Human Milk Oligosaccharides Protect against Necrotizing Enterocolitis by Inhibiting Intestinal Damage via Increasing the Proliferation of Crypt Cells
Abstract
Scope
Necrotizing enterocolitis (NEC) is a devastating disease that is highly lethal in premature infants. Human milk oligosaccharides (HMOs) efficiently reduce the incidence of NEC. However, the protective mechanism of HMO treatment is unknown. It is hypothesized that HMOs protect against NEC by inhibiting the damage to intestinal epithelial cells.
Methods and results
C57BL/6 pups are challenged with hypoxia and cold stress to induce NEC. All pups are sacrificed after 72 h. It is found that HMO administration reduces the concentrations of IL-8 in the serum and ileum of all NEC mice. Ileum toll-like receptor 4 (TLR4) protein expression and nuclear factor kappa-B (NFκB) pathway activation are inhibited. The proliferative ability of enterocytes in the ileum is restored as determined by labeling with proliferation markers (Ki67, SOX9). In a 3D culture intestinal crypt organoids study, HMO treatment improves the maturation of organoid cells and increases the ratio of proliferative cells under lipopolysaccharides (LPS) treatment. HMO treatment downregulates TLR4 expression in the organoid cells, thus reducing the effect of LPS.
Conclusion
HMOs protect intestinal epithelial cells from injury by accelerating the turnover of crypt cells by reducing the expression of TLR4 on intestinal epithelial cells.
1 Introduction
Necrotizing enterocolitis (NEC) is a devastating disease of newborn children, especially premature neonates. The incidence of NEC is ≈7% in very low birth weight infants with a mortality rate up to 30%.1, 2 Several risk factors have been reported: 1) hypoxic-ischaemic injury of intestinal epithelial cells; 2) immature gut of premature infants; 3) abnormal colonization of intestinal microbiota; and 4) formula feeding.3-6 Together, these risk factors cause dramatic intestinal dysfunction. The gut is an important immune organ, and the immune functions of the gut can be categorized into three groups: sensor, effector, and regulator.7 Toll-like receptor 4 (TLR4) is a sensor of LPS that induces the downstream effector and regulator. The overactivation of the TLR4-NFκB pathway results in excessive inflammation, which impairs the function of the epithelial barrier, resulting in increased intestinal permeability.8, 9 NEC can be alleviated by surgery, the sequelae of treatment always result in the malnutrition or growth delay of babies.10 Therefore, it is important to prevent NEC. Currently, breastfeeding is the most efficient way to prevent NEC.11 Human milk oligosaccharides (HMOs) are newly identified human milk compounds that lower the incidence and lethality of NEC, but which are not currently present in infant formula.12
HMOs are the third most abundant solid component of human milk; HMOs are present at the concentrations of 20–25 g L−1 in the colostrum and 5–10 g L−1 in term milk.13 Approximately 200 types of HMOs have been identified.14, 15 Most of these oligosaccharides are unique to human milk. The composition of HMOs is more diverse than the milk oligosaccharide compositions of other mammals.16 For comparison, the concentration of cow milk oligosaccharides is only 100 mg L−1, and ≈40–50 types of oligosaccharides are present in the colostrum milk of cows.17 Therefore, it is difficult to replace HMOs with the milk of domestic animals. Fructo-oligosaccharide (FOS) and galacto-oligosaccharide (GOS) are currently used to replace HMOs in infant formula; however, FOS and GOS oligosaccharides showed no protective effect against NEC.18, 19
Although a study by Jantscher et al. indicated that HMOs efficiently lowered the incidence of NEC in a rat model, the mechanism of how HMOs prevent NEC is still unknown.20 He et al. indicated that HMOs decreased the IL-8 secretion of enterocytes stimulated by LPS in vitro, which implies that HMOs may inhibit the excessive inflammation induced by endotoxin.21 However, the mechanism involved is unknown. In addition, it is not known whether HMOs protect against the damage to intestinal epithelial cells caused by excessive inflammation. Studies by Kunt et al. and Hester et al. showed that HMOs changed the cell cycle of enterocytes and inhibited the proliferation of enterocytes in vitro.22, 23 However, it is difficult to explain the physiological meaning of these results. We doubt that HMOs regulate the proliferation of enterocytes to avoid the damage induced by excessive inflammation.
