols content, observing that white-fleshed apple contained around eight-fold higher amounts of flavan-3-ols than red-fleshed apple (Table 1).
Aronia fruit administered as an infusion was selected as a rich source of ACN without the components of the apple matrix, containing cyanidin-3-O-galactoside and cyanidin arabinoside, the main ACN present in red-fleshed apple. Although, the daily dose of total PC through aronia infusion (4.10 mg/day/rat) was lower than the red-fleshed apple (11.6 mg/day/rat), the ACN dose was nearly the same (around 2 mg/day/rat). Half of the phenolic dose in aronia was ACN (46.5%), and the other half were phenolic acids, being caffeoylquinic acid and protocatechuic acid the most abundant (Table 1).
3.2 Phenolic Profile in Plasma, Urine, and Feces, and Their Metabolic Pathways
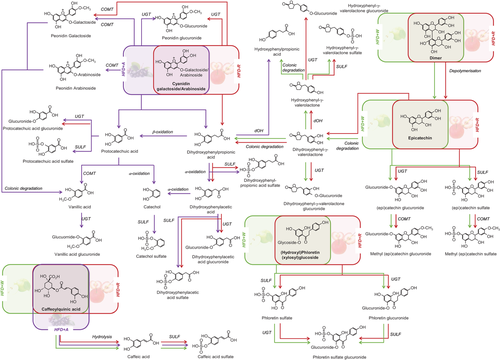
To study the metabolic fate of the PC administered through both apples and the aronia infusion, we analyzed the phenol biological metabolites in plasma, urine, and feces. As previously indicated, the concentration values of the metabolites quantified with their precursors are presented as putative results as their accuracy cannot be verified. Tables S3–S5, Supporting Information, show the putative concentration of the individual phenolic metabolites that presented a concentration significantly higher than the control diets (SCD or HFD) in plasma, urine, and feces, respectively. These data are presented independently for males and females and also as the average values. For a better understanding of the results, data have been summarized and represented by the sum of compounds of the main phenolic groups in Figure 1. The detected metabolites were derived from the phenolic families of ACN, phenolic acids (benzoic, phenylacetic (PAA), and phenylpropionic acids (PPA)), flavan-3-ols, and dihydrochalcones. The phenolic metabolites were mainly phase-II sulfated, glucuronided, and/or methylated conjugates, and also microbial catabolites from colonic degradation. In order to elucidate how each phenolic metabolite was generated from its precursor, in Figure 2, we propose the main metabolic pathways after the intake of the three administered products.
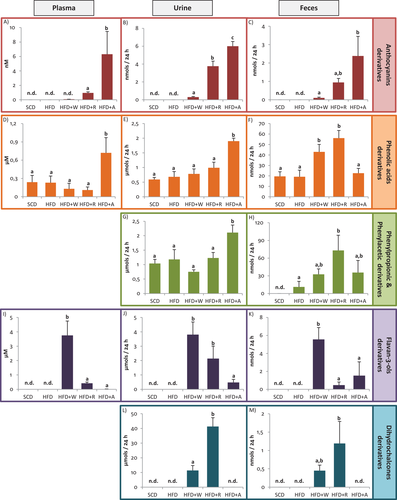
Additionally, in the present study, qualitative and quantitative differences on the metabolic fate of PC depending on the gender have been reported in Tables S3–S5, Supporting Information. The most significant differences between males and females are commented in sections below and also reflected in the metabolic pathways represented in Figures S2–S4, Supporting Information, which refer to urinary metabolites detected after HFD+W, HFD+R, and HFD+A, respectively. In addition, the significant differences between males and females observed in feces are shown in Figure S5, Supporting Information.
