Targeting on Gut Microbial Metabolite Trimethylamine-N-Oxide and Short-Chain Fatty Acid to Prevent Maternal High-Fructose-Diet-Induced Developmental Programming of Hypertension in Adult Male Offspring
Abstract
Scope
Alterations of gut metabolites, such as SCFAs and trimethylamine (TMA), and microbial composition are associated with the development of hypertension. Whether maternal 3,3-dimethyl-1-butanol (DMB, an inhibitor for TMA formation) treatment or the predominant SCFA acetate supplementation can prevent programed hypertension induced by a high-fructose diet (HFD) exposure during pregnancy and lactation in adult male offspring is examined.
Methods and results
Male offspring are divided into four groups: ND, normal diet; HFD, 60% HFD; ACE, HFD plus 200 mmol L–1 magnesium acetate in drinking water; and DMB: HFD plus 1% DMB in drinking water. Maternal HFD induces programed hypertension in adult male offspring, which is prevented by maternal acetate supplementation or DMB treatment. HFD-induced hypertension is relevant to increased plasma levels of TMA and acetate, and alterations of gut microbial composition. The protective effects of acetate supplementation are associated with decreased plasma TMA level and TMA-to-trimethylamine-N-oxide (TMAO) ratio, and increased renal expression of SCFA receptors. Maternal DMB treatment reduces plasma TMA, TMAO, acetate, and propionate levels.
Conclusion
Early intervention targeting on gut-microbiota-derived metabolites TMAO and SCFAs to reprogram hypertension may have significant impact to reduce the burden of hypertension.
1 Introduction
Intake of fructose in the form of high-fructose corn syrup and refined sugar has risen dramatically in the past few decades.1 Consuming a high-fructose diet (HFD) during pregnancy may cause adverse effects on both mother and their offspring.2, 3 Our previous reports demonstrated that offspring born to dams exposed to 60% HFD develop hypertension in adulthood.4, 5
The concept that adverse environments during fetal development can impair organogenesis, resulting in long-lasting effects on offspring and risk of adult diseases in later life is termed the developmental origins of adult health and disease (DOHaD).6 Among major organ systems, the kidney appears to play a decisive role in regulation of blood pressure (BP). During kidney development, maternal HFD caused permanent morphological changes and functional adaptation by so-called renal programming.7 Accordingly, renal programming hasbeen considered as a key mechanism driving programed hypertension.7, 8
On the other hand, the gut is the first organ in contact with dietary nutrients. Early-life nutritional insults may cause a microbial imbalance, namely dysbiosis.9 Dysbiosis in fetus and infant has negative effects and may have long-term consequences leading to many adult diseases.9 Emerging evidence shows that the development of hypertension is correlated with gut microbiota dysbiosis in several animal models of hypertension,10-12 including the HFD model.11 The major metabolites produced by the gut bacteria are SCFAs, with acetate, butyrate, and propionate predominating. The SCFA receptors are expressed in the kidney and have been reported to function as a regulator of BP.13 Additionally, gut microbiota-derived metabolites trimethylamine (TMA) and trimethylamine-N-oxide (TMAO) have been linked to hypertension.14 Dietary choline, betaine, and carnitine are metabolized by gut microbes to generate TMA, which in turn is metabolized by flavin-containing monooxygenases (FMOs) to generate TMAO.14 High blood TMAO levels are associated with increased risk of cardiovascular disease.15 Our objective in this study was first to determine whether maternal HFD-induced programed hypertension is associated with alterations of gut microbiota dysbiosis, SCFAs and their receptors, and TMA/TMAO metabolic axis.
A number of therapeutic interventions have been reported to reverse the adverse effects of developmental programming.16 Although modulating gut microbiota by probiotics was reported to prevent HFD-induced programed hypertension,17 whether targeting on gut microbiota-derived metabolites, such as TMAO and SCFAs, can prevent maternal HFD-induced hypertension in adult offspring remains unclear. 3,3-Dimethyl-1-butanol (DMB), an inhibitor for TMA formation through inhibition of microbial TMA lyases, was reported to reduce plasma TMAO levels in mice fed a Western diet.18 Additionally, a previous report showed that acetate supplementation alters the composition of gut microbiota and protect against hypertension in mineralocorticoid-excess hypertensive mice.19 Therefore, our second aim was to examine whether DMB (an inhibitor for TMA formation) or the predominant SCFA acetate supplementation can prevent maternal HFD-induced programed hypertension.
