Dietary Cocoa Prevents Aortic Remodeling and Vascular Oxidative Stress in Diabetic Rats
Abstract
Scope
The aim of the present study is to investigate the potential protective effect of a cocoa-rich diet on functional and structural vascular alterations in diabetes and the mechanism involved.
Methods and results
Male Zucker diabetic fatty (ZDF) rats are fed on a standard (ZDF-C) or cocoa-rich diet (ZDF-Co) from week 10 to 20 of life. Diabetic ZDF-C rats showed increased blood pressure and enhanced aortic stiffness, as demonstrated by the increased pulse pressure and the augmented aortic medial thickness with loss and disruption of elastic fibres. Interestingly, cocoa intake strongly avoided all these adverse effects and reduced aortic oxidative stress. Mechanistically, cocoa diet prevented sirtuin-1 (SIRT-1) depletion and increased NADPH oxidases (NOXs) and reactive oxygen species production as well as reduced active nuclear factor E2 related factor 2 (Nrf2) and their antioxidant products.
Conclusion
The results demonstrate for the first time that a cocoa-rich diet strongly prevents aortic stiffening and remodeling in diabetic animals and avoids aortic oxidative stress. It is suggested that this effect could be mediated via its effects on SIRT-1, NOXs, and Nrf2.
1 Introduction
Cardiovascular disease is the leading cause of morbidity and mortality in patients with type 2 diabetes (T2D).1 Diabetic macrovascular complications are associated with functional and structural changes in the vascular wall, resulting in early arterial stiffness and remodeling.2 Aortic stiffness is considered a marker of vascular dysfunction and a major predictor of poor cardiovascular outcomes.2 Therefore, alleviation of vascular stiffness is an important therapeutic target to reduce cardiovascular risk in diabetic patients.
Oxidative stress is one of the main pathogenic mechanisms contributing to arterial stiffening and vascular remodeling. In diabetes, overproduction of radical oxygen species (ROS) in the vascular system is mainly attributable to NADPH oxidases (NOXs) activation and/or to a decrease in cellular antioxidant defence mechanisms and close related key proteins.3 In particular, levels of sirtuin1 (SIRT-1), a NAD-dependent histone deacetylase, are decreased in diabetic patients and this reduction has been implicated in vasculopathy.4 Actually, SIRT-1 protects against ROS-mediated oxidative damage by suppressing NOXs activation in the vascular wall.5 Likewise SIRT-1 activation also increased the expression levels of the Nuclear factor-E2 related factor 2 (Nrf2) and its antioxidant target in human endothelial cells.6
Given the crucial role of ROS in arterial stiffness and remodeling, considerable efforts have been made to discover therapies to reduce oxidative stress in the vascular tissue. In this regard, dietary flavonoids constitute a large class of natural antioxidant compounds with demonstrated beneficial actions against several chronic diseases, including cardiovascular diseases.7 The favourable effect of flavonoids on arteries is emerging and it has been recently described that flavonoids could be associated with improved measures of vascular function, and in particular with arterial stiffness.8 Accordingly, cocoa flavanols have been related to numerous cardioprotective effects, including lowering of blood pressure (BP),9 anti-inflammatory10 and anti-atherosclerotic effects,11 as well as antidiabetic actions.12 Despite these facts, the effect of cocoa flavanols on arterial function in a situation of high cardiovascular risk such as diabetes remains unclear.
Therefore, this study was aimed at investigating the potential protective effect of a cocoa diet rich in flavanols on functional and structural vascular damage during diabetes and to explore the potential underlying mechanisms. To this end, we have used the Zucker diabetic fatty (ZDF) rats, a widely known and well-established model that displays many of the conditions of T2D in humans. These animals present arterial damage during the progression of the illnesses, making then highly appropriate to evaluate the effect of cocoa on vascular function in T2D.
2 Experimental Section
An extended version of the methods and protocols is included in the Supporting Information.
2.1 Animals and Experimental Design
Animals were treated according to the European (2010/63/EU) and Spanish (RD 53/2013) legislation on Care and Use of Experimental Animals and the experiments were approved by the Ethics Committee from Comunidad de Madrid (PROEX 304/15).
Male Zucker diabetic fatty (ZDF) rats and their Zucker lean controls (ZL) were obtained from Charles River Laboratories (L'arbresle, France) at 9 weeks of age. Animals were placed under standard controlled conditions (21 °C ± 1 °C; 12 h day/night cycle) and after 1 week of acclimation animals were randomly assorted into three different experimental groups (8 animals per group): (1) ZL group: ZL rats received a standard AIN-93G diet, (2) ZDF-C group: ZDF rats received a standard AIN-93G diet and (3) ZDF-Co group: ZDF rats received a cocoa rich diet. Details about cocoa diet composition and dosage regimen are described in the Supporting Information.