3.2.1 ACN Metabolism
As observed in Figure 1A,B, the concentration of ACN in plasma and urine, respectively, was significantly higher after HFD+A compared with HFD+R. In plasma samples (Figure 1A), cyanidin-3-O-galactoside was the main ACN detected after red-fleshed apple and aronia supplementation. Remarkably, peonidin galactoside was only detected in HFD+A group (Table S3, Supporting Information). As expected, ACN and its metabolites were higher after HFD+R and HFD+A than HFD+W, especially in urine samples (Figure 1B). Although the supplemented diets HFD+R and HFD+A provided the same daily amount and type of ACN (2 mg/day/rat), qualitative and quantitative differences were observed in the biological samples studied.
In urine samples, cyanidin-3-O-galactoside was also the main ACN detected in HFD+R; however, peonidin galactoside appeared to be the most abundant in HFD+A. Urinary peonidin arabinoside and peonidin galactoside in HFD+A appeared to be significantly higher than in HFD+R, whereas peonidin glucuronide was significantly higher in HFD+R (Table S4, Supporting Information). It is noteworthy, that in feces only the ACN parent compounds were detected, being cyanidin-3-O-galactoside the main ACN detected in HFD+R, and cyanidin arabinoside in HFD+A (Table S5, Supporting Information).
Based on the metabolites detected in the three intervention groups, we proposed a complete picture of the possible ACN metabolic pathways (Figure 2), highlighting differences between HFD+R (red arrows) and HFD+A (purple arrows) groups. After HFD+R and HFD+A, colonic catabolites from protocatechuic and dihydroxyphenylpropionic acids route were generated. Protocatechuic acid and dihydroxyphenylpropionic acid have described as the simple phenolic acids generated after the fission of B-ring (colonic degradation) of cyanidin glycosides.[24] Although similar metabolism was observed between HFD+R and HFD+A, a higher amount of metabolites were detected in urine and plasma after HFD+A, such as protocatechuic acid sulfate, vanillic acid glucuronide, and catechol sulfate (Figure 2, Tables S3 and S4, Supporting Information). It must be noted that the metabolites derived from dihydroxyphenylpropionic acid could also be generated by colonic degradation via valerolactones of flavan-3-ols (Figure 2).
3.2.2 Phenolic Acid Metabolism
The phenolic acid metabolites detected in plasma were protocatechuic acid sulfate and vanillic acid glucuronide, and together with caffeic acid sulfate, these were also the main excreted metabolites in urine after the three diet supplementations (Tables S3 and S4, Supporting Information). As seen in Figure 1D,E, the total phenolic acids concentration only appeared to be significantly higher after HFD+A intake compared to other groups, in plasma and urine samples. Our results are in accordance with a previous study in rats that reported phase II metabolites from protocatechuic acid and vanillic acid in plasma and urine after the intake of an ACN-rich extract.[25]
In feces, the metabolites detected were protocatechuic acid and hippuric acid. Although in plasma and urine, phenolic acids were only detected in higher amounts after aronia supplementation, in feces, these metabolites appeared in higher concentration after both apple supplementations compared to aronia (Figure 1D–F). It is remarkable that hippuric acid was only detected in feces (Table S5, Supporting Information). Regarding the possible metabolic routes of hippuric acid, we hypothesize three different pathways: 1) by protocatechuic acid derived from the colonic metabolism of cyanidin glycoside; 2) by hydroxyphenylpropionic acid, which has been reported to be a colonic metabolite of dihydrochalcones[26-28]; and 3) by dihydroxyphenylpropionic acid, which is a colonic metabolite of flavan-3-ols (Figure S5, Supporting Information).
As observed in Figure 1, phenolic acid metabolites were also detected in significant amounts in SCD and HFD (control diets). Although protocatechuic and vanillic acid metabolites have been reported as colonic metabolites from cyanidin and peonidin glycosides (B-ring fission), respectively,[24] vanillic acid glucuronide could also be generated by β-oxidation from ferulic acid, which was detected in significant amounts in SCD and HFD (control diets) (Table S6 and Figure S6, Supporting Information).