2 Experimental Section
2.1 Animal Study
The Institutional Animal Care and Use Committee of the Kaohsiung Chang Gung Memorial Hospital approved this protocol. Animal care and use was in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Virgin Sprague–Dawley (SD) rats were obtained from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). Rats were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facility with controlled temperature and 12:12 h light/dark cycles. Male SD rats were housed with individual females until mating was confirmed by the examination of a vaginal plug.
Pregnant rats (n = 12) were randomly divided into 4 groups and fed as follows during pregnancy and lactation period (i.e., a total of 6 weeks): 1) ND:,normal diet; 2) HFD, chow supplemented with fructose (60% diet by weight); 3) ACE, chow supplemented with fructose (60% diet by weight) plus 200 mmol L–1 magnesium acetate (Merck Millipore) in drinking water, refreshed three times per week, and 4) DMB, chow supplemented with fructose (60% diet by weight) plus 1% DMB in drinking water. The doses of magnesium acetate and DMB used here were based on previous studies conducted in rodents.18, 19 After birth, each litter was left with the mother until weaning; pups were not weighed at birth to prevent maternal rejection. Culling was performed to limit the litter size to eight pups per litter, as needed to standardize the received quantity of milk and maternal pup care. Because men are more prone to hypertension at a younger age,20 only male offspring from litters were used in subsequent experiments. Male pups were assigned to four groups (n = 7–8 per group): ND, HFD, ACE, and DMB. All male offspring in the four groups were fed a normal diet after weaning.
BP was measured in conscious rats on week 3, 4, 8, and 12 using an indirect tail-cuff method (BP-2000, Visitech Systems, Inc., Apex, NC, USA), as described previously.5 To ensure accuracy and reproducibility, the rats were allowed to adapt to restraint and tail-cuff inflation for 1 week prior to the experiment. BP measurements were taken between 13:00 and 17:00 each day on a blinded basis by the same experienced research assistant. Rats were placed on specimen platform, and their tails were passed through tail cuffs and secured in place with tape. Following a 10-min warm-up period, ten preliminary cycles of tail-cuff inflation were performed to allow the rats to adjust to the inflating cuff. For each rat, five cycles were recorded at each time point. Average of values from three stable measurements was taken. All rats were sacrificed at 12 weeks of age. Fresh feces samples were collected at week 3 and 12, frozen, and stored at −20 °C until extraction. Kidneys and heparinized blood samples were collected at the end of the study. Perfused kidneys were harvested, decapsulated, divided into cortex and medulla, flash frozen in liquid nitrogen, and stored at −80 °C until assayed.
2.2 GC–Flame Ionization Detector
Plasma acetate, butyrate, and propionate levels were measured using GC–MS (GCMS-QP2010; Shimadzu, Kyoto, Japan) with flame ionization detector (FID), as described previously.17 Separation was performed on the SGE BP GC column (21 × 0.5 µm, 30 m × 0.53 mm; Shimadzu). The working solutions of acetate, butyrate, and propionate used as internal and external standards were at the concentration of 10 mm and kept at −20 °C freezer. Dry air, nitrogen, and hydrogen were supplied to the FID at 300, 20, and 30 mL min–1, respectively. An aliquot of 2 µL sample was injected into the column. The inlet and FID temperature were set at 200 and 240 °C, respectively. The total running time was 17.5 min. Analytical standard grades used as internal standards for acetate and propionate were obtained from Sigma–Aldrich (St. Louis, MO, USA) and for butyrate was from Chem Service (West Chester, PA, USA).