Recently, 3D crypt organoid cultures have become a useful method to study the physiological functions of the intestine in vitro.24 Compared with the traditional 2D culture of cells, 3D crypt organoid cultures simulate the microenvironment of the intestine in vivo.25 In contrast with 2D intestinal epithelial cells, 3D crypt organoid cultures are established by separating crypts from fresh intestinal epithelial cells.26 Thus, these cultures reflect the character and responses of crypt stem cells; cells differentiate when the microenvironment is challenged, similar to in vivo conditions.27 This new approach has been applied to drug metabolism and pathological studies, including NEC research.28 LPS changes the proliferation of 3D crypt organoid cultures by upregulating TLR4 expression, while human milk has a protective effect.29, 30 The influence of HMOs on 3D crypt organoid cultures treated with LPS is unknown.
In the present study, we hypothesized that HMOs attenuate the intestinal damage and inflammation in mice challenged with NEC by regulating the proliferation of intestinal epithelial cells. We induced NEC in vivo by hypoxia and cold stress, together with LPS gavage. LPS was used to simulate the damage of NEC to intestinal epithelial cells in 3D crypt organoids. The aim of this study was to investigate the preventative mechanism of HMOs against the excessive intestinal inflammation caused by NEC.
2 Experimental Section
2.1 Materials
Human milk samples were collected from ten lactating healthy mothers less than 4 weeks after delivery. C57BL/6 mouse pups were purchased from Charles River (Beijing, China). All human and animal experiments were approved by the Ethics Committee of the Military General Hospital of Beijing PLA (No.100).
2.2 HMO Purification and Profiling by HPLC-MS
HMOs were isolated from human milk according to a published method with some modifications.31 Briefly, the lipid layer was skimmed after 14 000 × g centrifugation at 4 °C for 30 min. The collected milk serum was ultrafiltrated through a 10-kD membrane (Sartorius, Gottingen, Germany) to remove most of the proteins and remaining lipid. After freeze-drying, the fraction was separated with an Epoxy aspartic column (10 × 250 mm, 5-µm particle size, Dalian, China) using HPLC, and lactose was removed by identifying the retention time of lactose by LC-QTOF-MS (Agilent 6545, California, USA) with an Epoxy aspartic acid column (10 × 150 mm, 5-µm particle size, Dalian, China). The parameters of HPLC separation used ultrapure water as solvent A and acetonitrile solvent B. The gradient was performed as follows: 0–40 min, 80–50% B; 40–45 min, 20% B; 45–55 min, 80% B. Both the MS and MS/MS spectra were acquired in the negative-ion mode with an acquisition rate of 1 s per spectrum over a mass range of m/z 300–2000 (for MS) and m/z 50–2000 (for MS/MS). The drying gas temperature was 350 °C with a flow rate of 8.0 L min−1. The collision energy of 30 V was used for collision-induced dissociation. Endotoxin was reduced to less than 0.01 EU mg−1 by Endotoxin Removal Spin Columns (0.5 mL, Thermo Fisher, USA), which eliminated the effects of LPS.32 The concentration of LPS was determined by using an endotoxin detection kit (Xiamen Bioendo Technology, Xiamen, China).