3.2.3 PAA and PPA Metabolism
After the three supplemented diets (HFD+W, HFD+R, and HFD+A), significant differences in PAA and PPA were only observed in urine and feces. In urine samples, the total concentration of PPA and PAA was only significantly higher after HFD+A (Figure 1G), being dihydroxyphenylacetic acid sulfate the most abundant metabolite (Table S4, Supporting Information). PPA and PAA metabolites could be generated mainly from ACN by B-ring fission (colonic degradation) in HFD+A, but also from flavan-3-ols in diets supplemented with both apples (Figure 2).
Similarly to phenolic acid derivatives, PAA and PPA presented a different response in feces compared to urine, showing significantly higher concentration in urine after HFD+A and in feces after HFD+R (Figure 1G,H). Specifically, m-hydroxyphenylacetic was the predominant metabolite in feces increasing its concentration significantly after HFD+R being only detected in females (Table S5 and Figure S5b, Supporting Information).
3.2.4 Flavan-3-ol Metabolism
Flavan-3-ols were more abundant in the white-fleshed apple diet (Table 1), which was clearly reflected in biological samples, especially in plasma, observing significant higher levels of all the derived metabolites after HFD+W (Figure 1I). The sum of flavan-3-ol metabolites includes both phase II and colonic metabolites (valerolactones). The monomers (catechin and epicatechin) could also be generated from dimer by depolymerization (Figure 2).
In plasma, all the detected metabolites were significantly higher after HFD+W than in other groups, being catechin glucuronide the most abundant (Table S3, Supporting Information).
Similarly, in urine samples, the concentrations of all the individual metabolites were significantly higher in the HFD+W than other groups (Table S4, Supporting Information). In this case, hydroxyphenyl-γ-valerolactone sulfate was the most abundant metabolite. It is noteworthy that significant gender differences were reported in urine in some flavan-3-ols metabolites in the HFD+W group (Table S4, Supporting Information).
In feces samples, (epi)catechin, dimer, trimer, and methyl catechin sulfate were detected, being all metabolites also significantly higher after HFD+W compared to other groups (Table S5, Supporting Information).
3.2.5 Dihydrochalcone Metabolism
Dihydrochalcones, and particularly phloretin, are a specific phenolic group from apples, and this was reflected in our results, observing that their derived metabolites were only detected after both apples supplementation. It was also noted that dihydrochalcones were the most abundant metabolites excreted in urine after the supplementation with both apples (HFD+R and HFD+W), representing around the 90% of total metabolites excreted in the HFD+R group. The concentration of dihydrochalcones in red-fleshed apple diet was around four-fold higher than in white-fleshed apple (Table 1), and this fact was reflected in urine and in feces observing that the total urine dihydrochalcone concentration in HFD+R was significantly higher than in HFD+W (Figure 1L,M). In urine samples, phloretin sulfate was the most abundant metabolite (Table S4, Supporting Information).
In feces, only the parent compounds, (hydroxyl) phloretin and (xylosyl) glucoside, were detected after supplementation of both apples. The total concentration in HFD+R was similar to HFD+W and the concentrations of the three compounds followed the same trend (Table S5, Supporting Information).
3.3 Cardiometabolic Effects of the Diet Supplementations: Aorta, Kidney, Liver, and Heart Histological Analysis
3.3.1 Aorta Samples: Differences in Thickness
To evaluate possible deposition of lipids in the aorta, which can lead to the formation of atherosclerotic plaques, its thickness was measured in sections stained with hematoxylin-eosin. Results showed significant differences between groups. The aorta thickness of the HFD group (131 ± 6.92 µm) was significantly higher than the other groups (p < 0.05) (Figure 3A). The aorta thickness in HFD+W (109 ± 5.76 µm) was similar to those in SCD group (107 ± 5.64 µm) and similar to those in HFD+R (113 ± 5.94 µm). In the HFD+A group (118 ± 6.21 µm) a significant improvement was observed compared to the HFD group, although the effect was not as effective as HFD+R or HFD+W.