2.3 LC–MS Analysis
Plasma TMA and TMAO levels were measured by LC–MS analysis using an Agilent 6410 Series Triple Quadrupole mass spectrometer (Agilent Technologies, Wilmington, DE, USA) equipped with an ESI source. TMA and TMAO were monitored in multiple reaction monitoring mode using characteristic precursor–product ion transitions: m/z 60.1→44.1 and m/z 76.1→58.1, respectively. An Agilent Technologies 1200 HPLC system was equipped with a binary pump and an autosampler. Chromatographic separation was performed on a SeQuant ZIC-HILIC column (150 × 2.1 mm, 5 µm; Merck KGaA, Darmstadt, Germany) protected by an Ascentis C18 column (2 cm × 4 mm, 5 µm; Merck KGaA). The mobile phase containing methanol with 15 mmol L–1 ammonium formate (phase A) and acetonitrile (phase B) was used at a ratio of 20:80 (phase A: phase B) with a flow rate of 0.3–1 mL min–1. Diethylamine was added to plasma samples as an internal standard.
2.4 Analysis of Gut Microbiota Composition
Metagenomic DNA was extracted from frozen fecal samples after centrifugation. According to the manufacturer's protocol, all PCR amplicons were mixed together and sent to the Biotools Co., Ltd. (Taipei, Taiwan) for sequencing using Illumina Miseq platform (Illumina, California, USA). The sequences were analyzed using QIIME version 1.9.1. Sequences with a distance-based similarity of 97% or greater were grouped into operational taxonomic units (OTUs) using the USEARCH algorithm. The phylogenetic relationships were determined based on a representative sequence alignment using Fast-Tree. Shannon's index accounting for both abundance and evenness of the taxa present was analyzed by QIIME version 1.9.1. The β-diversity changes in gut microbiota were evaluated across groups by the Partial Least Squares Discriminant Analysis (PLS-DA) and the Analysis of Similarities (ANOSIM).21 To determine the significantly differential taxa, linear discriminant analysis effect size (LEfSe) was applied to compare samples between groups. The threshold of the linear discriminant was set to 4. For each of the differential features detected by LEfSe, a linear discriminant analysis value, which represented the differences of the feature between the tested groups, was calculated. Predictive assessment of the microbial community functional potential (PICRUSt) was used to predict the abundance of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways based on the 16S rRNA gene sequencing data.22
2.5 Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted from kidney cortex according to previously described methods.11 Two-step quantitative real-time PCR was conducted using the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA, USA) and the iCycler iQ Multi-color Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). One microgram of RNA reverse transcribed into cDNA using the primers indicated in Table S1, Supporting Information. Three SCFA receptors, G-protein coupled receptor 41 (GPR41), GPR43, and olfactory receptor 78 (Olfr78), and one succinate receptor GPR91 belonging to sensory receptors were analyzed. The 18S rRNA gene (Rn18s) was used as a reference. The cycling conditions were one cycle of 3-min denaturation at 95 °C, followed by 45 three-segment cycles of amplification (95 °C for 10 s, 55–60 °C (gene dependent) for 20 s, 72 °C for 1 s) where the fluorescence was automatically measured during PCR, and one three segment cycle of product melting (95 °C for 5 s, 65 °C for 30 s, 97 °C for 5 min). All samples were run in duplicate. The comparative threshold cycle (CT) method was used to calculate relative gene expression. For each sample, the average CT value was subtracted from the corresponding average Rn18s value, calculating the ΔCT. ΔΔCT was calculated by subtracting the average control ΔCT value from the average experimental ΔCT. The fold-increase of the experimental sample relative to the control was calculated using the formula 2−ΔΔCT.
2.6 Statistical Analysis
Data are expressed as the mean ± SD. For most parameters, statistical analysis was performed using one-way analysis of variance (ANOVA) and LSD post hoc test for multiple comparisons. BP was analyzed by two-way repeated-measures ANOVA and Tukey's post hoc test. A p-value < 0.05 was considered statistically significant. Analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (Chicago, IL, USA).