2.3 NEC Animal Experiments
Ninety 7-day-old neonatal C57BL/6 mice were randomly assigned to six groups: breastmilk-fed mice were used as healthy controls (BF); formula-fed mice with hypoxia stimulation were used as one disease control (FF); and formula-fed mice with both hypoxia and 4 mg kg−1 d−1 LPS (O111:B4, Sigma, St. Louis, USA) according to the method of Besner33 were used as another disease control (FF+LPS). The NEC model protocol was performed as described previously.33 Briefly, after gavage, the mice were immediately exposed to 10 min of hypoxia (95% N2+5% O2), then exposed to cold stress at 4 °C for 10 min, three times daily. All animals were kept for 3 days and sacrificed 30 min after the last gavage and NEC model exposure. Then, 1.0-cm sections of the ileum were fixed for haematoxylin-eosin (H&E) staining. NEC was diagnosed by the results of NEC scores. For scoring, each sample was blindly judged by two investigators using a score of 0 to 4 according to a published method.34 Briefly, grade 0 indicated no damage; grade 1 indicated epithelial cell lifting or separation; grade 2 indicated necrosis to the mid villous level; grade 3 indicated necrosis of the entire villus; and grade 4 indicated transmural necrosis. A grade of 2 or higher was considered representative of NEC.
2.4 Dosage Regimen
A dose of 20 g L−1 HMOs was administered according to their concentration in colostrum and their effectiveness in protecting the two treatment groups against NEC (FF+HMOs, FF+LPS+HMOs).13 In addition, 20 g L−1 infant formula oligosaccharides (IFOS; 9 GOS:1 FOS, Shanghai Yuanye Biological, Shanghai, China) was used as a prebiotic control (FF+IFOS). Rodent formula simulated the protein and caloric content of rodent breast milk as follows: 35 g Abbott milk powder (Similac, Abbott, Chicago, USA) and 4 g albumen powder (Tangchenbeijian, Guangzhou, China) were dissolved in 100 mL water; the total calories in formula milk were 8.2 MJ/L.T. The pups in all groups except the BF group were housed in a water bath at 37 °C. Formula (0.1 mL) was administered by gavage every 8 h beginning on day 1, and the volume administered was increased by 0.05 mL every other day until the end of treatment.
2.5 IL-8 and IL-6 ELISA
The systemic inflammation in NEC was analyzed by measuring the concentration of IL-8 (CXCL15) in serum using an ELISA kit (CXCL15 Mouse ELISA Kit, Invitrogen, Massachusetts, USA). The inflammatory response of the intestine was measured as the concentrations of IL-8 and IL-6 in the ileum by ELISA kits (IL-6 Mouse ELISA Kit, Invitrogen, Massachusetts, USA). The serum was collected after centrifugation at 3000 × g for 15 min at room temperature. Approximately 100 mg ileum tissue was lyzed in RIPA buffer (Beyotime, Shanghai, China), and the supernatant was collected. All samples were stored at −80 °C before analyses. The measurements were performed according to the manufacturers’ instructions. Values were normalized based on volume (for serum) or the concentration of protein (for the ileum).
2.6 Intestinal Crypt Organoid Culture
The protocol used for the separation of intestinal crypt organoids was based on a previous method.26 Briefly, the small intestinal tissues of the ileum from three 6-week-old C57BL/6 male mice were dissociated and cut into <2 mm pieces. The crypts were released by incubation in 2 mM EDTA for 30 min on ice. Then, a 70-µm strainer was used to separate the crypts from individual epithelial cells. Each well of a 24-well plate was plated with 1000 crypts mixed with 50 µL Matrigel (BD, New York, USA). After polymerization with Matrigel, 500 µL IntestiCult Organoid Growth Medium (Mouse, Stem Cell, Vancouver, Canada) was added to each well. The culture medium was changed every 2 days, and after 7 days the organoids were harvested with cold DMEM/F12 (Gibco, Thermo Fisher, USA), mechanically dissociated into a single crypt and re-plated in fresh Matrigel. After seeding for 5 days, 50 µg mL−1 LPS (Sigma, USA) was added to the medium to induce inflammation.