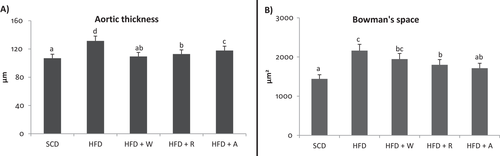
3.3.2 Kidney Samples: Bowman's Space
It has been proven that the chronic administration of dietary lipids alters the renal structure and one of the parameters that is altered is the Bowman's space that increases and leads to glomerular atrophy and functional loss of glomeruli and tubules.[29, 30] To assess possible protective effects against HFD, differences in Bowman's space were evaluated.
Results showed significant differences in the Bowman's space area among groups (Figure 3B). The Bowman's space of the HFD group (2162 ± 161 µm2) was significantly higher than the other groups (p < 0.05) except with the HFD+W group (1946 ± 145 µm2) in which the values are statistically similar. With respect to the HFD+R group (1801 ± 134 µm2), significantly lower values were observed compared to the HFD group. Finally, in the HFD+A group (1713 ± 128 µm2), values were significantly similar to those of SCD (1441 ± 107 µm2).
3.3.3 Fatty Liver Development by Oil Red O Staining
To evaluate possible attenuation effects of diet supplementations on fatty liver development, samples were stained with Oil Red O staining to assess the lipid accumulation. As it can be observed in Figure 4, HFD led to an increase in cellular lipid accumulation compared to SCD. However, the diet supplementation with either apples or aronia did not reduce the lipid accumulation, inflammation, or modified hepatocytes, in comparison to the HFD group.
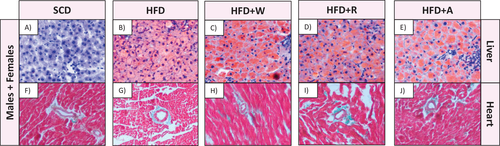
3.3.4 Heart Masson's Trichrome Staining
To assess possible protective effects of diet supplementations on the development of heart fibrosis that occurs in a chronic inflammatory process, heart samples were stained with Masson's trichrome stain to observe collagen deposition. As seen in Figure 4, this staining revealed that widths of cardiac myocytes and the collagen deposition (blue color) in the perivascular area that leads to cardiac fibrosis increased similarly after HFD, HFD+R, HFD+W, and HFD+A compared to the SCD group. No differences were observed between the groups supplemented with apples or aronia infusion.
3.4 Plasma Biochemical Parameters
Data of various biochemical parameters including insulin, glucose, lipid profile, and liver enzymes are presented as mean ± SD values in Table 2. In general, high intra-individual variability was observed in all parameters. The effects of the HFD were reflected in a significant increase of TC, non-HDLc, ALT, AST, and insulin values (p < 0.05) compared to SCD. Results showed that the supplemented diets with apples or aronia did not attenuate these effects, observing no significant reductions compared to HFD in all parameters (n = 6). Nevertheless, we observed a reduction of insulin levels when comparing HFD with all the supplemented diet groups in males (n = 3), but only in HFD+R and HFD+W groups, this insulin reduction was significant (p < 0.05) (Table S7, Supporting Information).