3 Results
3.1 Effect of HFD, ACE, and DMB on Morphological Values and BP
Maternal HFD, ACE, and DMB had no effect on litter sizes (pups per litter: ND = 11.2 ± 1.1; HFD = 10.8 ± 1.8; ACE = 10.8 ± 1.5; DMB = 11.2 ± 1.3).
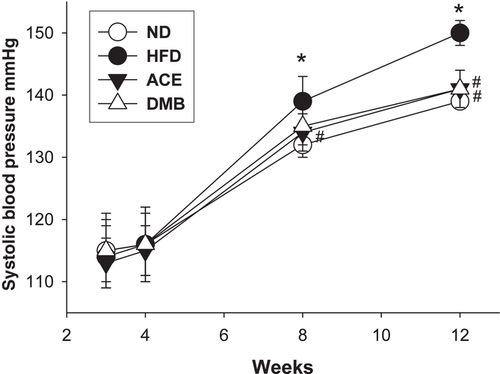
The ACE group had a lowest body weight (BW) among the four groups. HFD caused an increase in kidney weight, while this increase was restored by ACE therapy (Table 1). The ratio of kidney weight-to-body weight were comparable among the four groups. As shown in Figure 1 systolic BP significantly increased (≈10 mm Hg) in HFD group compared with that in ND group from week 8 through 12. At 12 weeks of age, these HFD-induced increases in systolic and diastolic BPs and mean arterial pressures were prevented by maternal ACE or DMB therapy.
| ND | HFD | ACE | DMB | |
|---|---|---|---|---|
| N = 8 | N = 8 | N = 7 | N = 8 | |
| Mortality (%) | 0% | 0% | 0% | 0% |
| Body weight (BW) (g) | 400 ± 20 | 436 ± 39 | 346 ± 21*,# | 414 ± 32† |
| Left kidney weight (g) | 1.72 ± 0.12 | 1.88 ± 0.27* | 1.48 ± 0.14# | 1.76 ± 0.74† |
| Left kidney weight per 100 g BW | 0.43 ± 0.04 | 0.43 ± 0.03 | 0.43 ± 0.04 | 0.42 ± 0.18 |
| Systolic blood pressure (mm Hg) | 139 ± 1 | 150 ± 2* | 141 ± 3# | 141 ± 3# |
| Diastolic blood pressure (mm Hg) | 65 ± 8 | 71 ± 4* | 65 ± 4# | 70 ± 5 |
| Mean arterial pressure (mm Hg) | 89 ± 5 | 97 ± 3* | 90 ± 4# | 93 ± 3# |
- Data are shown as mean ± SD. *p < 0.05 versus ND; #p < 0.05 versus HFD; †p < 0.05 versus ACE. ND, mother rats received normal diet; HFD, mother rats received 60% HFD; ACE, HFD-treated mother rats received 200 mmol L–1 ACE in drinking water; DMB, HFD-treated mother rats received 1% DMB in drinking water.
3.2 Effect of High-Fructose Diet, ACE, and DMB on TMA and TMAO Levels
We first determined TMA and TMAO levels and their ratio in the plasma and urine (Table 2. Plasma TMA levels were higher in the HFD group versus ND group. While these increases were prevented by ACE or DMB treatment. Additionally, DMB treatment results in the lowest plasma TMAO level among the four groups. The TMA-to-TMAO ratio was lower in the ACE group compared to the HFD group. In the urine, ACE and DMB similarly caused a reduction of TMAO level compared to the ND group. However, urinary TMA level and TMA-to-TMAO ratio were not different between the ND, HFD, ACE, and DBM groups.