2.7 RNA Isolation and qPCR
Total RNA from animal tissue was extracted using a Total RNA extraction kit according to the manufacturer's protocol. RNA was reverse transcribed to cDNA using a 5 × All-in-One RT Premix Kit. qPCR was performed with the SYBR Green qPCR Master Mix. All reactions were performed in triplicate, and the detected mRNA expression was normalized to gapdh. Primer sequences are shown in Table 1. All primers were designed according to a previous report.29, 35, 36
| Gene | Primer sequence |
|---|---|
| Gapdh | 5′-3′ TGAAGCAGGCATCTGAGGG |
| 3′-5′ CGAAGGTGGAAGAGTGGGAG | |
| Il-6 | 5′-3′CCAATTTCCAATGCTCTCCT |
| 3′-5′ ACCACAGTGAGGAATGTCCA | |
| Tlr4 | 5′-3′TCAGAACTTCAGTGGCTGGA |
| 3′-5′GGTAAGAAAGGCTCCCCAGT | |
| Il-1β | 5′-3′AATGCCACCTTTTGACAGTGAT |
| 3′-5′TGCTGCGAGATTTGAAGCTG | |
| Il-8 | 5′-3′TTTCCACCGGCAATGAAG |
| 3′-5′TAGAGGTCTCCCGAATTGGA |
2.8 Western Blot Analysis
Total protein from tissue was lyzed with RIPA buffer and subjected to Western blot analysis. The nuclear protein was extracted (Beyotime, China) according to a published method.37 Protein samples were separated with 10% SDS-PAGE and transferred to 0.45 mm PVDF membranes (Millipore, Billerica, USA) that were incubated overnight at 4 °C with anti-TLR4 (1:1000, ab22048, Abcam, Cambridge, USA), anti-pNF-κB-p65 (1:1000, 3033, CST, Danvers, USA), anti-IKBα (1:1000, 9242, CST, USA), anti-pIKBα (1:1000,2859,CST, USA), anti-histon3 (1:1000, 4499, CST, USA), and anti-β-actin (1:5000, bs0061R, Bioss, Beijing, China). After washing, the membranes were incubated with secondary antibodies (goat anti-rabbit/goat anti-mouse, 1:10000, Beyotime, China) for 1 h. The proteins were detected by enhanced chemiluminescence (ECL) (Millipore, USA) using a gel imaging system (Amersham Imager 600, GE, USA).
2.9 Immunofluorescence Staining
For tissue immunofluorescence staining, paraffin-embedded distal ileum samples were cut into 5-µm thick slices. After deparaffinization, rehydration, and washing, the sections were blocked with blocking buffer (Beyotime, China) for 1 h. Then, the sections were incubated with anti-TLR4 (1:100, 19811-1-AP, Proteintech, Rosemont, USA), anti-Ki67 (1:200, ab16667, Abcam, USA) or anti-sox9 (1:100, ab185230, Abcam, USA), and anti-NFκB-p65 (phospho-Ser536) (1:100,YP0191, Immunoway, Plano, USA) primary antibody overnight at 4 °C. The sections were incubated with secondary antibodies, goat anti-rabbit cy3 (1:1000, ab6939, Abcam, USA), and goat anti-rabbit Alexa Fluor488 (1:1000, ab150077, Abcam, USA), for 1 h at room temperature, and then the nucleus was stained with DAPI (C0065, Solarbio, Beijing, China). The slides were cover slipped, and images were captured with a fluorescence microscope (Leica DM4/6B, Leica, Germany).
For cultured organoid immunofluorescence staining, organoids were seeded on coverslips. After treatment, organoids were fixed with 4% paraformaldehyde for 1 h at 4 °C and incubated with 0.1% Triton X 100 for another 1 h at room temperature. After incubation with anti-TLR4 or anti-Ki67 antibodies overnight at 4 °C, the organoids were incubated with secondary antibodies for 1 h at room temperature. Then, the organoids were mounted on coverslips on slides, and images were captured with a laser scanning confocal microscope (Olympus Confocal Microscope, Olympus, Japan).