| TC [mg dL−1] | TG [mg dL−1] | HDLc [mg dL−1] | Non-HDLc [mg dL−1] | ALT [U L−1] | AST [U L−1] | Insulin [µg L−1] | Glucose [mg L−1] | |
|---|---|---|---|---|---|---|---|---|
| HFD | 325 ± 150 | 141 ± 44.0 | 41.8 ± 12.5 | 283 ± 144 | 149 ± 70.0 | 381 ± 127 | 3.26 ± 2.25 | 218 ± 18.0 |
| SCD | 71.4 ± 10.0 (0.009) | 96.4 ± 76.0 (ns) | 53.5 ± 5.07 (ns) | 17.8 ± 6.05 (0.006*) | 43.8 ± 12.1 (0.013*) | 175 ± 56.3 (0.005*) | 0.61 ± 0.23 (0.035*) | 243 ± 36.2 (ns) |
| HFD+W | 352 ± 184 (ns) | 103 ± 23.4 (ns) | 44.4 ± 23.5 (ns) | 307 ± 187 (ns) | 174 ± 105 (ns) | 452 ± 229 (ns) | 2.52 ± 1.44 (ns) | 228 ± 12.0 (ns) |
| HFD+R | 390 ± 261 (ns) | 152 ± 116 (ns) | 42.7 ± 16.2 (ns) | 347 ± 250 (ns) | 156 ± 71.5 (ns) | 349 ± 78.8 (ns) | 2.28 ± 1.21 (ns) | 257 ± 46.7 (ns) |
| HFD+A | 386 ± 196 (ns) | 174 ± 80.0 (ns) | 41.8 ± 8.78 (ns) | 344 ± 198 (ns) | 167 ± 95.8 (ns) | 404 ± 144 (ns) | 1.73 ± 1.60 (ns) | 235 ± 47.8 (ns) |
- Concentration is expressed as mean ± standard deviation (n = 6). Data were analyzed using Student's t-test comparing each treatment versus HFD. *p ˂ 0.05 each treatment versus HFD. A, Aronia melanocarpa; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDLc, high-density lipoprotein cholesterol; HFD, high-fat diet; non-HDLc, non-high-density lipoprotein cholesterol; ns, non-significant; R, red-fleshed apple; SCD, standard chow diet; TC, total cholesterol; TG, triglycerides; W, white-fleshed apple.
4 Discussion
In the present study, we investigate the response to a sustained intake of red-fleshed apple biofortified in ACN on the phenolic metabolism and their cardiovascular effects associated. For this purpose, we used a Wistar rat model fed with an HFD to induce hypercholesterolemia supplemented with red-fleshed apple and compared with HFD supplemented with a common white-fleshed apple without ACN. To evaluate the impact of the components of the apple matrix, a group supplemented with ACN-rich infusion was included. The metabolites detected after the ingestion of red-fleshed apple, white-fleshed apple, and A. melanocarpa were also similar to those found in several studies carried out in humans,[21, 31, 32] what enables a possible translation of the observed effects in humans.
Regarding the results on absorption and metabolic fate of ACN, although the amount and profile of ACN in red-fleshed apple and aronia infusion were the same, the metabolic profile detected in biological samples differed quantitatively and qualitatively. The differences observed could be related to the effect of the apple matrix. In apple fruit, the ACN are bound to fiber and saccharides, whereas in the aronia infusion, the ACN are in their free forms more available, which favor their gastrointestinal absorption and metabolism. The metabolites observed in the present study were in agreement with previous studies describing the metabolic fate of ACN after the intake of red-fleshed apple in a postprandial study in humans,[21] and aronia in rats.[33, 34] The apple matrix effect was also observed in phenolic acids, PPA and PAA metabolites, which appeared in higher concentration in urine after HFD+A, and in feces after HFD+R. This fact indicates that ACN and PC linked to apple fiber are absorbed more slowly compared to aronia infusion, and therefore, they reach the large intestine where they are catabolized by the colonic microbiota appearing in bigger amounts in feces. Regarding the other phenolic metabolites, our results from flavan-3-ols are in agreement with previous studies, reporting similar phase II metabolites and colonic metabolites (valerolactones) after the intake of flavan-3-ol rich extracts, such as apple[21] or wine.[35] The detected dihydrochalcone metabolites were also reported by other studies in biological samples after the intake of cider,[36] and red-fleshed apple snack in humans.[21, 23] To our knowledge, the colonic metabolism of dihydrochalcones has been scarcely studied, and the obtained results were in agreement with previous studies.[26-28]
When studying the metabolic fate of PC after apple and aronia supplementation, a gender effect was also reported. In general terms, the differences between males and females detected in urine samples (Table S4, Supporting Information) could be summarized as: i) the methylated, glucuronided, methylglucuronided, and methylsulfate conjugates were found in higher concentrations or predominantly in females and ii) the sulfate conjugates in males. These results were in agreement with those reported in previous studies in rat model[37, 38] and in humans[39] showing that the sulfatation and glucuronidation were more intense in males than in females.[39] Contrary to our results, several studies have reported that some isoforms of catechol-O-methyltransferases enzymes may be more active in males than in females.[38, 40, 41] In quantitative terms, the most significant differences between genders were observed in phenolic acid metabolites, observing that females absorbed and excreted significant higher amounts of these metabolites after all the supplemented diets. These differences could be explained by the sex-dependent expression of many isoforms of gastrointestinal enzymes or/and transporters that participate in the absorption of phenolics.[42] For example, in rats, β-glucosidase activity is greater in females than in males.[43] However, the differences between genders observed in the present study should be confirmed in future studies with a greater number of animals that would allow more conclusive results.