| ND | HFD | ACE | DMB | |
|---|---|---|---|---|
| Plasma | ||||
| TMA (ng mL–1) | 443 ± 57 | 614 ± 199* | 514 ± 146# | 407 ± 100# |
| TMAO (ng mL–1) | 257 ± 58 | 272 ± 35 | 290 ± 43 | 193 ± 43*,#,† |
| TMA-to-TMAO ratio | 1.8 ± 0.4 | 2.3 ± 0.5 | 1.8 ± 0.4# | 2.2 ± 0.8 |
| Urine | ||||
| TMA (ng mL–1 kg–1 BW d–1) | 1.07 ± 0.18 | 1.07 ± 0.13 | 1.17 ± 0.22 | 1.04 ± 0.2 |
| TMAO (ng mL–1 kg–1 BW d–1) | 41.6 ± 7.4 | 35.1 ± 5.3 | 30.9 ± 12.3* | 29.1 ± 12.8* |
| TMA-to-TMAO ratio | 0.026 ± 0.004 | 0.031 ± 0.004 | 0.067 ± 0.089 | 0.065 ± 0.092 |
- Data are shown as mean ± SD. N = 7–8 per group. *p < 0.05 versus ND; #p < 0.05 versus HFD; †p < 0.05 versus ACE. ND, mother rats received ND; HFD, mother rats received 60% HFD; ACE, HFD-treated mother rats received 200 mmol L–1 ACE in drinking water; DMB, HFD-treated mother rats received 1% DMB in drinking water.
3.3 Effect of High-Fructose Diet, ACE, and DMB on SCFAs and SCFA Receptors
It was reported previously that SCFAs and their receptors are involved in the development of hypertension.13 We next investigated whether HFD affects plasma SCFAs levels and their receptors expression in the kidney. HFD significantly increased plasma acetate level, which was restored by maternal DMB therapy (Figure 2A). We observed that plasma butyrate levels were not different among four groups (Figure 2B). However, plasma propionate levels were lower in the DMB groups than those in the ND and HFD group (Figure 2C). Additionally, maternal ACE treatment significantly increased renal mRNA expression of GPR41 [fold change (FC) = 3.07], GPR43 (FC = 2.17), Olfr78 (FC = 1.99), and GPR91 (FC = 3.78) compared with those in ND group (Figure 2D). We observed that renal mRNA expression of Olfr78 (FC = 2.28) and GPR91 (FC = 3.81) was higher in the DMB group than that in the ND group.

3.4 Effect of HFD, ACE, and DMB on Gut Microbiota
We further analyzed gut microbiota composition of offspring at 3 weeks and 12 weeks of age, to determine the role of the gut microbiota in hypertension of developmental origin in response to HFD, ACE, and DMB. For alpha diversity, there was no significant difference between ND and HFD group at 3 weeks of age (Figure 3A), However, we found that the alpha diversity was significantly increased in ACE (p = 0.001 for Shannon index) and DMB (p < 0.001 for Shannon index) group compared to HF group as well as ND group (both p < 0.001 for Shannon index; Figure 3A). The β-diversity analysis indicates the extent of similarity between microbial communities by measuring the degree to which membership or structure is shared between communities. The score plots of PLS-DA analysis showed that most groups were well separated except the ANOSIM analysis between ND and HFD group did not reach significance (p = 0.159) (Figure 3B).
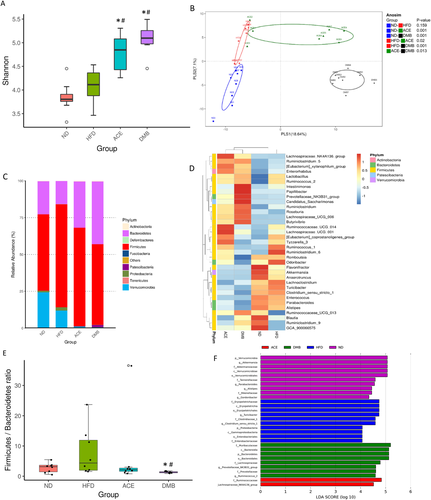
We observed that the main phyla in the four groups studied were Firmicutes, Bacteroidetes, Verrucomicrobia, Actinobacteria, and Proteobacteria. At the phylum level (Figure 3C), HFD significantly reduced Verrucomicrobia abundance versus ND group (Figure 3C; p = 0.033). Maternal ACE (p = 0.037) or DMB (p = 0.049) resulted in a further reduction of Verrucomicrobia abundance. Maternal acetate supplementation significantly decreased Firmicutes (p = 0.045) compared to the HFD group. DMB treatment decreased Firmicutes (p < 0.001), while it increased abundance of Bacteroidetes (p ≤ 0.001), and Actinobacteria (p = 0.03).