2.10 Statistical Analysis
Data are expressed as the mean ± SD. Statistical comparisons were made by one-way analysis of variance (ANOVA) with Dunnett's T3 tests or non-parametric test with Chi-squared test. Comparisons with p-values less than 0.05 were considered significant. Statistical analysis was performed with SPSS 18.0. Graphs were generated by GraphPad Prism 7.0.
3 Results
3.1 HMOs Purification and Endotoxin Removal
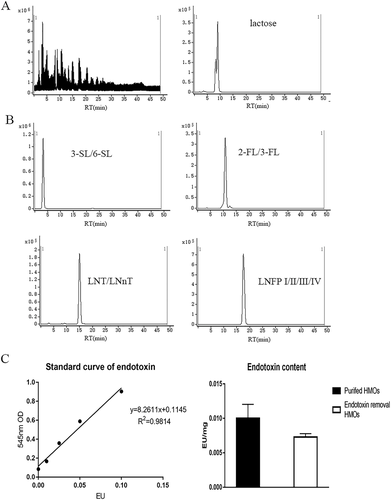
Crude HMOs contained 90% lactose. According to a previous report, ≈10 oligosaccharides in human milk provided nearly 90% of the total HMO content.38 We separated these oligosaccharides by LC-QTOF-MS according to their degree of polymerization (DP), and their retention times are shown in Table 2. Oligosaccharides were separated from low DP to high DP. Sialic acid oligosaccharides were separated before neutral oligosaccharides and fucosylated oligosaccharides.
| Isomer | m/z | RT [min] |
|---|---|---|
| 3′-Sialyllactose, 6′-Sialyllactose | 632.2257 | 3.2 |
| Disialyl-lacto-N-tetraose | 1288.4489 | 3.9 |
| Sialyl-lacto-N-tetraose a/b/c | 997.3597 | 3.9 |
| Lactose | 683.1492 | 8.3 |
| 2′-Fucosyllactose, 3′- Fucosyllactose | 487.1183 | 11.4 |
| GOS | 503.1128 | 12.6 |
| Lactodifucotetraose | 679.1747 | 14.3 |
| Lacto-N-tetraose, Lacto-N-neotetraose | 706.1865 | 15.6 |
| Lacto-N-fucopentaose I/II/III/IV | 852.2402 | 20.7 |
We separated lactose from HMOs as shown in Figure 1A. Although we could not separate the isomers of each DP, we successfully extracted HMOs and collected different types of HMOs (Figure 1B). To prevent the presence of endotoxin from affecting the results, endotoxin was reduced to <0.01 EU mg−1 (Figure 1C). Lyophilized HMO powder was used in the following experiment.
3.2 HMO Treatment Significantly Reduces the Incidence and Lethality of NEC
Compared with the BF group, the FF, FF+IFOS, and FF+LPS groups had increased mortality (Figure 2C). The mean NEC scores were markedly increased and pneumatosis was significantly increased in the intestines of these groups compared with that of the BF group (Figure 2B,D). These results indicated that LPS significantly aggravated NEC in our model and that IFOS had a limited effect against NEC.
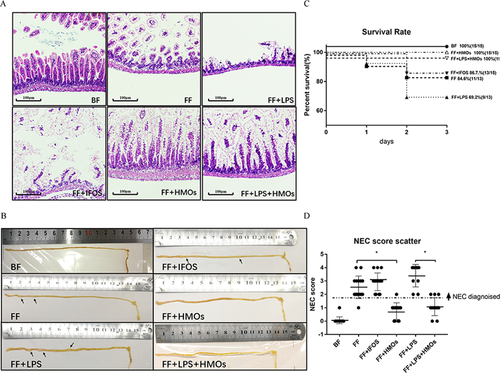
According to the H&E staining of the ileum, HMO treatment effectively prevented ileum collapse and intestinal flatulence in all NEC models (Figure 2A,B). The mean NEC scores of the FF+HMOs and FF+LPS+HMOs groups were reduced to similar scores as the BF group. These results showed that HMO treatment alleviated the injury associated with NEC.