Concerning the cardioprotective parameters studied, it is important to highlight that we did not observe the presence of atherosclerotic plaques nor the presence of large lipid deposits in the aortas of rats after supplementing with HFD. This could probably be related to the hypo-responsive character of rats to HFD and also the short duration of the study, which appeared to be insufficient for the formation of atherosclerotic plaques. For this reason, we measured the aortic wall thickness, which is considered a valuable biomarker of early atherosclerosis and a predictive value of future vascular events, the presence of plaques or aorta stenosis.[44, 45] In this sense, several pathological studies have demonstrated that the earliest phases of atherosclerosis result in the vascular thickening, and therefore, the maintenance of a healthy cardiovascular risk profile is strongly associated with lower aortic thickness. A significant reduction was reported in aortic thickness as a consequence of apple (red- and white-fleshed) and aronia intake compared with control HFD, indicating a protective atherogenic effect of the three administered products.
Particularly, the effect observed in HFD+W group could be mainly due to flavan-3-ols metabolites, being catechin glucuronide and hydroxyphenyl-γ-valerolactone sulfate, the most abundant metabolites detected in plasma and urine samples, respectively (Tables S3 and S4, Supporting Information). The anti-atherosclerotic potential of flavan-3-ols from apples has already been demonstrated in mice, after the administration of cider apple extracts in apo-E deficient mice.[46] Similarly, the diet supplementation with an apple phenolic extract rich in flavan-3-ols for 12 weeks in hypercholesterolemic male mice[47] produced a reduction of the mean atherosclerotic lesion area in the aortic sinus. Similar effects were also observed in male hypercholesterolemic rabbits after the sustained intake of apple juices producing a significant reduction of the atherosclerotic lesions of coronary arteries.[1] Also, in agreement with our results, after the sustained administration in Sprague Dawley rats of a persimmon fruit extract rich in catechin derivatives, a significant decrease in aortic thickness was observed.[48]
Regarding the HFD+R group, ACN and dihydrochalcones were the most abundant PC and also their metabolites in plasma and urine, so we hypothesize that the reduction of the aortic thickness could be mainly related to ACN and dihydrochalcones, with a minor contribution of flavan-3-ols. Similar effects have been reported in rabbits and mice studies supplementing with apples in which dihydrochalcones were studied.[1, 46, 47] The potential anti-atherosclerotic role of ACN was also consistent with other studies in which lower fatty deposition in the arteries of hypercholesterolemic male rabbits was observed after the intake of sun-dried berries for 8 weeks,[11] or after the administration of red-fruit juices like raspberry in hypercholesterolemic hamsters.[12]
In the case of aronia supplementation, the attenuation effects observed in the aorta can be attributed to ACN, as these were the main PC administered through the aronia infusion and was also reflected in a higher amount of their derived metabolites in plasma and urine. These results are in agreement with a previous study[13] in which it was observed a decrease in epididymal fat accumulation in hypercholesterolemic Wistar rats after the administration of aronia fruit (at two different concentration, 3 or 6 mg daily) for 6 weeks.