At the genus level (Figure 3D), HFD decreased abundance of genus Akkermansia (p = 0.014). Maternal ACE treatment increased abundance of genus Rothia (p = 0.002), and decreased Eubacterium (p = 0.04) and Tubicibacter (p = 0.002) versus HFD group. Similarly, DMB increased abundance of genus Rothia (p < 0.001), and decreased Eubacterium (p = 0.01) and Tubicibacter (p < 0.001). Results of a previous study reported that Firmicutes to Bacteroidetes (F/B) ratio might act as a microbial marker for gut dysbiosis and hypertension.10 We observed a lower F/B ratio in the DMB group compared to the ND and HFD group (Figure 3E). Additionally, we performed LEfSe algorithm to identify metagenomic biomarkers (Figure 3F). The results identified genus Akkermansia (LDA score: 5.1) in ND group, genus Tubicibacter (LDA score: 4.7) in HFD group, and phylum Bacteroidetes (LDA score: 5.2) and genus Prevotellaceae_NK3B31_group (LDA score: 4.6) in DMB group.
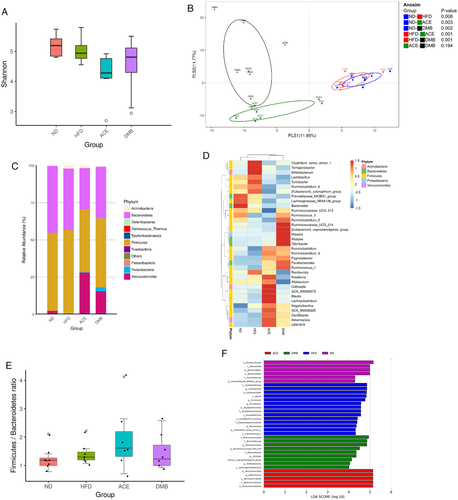
Unlike in week 3, the alpha diversity was decreased in ACE group compared to ND (p = 0.004 for Shannon index) and HF group (p = 0.031 for Shannon index) in week 12 (Figure 4A). The PLS-DA score plots showed the composition of microbial communities of most groups was found to be distinct, except that the difference was not significant between ACE versus DMB group (Figure 4B). As shown in Figure 4, HFD increased phylum Actinobacteria (p < 0.001). Maternal ACE treatment significantly increased phylum Verrucomicrobia (p = 0.007), and decreased Firmicutes (p = 0.017) and Bacteroidetes (p = 0.027) compared to the HFD group. DMB treatment increased phylum Verrucomicrobia (p = 0.004) and Proteobacteria (p = 0.001), while it decreased Actinobacteria (p = 0.035) versus HFD group. At 12 weeks of age, DMB treatment caused an increase in genus Akkermansia (p = 0.014; Figure 4D), whereas it significantly reduced genus abundance of Rothia (p < 0.001), Prevotellaceae_NK3B31_group (p < 0.001), and Tubicibacter (p < 0.001). ACE therapy decreased genus Eubacterium (p = 0.002) and Prevotellaceae_NK3B31_group (p < 0.001). Unlike in week 3, The F/B ratio was not different among the four groups in week 12 (Figure 4E). LEfSe algorithm identified genus Akkermansia (LDA score: 5.2) in ACE group (Figure 4F).
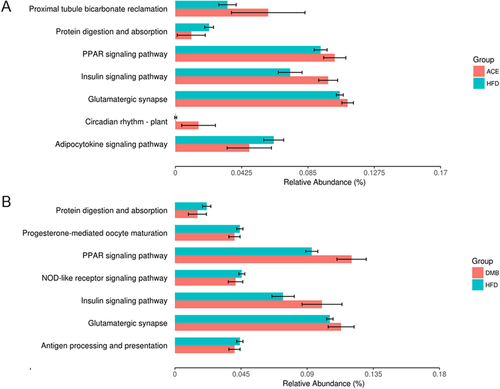
As shown in Figure 5, seven KEGG pathways from the level 3 analysis were identified between HFD and ACE group. Similarly, seven KEGG pathways were identified from HFD versus DMB group (Figure 5B). Among them, four KEGG pathways namely protein digestion and absorption, peroxisome proliferator-activated receptor (PPAR) signaling pathway, insulin signaling pathway, and glutamatergic synapses are shared.