3.3 HMO Treatment Downregulates Proinflammatory Cytokine Expression in the Ileum by Inhibiting the Activation of the TLR4/NF-κB Pathway
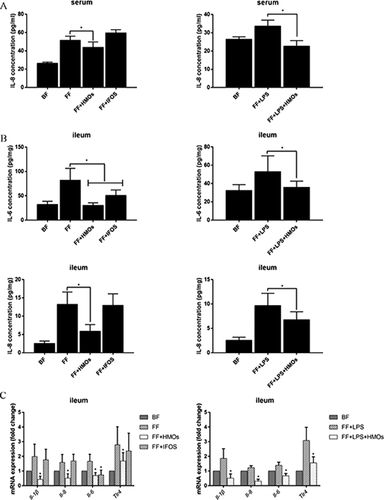
Several proinflammatory cytokines and proteins closely related to endotoxin stimulation were measured.39 The IL-8 serum concentration of the HMO-treated groups, but not the IFOS-treated groups, was significantly reduced compared with that of the FF and FF+LPS groups (Figure 3A). In the ileum, HMOs also reduced IL-6 and IL-8 levels (Figure 3B). Although IFOS alleviated IL-6 upregulation in the ileum, IFOS did not influence the IL-8 level in the serum or ileum. The mRNA expression results in the ileum are shown in Figure 3C. The expression of Il-1β, Il-6, Il-8, or Tlr4 in the FF+HMOs and FF+LPS+HMOs groups was lower than that in the FF and FF+HMOs groups. We speculated the regulation of IL-8 might be more important than the regulation of IL-6 in the HMO-mediated prevention of NEC.
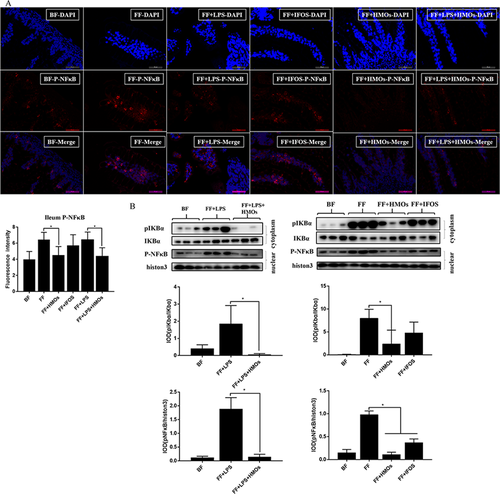
The immunofluorescence results indicated that HMOs significantly reduced pNF-κB in the villi (Figure 4A). We also detected low pIKBα expression and found that pNF-κB in the nuclei remained at a low level in the FF+HMOs and FF+LPS+HMOs groups (Figure 4B). We found that the IFOS-treated groups also had a low level of pNF-κB in the nuclei; however, the nuclear pNF-κB in the IFOS-treated groups remained higher than that in the HMO-treated groups, which could account the downregulation of IL-6. We concluded that HMO supplementation significantly inhibited the activation of NF-κB in the ileum.
3.4 HMOs Inhibited TLR4 Protein Expression in the Epithelial Cells of the Ileum
TLR4 is a key protein in NEC. The activation of TLR4 induces immune responses in the immature neonatal intestine. The expression of the TLR4 protein in ileum crypts and villi is shown in Figure 5A. The results of the immunofluorescence staining indicated that TLR4 expression in ileum intestinal epithelial cells was decreased in the HMO-treated groups compared with that in the FF and FF+LPS groups. HMO treatment inhibited TLR4 protein expression (Figure 5B). We used LPS to simulate an inflammatory environment in 3D crypt organoid cultures (Figure 5C). HMOs alleviated the LPS-induced upregulation of TLR4 and dispersed the organoid cells, which were mature differentiated cells that originated from stem cells and represented the cells located on the tip of villi in the ileum. These cells expressed less TLR4 protein after LPS was added. Thus, HMO treatment might affect cell maturation and promote cell renewal rather than decrease TLR4 protein expression.