Although the sample size is very small (n = 3), significative differences can be observed (p < 0.05) in the aortic thickness between males and females (Figure S7, Supporting Information). In relation to this, contradictory results are described in the literature regarding the anti-atherogenic effects of ACN. Some studies with males of different species such as rabbits,[11] hamsters[12] or Wistar rats[13] have reported the anti-atherogenic effects of ACN, whereas other studies[49, 50] did not report histological differences in aorta after the sustained administration of A. melanocarpa (106.8 mg ACN per 100 mL water) in Wistar rat males. Regarding the HFD+W group, a reduction of aortic thickness was detected in both males and females, which corroborates the protective effect of flavan-3-ol in the development of atherosclerotic lesions. The attenuation effects in the reduction of aorta thickness were not related with the plasma lipid profile, since no significant differences were observed in TC, TG, HDL, and non-HDL after the three supplemented diets compared with HFD group. This fact could indicate that the absolute cholesterolemia is not an indicative parameter of the aortic fatty streak deposition.[51, 52]
Regarding the studied effects on kidney, an attenuation effect in the glomerular atrophy and functional loss of glomeruli and tubules was observed after the three supplemented diets compared to HFD although this attenuation was only significant after the intake of the supplemented diets with red-fleshed apple and aronia (Figure 3B). Curiously, when data was analyzed separately by genders, this attenuation in the renal structure appeared to be significant only in female samples (Figure S7c,d, Supporting Information) and in this case, the reductions were significant after the administration of all the supplemented diets with respect to HFD. This fact could probably be related to the gender-related differences in the phenol bioavailability, as we observed higher amounts of urinary phenolic metabolites in females compared to males. Although the differences were not significant, the total excretion of phenolic metabolites in urine was greater in females after the three supplementation diets (Table S4, Supporting Information), suggesting that higher accumulation of phenolic metabolites in the kidney could lead to a major protection from kidney glomerular atrophy.
As it was discussed for the aorta, the attenuation effects reported in kidney after the supplemented HFD diets could be mainly related to ACN, flavan-3-ols, and dihydrochalcones. The effect observed after HFD+W group could be mainly attributed to the flavan-3-ol metabolites, being hydroxyplenyl-γ-valerolactone sulfate (urine) and catechin glucuronide (plasma and urine) the main metabolites detected. In addition, the protective effect observed in kidney structure after both apples supplementation, especially after HDF+R, could be also attributed to the dihydrochalcone metabolites detected in high concentration in urine, being phloretin sulfate the main metabolite detected. These results are consistent with the studies reported in the literature. After the intake of a dried apple rich in flavan-3-ols and dihydrochalcones, kidney function improved, and the concentration of uric acid, urea, and creatinine decreased in eight Sprague Dawley hyperlipidemic rats.[2] In addition, after apple supplementation (approximately 20% of the daily intake) of obese Zucker rats, the glomerulopathy with the consequent proteinuria was reduced.[53]
In addition, ACN could also be responsible for the effects observed in the HFD+A group, and to a lesser extent in the HFD+R group as commented for aorta results, which could be in agreement with previous studies. After the administration of black soybean in hypercholesterolemic Sprague Dawley rats, ACN reversed the effects of HFD on body weight, serum lipids and decreased the weights of epididymal and perirenal fat pats.[54] In another study in Wistar rats, after the supplementation with A. melanocarpa extract (with phenol doses approximately eight-fold higher than those administered in our study), the antioxidant status was improved, especially, the concentration of a lipid peroxidation indicator, the thiobarbituric acid reactive substances in the kidneys.[55]