4 Discussion
This study provides a new insight into the mechanisms responsible for the maternal high-fructose diet-induced programed hypertension with particular emphasis on gut microbiota-derived metabolites TMAO and SCFAs. To our knowledge, this is the first study to examine the potential benefit of maternal supplementation with acetate or 3,3-dimethyl-1-butanol in HFD-induced programed hypertension. Consumption of various degrees of HFD ranging from 10% to 60% by pregnant dams have been reported to cause hypertension in their adult offspring.2-5, 23 Consistent with previous reports, we found that maternal HFD produced elevation of BP in 12-week-old male offspring. We observed that maternal acetate supplementation results in a lower body weight than that in the HFD group. Our data are in agreement with a previous report showing that acetate supplements reduced body weight and fat storage in adult rats.24 Whether maternal acetate supplementation induces a reprogramming effect on antiobesity awaits further evaluation.
Several mechanisms have been reported involved in HFD-induced hypertension in adult and offspring.2, 25 In the current study, we provide further evidence for an association between HFD-induced programed hypertension and gut microbial metabolites, TMA and acetate. TMA is a microbiota-derived precursor of TMAO. Diet plays a key role in TMA and TMAO formation. For the first time, our data reveal that maternal HFD increases plasma TMA level in adult offspring. DMB was shown to inhibit microbial TMA lyases, subsequently both inhibiting TMA production and reduce TMAO levels.26 These observations were consistent with our findings showing that maternal DMB therapy reduced plasma TMA and TMAO levels in adult offspring born of mothers fed with HFD. Given that gut microbiota-driven TMA-TMAO pathway has been linked to cardiometabolic diseases,27 our results suggest the beneficial effects of DMB on HFD-induced programed hypertension might be due to, at least in part, modulation of TMA–TMAO pathway. In rodents, specific bacterial taxa in the gut were associated with greater production of TMA and TMAO in response to diverse diets.28 In the genus level, TMA and TMAO levels are positively correlated with Prevotellaceae, while they are negative correlated with Akkermansia.28 Here, we found DMB reduced plasma TMA and TMAO levels, combined by increased abundance of genus Akkermansia and decreased Prevotellaceae. It is possible that the changes in specific microbial abundance contributed to the balance of TMA–TMAO pathway.
In the present study, HFD-induced hypertension is related to elevation of plasma acetate levels. With regard to the current literature, there is no reported direct interaction between fructose and SCFAs. Although it is presumably fructose consumption influences the microbiota and therefore affects the composition of SCFAs, we are the first to demonstrate the long-term influence of mother fed with HFD on plasma acetate level of their adult offspring. Given that acetate has vasodilatory effect and that acetate supplementation prevents the development of hypertension in the current study, the elevation of acetate might be a counterbalancing mechanism rather than a cause of programed hypertension. Acetate is a ligand for Olfr78, GPR41, and GPR43.13, 29, 30 Olfr78 and GPR43 mediates vasoconstriction, which can be counteracted by the vasodilatory action of GRP41.29, 30 Although maternal acetate supplementation had neglectable effects on SCFAs levels, it upregulated SCFA receptors, Olfr78, GPR41, and GPR43, as well as the succinate receptor GPR91 in offspring kidney. Thus, the beneficial effects of ACE against HFD-induced programed hypertension might be related to regulation of several sensory receptors and overall balance of vasoconstriction and vasodilation shifts in favor of vasodilatation.29, 30
Moreover, the beneficial effects of ACE and DMB therapies might be owing to alterations of the gut microbiota composition. Pregnant and lactating supplementation with HFD, ACE, and DMB to dams causes long-term alterations on their offspring's gut microbiota at 3 and 12 weeks of age. We observed gut microbiota compositions are distinct between week 3 and 12, which are in agreement with previous reports showing that gut microbiota changes with age.31 Despite ACE and DMB treatments show similar protective effects against programed hypertension, they elicit distinct alterations on gut microbial compositions.