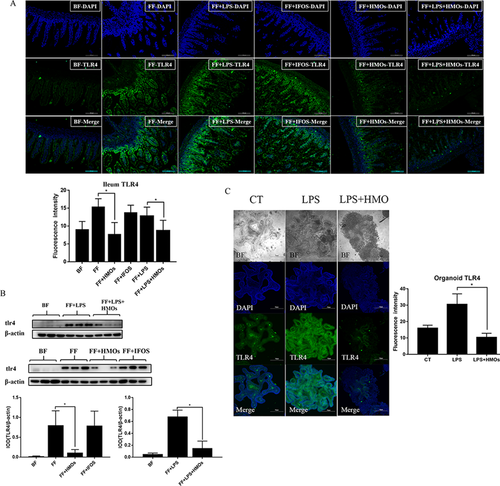
3.5 HMO Treatment Restores the Proliferative Activity of the Crypt Cells in the Ileum
Staining for Ki67, a marker of proliferating cells,40 revealed that the FF and FF+LPS groups had significantly fewer Ki67-positive cells than the BF group (Figure 6A). HMO administration restored the ratio of Ki67-positive cells, whereas IFOS treatment had no effect. Thus, HMO treatment had a protective effect against decreased proliferation in the ileum. Staining for SOX9, a marker of intestinal stem cells, showed that HMO treatment prevented damage to stem cells during NEC (Figure 6B).41 The ratio of SOX9-positive cells in the HMO-treated groups was increased compared with that in the FF and FF+LPS groups. This result indicated that HMO treatment prevented NEC-induced damage to stem cells. Proliferating cells in the intestine are located in the crypts. Immunofluorescence staining for Ki67 in 3D crypt organoid cultures indicated that LPS treatment reduced the number of Ki67-positive cells (Figure 6C). HMO treatment promoted the maturation of crypt organoid cells and restored the number of Ki67-positive cells in the crypt organoids.
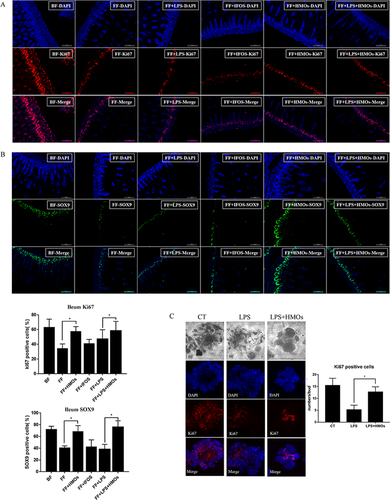
4 Discussion
Under normal conditions, inflammation in the intestine helps neonates defend against the invasion of pathogens. However, neonatal intestinal and immune systems are immature, which results in excessive inflammation.42 A previous study reported that the damage of intestinal epithelial cells caused by excessive inflammation is the main mechanism of NEC-induced intestinal injury.43 HMOs protect against NEC but the mechanism involved remains unclear. Bode et al. reported that HMOs inhibited leukocyte adhesion to endothelial cells to inhibit tissue damage.44 Good et al. had pointed out that HMOs attenuates NEC by enhancing mesenteric perfusion in neonatal intestinal.45 These results all indicated that HMOs could through indirect way to benefit the intestinal barrier to prevent NEC. In this study, we found that HMO treatment directly downregulated TLR4 expression and inhibited NF-κB activation in intestinal epithelial cells during NEC.
He et al. reported that HMOs did not influence the expression of TLR4 after stimulation with LPS in vitro.21 However, we found that the FF+HMOs and FF+LPS+HMOs groups had low expression of TLR4 mRNA and protein in vivo. Furthermore, HMO treatment downregulated TLR4 expression in cultured crypt organoids after LPS stimulation. We speculated these differences might be explained by the different in vitro cell models used. 2D cell lines partially reflect a single type or state of intestinal epithelial cells. The in vitro study used different types of cells that differentiated from crypt stem cells;27 therefore, these cells might have different responses to inflammation, and complicated interactions between different types of cells might exist.