It is noteworthy that, apart from the effects observed in aorta and kidney, a significant decrease in plasma insulin values was also observed in males supplemented with both apples (3.49 ± 0.63 and 3.22 ± 0.34 µg L−1 in HFD+W and HFD+R, respectively) compared with HFD group (5.27 ± 0.34 µg L−1) (S7, Supporting Information). These results are in agreement with the results reported in the literature.[13, 56] After the administration of cranberry extract rich in ACN and flavan-3-ols[56] or aronia extract[13] in obese mice, the insulin values decreased with respect to the control HFD. One of the effects of HFD diets is their induction of adipose tissue dysfunction. This can alter diverse factors, inducing to systemic insulin resistance, which is a major contributor to the development of type 2 diabetes.[57] The anti-insulin resistance effect of PC is partly due to their ability to reverse this dysregulation. It has also been reported that after chokeberry extract consumption rich in ACN, the Ppargamma mRNA expression increased, the Fabp4, Fas, and Lpl mRNA levels were suppressed, a decrease of mRNA expression of TNF-α and Ilb and Il6 was induced, and in consequence, the plasma levels of TNF-α and IL-6 were decreased.[13] In other studies in diet-induced insulin-resistant animals, after the administration of polyphenols from cinnamon,[58] and green tea,[59] Fas and Lpl mRNA expression and other genes related with lipogenesis were inhibited leading to improve systemic insulin sensitivity and dyslipidemia by enhancing insulin signaling.
The obtained results in liver and heart stainings are in agreement with plasma ALT and AST values, since no significant differences were observed concerning HFD after diet supplementations (Table S7, Supporting Information). These results may be due to the fact that the HFD diet was so powerful that the attenuation effect of PC administered in relatively low doses could not be appreciated at the macromolecular level. These results contrast with another study[53] performed in another rat model (obese Zuckers rats) in which after the administration of a similar dose of lyophilized apple, a cardioprotective (decreasing TG in the heart) and hepatoprotective (limiting liver steatosis) effects were observed.
5 Conclusions
Our study showed the in vivo cardiometabolic protective effects of both red-fleshed and white-fleshed apples and aronia infusion supplementation in hypercholesterolemic rats, specifically in the reduction of the aorta thickness, the improvement of the renal function, and the reduction of insulin levels. In the case of apples, ACN or flavan-3-ols, together with dihydrochalcones, compose a phenolic phytocomplex that could act synergistically in the attenuation of cardiovascular outcomes. In addition, our findings support that ACN without apple matrix through aronia infusion can induce cardioprotective effects. Moreover, an apple matrix effect was reported between red-fleshed apple and aronia infusion observing a higher bioavailability and excretion of ACN after aronia supplementation.
A gender effect was also reported probably related with the differences observed in phenol metabolism, specially noted in the improvement of kidney function in females that could be related with the higher phenol bioavailability. So our results suggest that differences in the metabolic fate of PC underlie the possibility that PC can differently influence the health outcomes. However, the results regarding the impact of gender can be considered preliminary and should be confirmed in future studies with a larger number of animals.
Acknowledgements
This study was supported by the Spanish Ministry of Industry, Economy and Competitiveness through the AGL2016-76943-C2-1-R and AGL2016-76943-C2-2-R projects (co-funded by the European Social Fund, European Union). I.A.L. enjoyed a post-doctoral contract (2017PMF-POST2-19) from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement and from the Universitat Rovira i Virgili (URV). S.Y. was supported by a grant from the University of Lleida. Ú.C. has a Pla estratègic de recerca i innovació en salut (PERIS) post-doctoral grant (SLT002/16/00239; Catalunya, Spain) from Generalitat de Catalunya. A.P. enjoys a post-doctoral grant (PTQ-15-08068; Spain). L.R. is a Serra Húnter Fellow. In addition, the authors were grateful to NUFRI SAT (Mollerussa, Lleida, Catalonia, Spain) for providing the red-fleshed apples; to Ana Martínez (Department of Medicine, University of Lleida) for helpful with the histological stains; and finally, to SCT-Estabulari of the University of Lleida where the animal experiment was carried out. A few spelling and layout mistakes were corrected on May 14, 2021.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