A decrease in microbial richness and diversity, and an increased F/B ratio have been linked to hypertension in the spontaneously hypertensive rat.10 Our results showed that ACE and DMB treatments protect HFD-induced hypertension is related to reshape the microbiota composition and increase microbial diversity. Although the F/B ratio was reported as a microbial marker for established hypertension,10 which was not supported by our results in this HFD-induced programed hypertension model. Interestingly, DMB was capable of increasing genus Akkermansia in the gut, which has been reported as a beneficial gut microbe.32 Also, LEfSe algorithm identified genus Akkermansia is a microbial marker in the ACE group. These findings agree with our recent report showing that increased abundance of Akkermansia by probiotic and prebiotic therapies attributed to their protective effects on HFD-induced programed hypertension.17 Whether dietary interventions to promote abundance of Akkermansia can be a strategy to prevent programed hypertension remains to be determined.
Nevertheless, it remains unclear which biological pathways affected by microbiota dysbiosis could be related to the pathogenesis of programed hypertension. It is noteworthy that PPAR signaling is identified as a significant KEGG pathway shared by ACE versus HFD and DMB versus HFD. It has been demonstrated that PPAR signaling is a common pathway contributing to hypertension in a variety of programming models,33, 34 including maternal HFD exposure.35 Therefore, the beneficial effects of ACE and DMB might be related to PPAR signaling pathway. However, the exact underlying mechanisms are required to be further clarified.
As hypertension is a disease of multifactorial origins, several maternal insults have been reported to induce the developmental programming of hypertension in animal models as we reviewed elsewhere.36 Given that the worldwide fructose consumption has grown in the last half-century and that its growth has been paralleled by an increase in metabolic syndrome (MetS)-related disorders, a maternal HFD model is being developed into a commonly used animal model to induce MetS of developmental origins. Since hypertension is a key feature of MetS, research into effective reprogramming strategies for HFD-induced programed hypertension may also have an impact on other MetS-related phenotypes.
The present study also had limitations. First, only male offspring was recruited in this study. Whether sex differences exist in the reprogramming effects of ACE and DMB deserve further evaluation. Second, we did not conduct the control treated with ACE or DMB group because dietary magnesium acetate supplementation and DMB administration used in controls showed a good safety profile in previous studies.37, 38 Nevertheless, the long-term programed effects of ACE or DMB on normal controls deserve further elucidation.
The gut-kidney axis has recently started to gain importance in the development of hypertension.39 To the best of our knowledge, we have shown for the first time that maternal acetate supplementation or DMB treatment prevented HFD-induced hypertension in adult offspring via reshaping gut microbiota, regulating TMA–TMAO metabolic pathway, and mediating SCFAs and their receptors. Our findings provide a new insight into the potential role of gut microbiota responsible for the HFD-induced programed hypertension and indicate that the targeting on gut microbiota-derived metabolites TMAO and SCFAs may be reprogramming strategies to reverse the programming processes.
Acknowledgements
C.H. contributed to concept generation, data interpretation, manuscript drafting, critical manuscript revision, and article approval. G.C. contributed to data interpretation, manuscript drafting, critical manuscript revision, and article approval. S.L. contributed to data interpretation, manuscript drafting, critical manuscript revision, and article approval. C.H. contributed to data interpretation, manuscript drafting, critical manuscript revision, and article approval. Y.T. contributed to concept generation, data interpretation, critical manuscript revision, and article approval. This work was supported by Grant CMRPG8H0081 from Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan and Cheng Shiu University, Kaohsiung, Taiwan.
Conflict of Interest
The authors declare no conflict of interest.