TLR4 is critical for the development of NEC. During NEC, pathogen or endotoxin signaling activates the TLR4 receptor and downstream NF-κB signaling.46 When TLR4 was knocked out, animal models failed to respond to LPS.47 Leaphart et al. reported that rats with reduced TLR4 expression had a lower incidence of NEC and reduced symptoms than did rats with normal TLR4 expression.48 The TLR4-NF-κB pathway regulates inflammatory responses in the intestine.49 We found that HMO treatment significantly influenced TLR4 expression in the ileum. Thus, HMOs might regulate TLR4 expression to prevent excessive inflammation. The regulatory mechanism of TLR4 expression by HMOs is unclear. Huang et al. reported that a low environmental pH attenuated the effect of LPS to induce nitric oxide expression in vitro.50 There are various sialic-oligosaccharides in HMOs, and it is possible that HMOs might change the environment of intestine and attenuate the effect of LPS. HMOs may also prevent endotoxins or pathogens from binding to TLR4. Although we did not observe binding between HMOs and LPS in vitro, such binding might be possible in vivo. However, we found that both the FF+HMOs and FF+LPS+HMOs groups downregulated the expression of TLR4 in ileum. We thought the regulatory mechanism of HMOs is independent of LPS.
Our results showed that LPS treatment reduced the proliferation of intestinal crypt cells both in vivo and in vitro. This result might be explained by the damage induced by proinflammatory cytokine-mediated excessive inflammation or by the LPS-induced activation of NF-κB.29 HMO treatment reversed this damage in vivo. In crypt organoids, HMO treatment increased the numbers of proliferative cells and dispersed the organoids. This is the first report of this phenomenon. Kuntz et al. reported that HMOs accelerated the growth of HT29, Caco2, or HIEC cells and improved their maturation.51 3D cultures of crypt organoid cells were observed to migrate from the crypt bottom to the crypt top in vivo. HMOs stimulated cell migration to the outside region of the organoids. Although the area of the crypt organoids was smaller in the HMO-treated group than in the LPS-stimulated group, there were more proliferative cells in the HMO-treated group. We speculated that HMOs accelerated the renewal of crypt cells. Cells damaged by inflammation would therefore be replaced. The new cells expressed low TLR4 levels during LPS treatment. Therefore, their response might be reduced when stimulated by TLR4 agonists. Finally, the intestine of HMO-treated mice had reduced sensitivity to inflammation to prevent NEC. The mechanism involved in this reaction remains to be clarified.
Although HMOs have been known for nearly a century, only two types of HMOs:2′-fucosyllactose (2-FL) and lacto-N-neotetraose (LNT) are currently permitted for use in infant formula.52 The functions of 2-FL and LNT remain poorly defined. In this study, we used two NEC models (hypoxia, hypoxia+LPS) to investigate the effect of HMOs. Our results indicated that the beneficial effect of HMOs against NEC was independent of the NEC modeling methods. The mechanism of HMOs in the alleviation of NEC may be closely correlated with the pathogenesis of NEC. Further studies are needed to analyze the specific components of HMOs that could lower the expression of TLR4.
In summary, HMOs inhibited proinflammatory cytokine expression and NF-κB activation as well as renewed crypt cells to maintain the low level of TLR4 expression. These results provide a new understanding of the anti-inflammatory effects of HMOs against NEC.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31401668, 31601443), Beijing Science and Technology Project (Z181100009318005), and 111 Project from the Education Ministry of China (No. B18053). C.W. designed and conducted the experiment as well as performed data analysis. J.Y. performed the HMO extraction and analysis. F.L., J.C., and Y.L. collected human milk. The manuscript was written by C.W. and revised by M.Z., H.G., and F.R.
Conflict of Interest
The authors declare no conflict of interest.