2.2 Biochemical Determination
At 20 weeks of age, animals were fasted overnight and blood samples were collected for glucose, insulin, triacylglycerols (TG), total cholesterol (T-Cho), HDL-Cho, and LDL-Cho analysis.
2.3 Glucose Tolerance Test (GTT)
One week before the end of the study, an overload of glucose (2 g per kg b.w.) was i.p. administered in overnight-fasted animals. Blood samples were obtained from the tail vein before and 30, 60, 90, and 120 min after the glucose overload. Glucose was determined using an Accounted Glucose Analyzer (LifeScan España, Madrid, Spain). The integrated glucose response (AUC) over a period of 120 min after glucose overload was also calculated.
2.4 Hemodynamic Measurements and Vascular Reactivity
Rats were anesthetized (80 mg per kg ketamine and 8 mg per kg xylacine i.p.), tracheostomyzed and ventilated with room air (tidal volume 9 mL kg−1, 60 breaths per min, and a positive end-expiratory pressure of 2 cm H2O, Nemi Scientific Inc, Medway, USA). Systolic, diastolic, and mean systemic arterial pressures (sSAP, dSAP, and mSAP) were measured by cannulation of the carotid artery in closed chest animals. After completion of hemodynamic measurements rats were euthanized by cardiac puncture and thoracic aortic sections were dissected for the different experiments. Evaluation of vascular reactivity is detailed in the Supporting Information.
2.5 Histological and Immunohistochemical Analyses of Aortic Rings
For histological analysis, aortic samples were fixed in paraformaldehyde (4%), embedded in paraffin, and cut into 4-µm-thick sections. Aorta sections were stained with haematoxylin and eosin (H&E) and media thickness and the internal diameter were measured using image-J software (Fiji image J; 1.52i, NIH, USA). Aortic medial thickness was measured in cross-sections by ten consecutive measurements in a systematic manner to evaluate all segments of the circumference of the aorta. Estimation of the aorta internal diameter was performed by measuring four inner diameters at right angle for each cross-section of the thoracic aorta. At least three different cross-sections of the aorta were analyzed for each rat. The ratio between aortic thickness and the internal diameter was calculated.
2.6 Determination of ROS
ROS were quantified by the DCFH assay based on the oxidation of dichlorofluorescein (DCF) that emits fluorescence.13
2.7 Western Blott Analysis
To detect ERKs, p-ERKs, JNKs, p-JNKs, and NOX-1, NOX-2, and NOX-4, frozen aortic rings were homogenized 1:10 (w:v) in extraction buffer. Aortic homogenates were centrifuged at 14 000 × g for 60 min. Protein concentration was determined by the Bradford assay and stored at −80 °C until use for Western blot analyses.
2.8 Protein Carbonyl Levels
Protein oxidation was measured as carbonyl levels in aortic homogenates. Protein carbonyl content was determined spectrophotometrically following derivatization with 2,4-dinitrophenylhydrazine as described.14
2.9 GSH Levels and GPx and GR Activities
The content of reduced glutathione (GSH) was quantitated by fluorometric assay as previously described.14 The method is based on the reaction of GSH with OPT at pH 8.0, which generates fluorescence. Determination of GPx activity is based on the oxidation of GSH by GPx, using tert-butylhydroperoxide as a substrate, coupled to the disappearance of NADPH by GR.14 GR activity was determined by following the decrease in absorbance due to the oxidation of NADPH utilized in the reduction of oxidized glutathione.14
2.10 Statistical Analysis
Data were tested for normality and homogeneity of variances by the D'Agostino and Pearson and Levene tests, respectively; for multiple comparisons, one-way ANOVA was followed by a Bonferroni test when variances were homogeneous or by the Tamhane test when variances were not homogeneous. The level of significance was p < 0.05. A SPSS 23.0 version program was used.
3 Results and Discussion
In this work, we investigated whether cocoa intake may prevent the alterations on hemodynamics, arterial stiffness, and vascular remodeling during diabetes and the potential mechanism involved. To this end, we have used Zucker diabetic rats that were fed on a cocoa-rich diet (ZDF-Co) from weeks 10 to 20 of life and their respective controls fed on standard diet (ZDF-C). During the study period, food intakes and body weight increased significantly in both diabetic groups (ZDF-C and ZDF-Co) compared to non-diabetic ZL animals, confirming the hyperphagic and obese state of male ZDF rats. However, the body weight of animals fed on the cocoa diet (ZDF-Co) was slightly but significantly reduced (about 10 %) despite the fact that they had food intakes similar to their corresponding ZDF-C animals (Table 1). Likewise, both ZDF groups showed hyperglycaemia, hyperinsulinaemia, and glucose intolerance (AUC) compared to ZL rats, which also confirmed their diabetic state. Interestingly, the severity of these metabolic disorders was clearly reduced by the cocoa diet and, consequently, the levels of HbA1c and insulin resistance (HOMA-IR) were significantly improved in ZDF-Co as compared to the ZDF control animals (Table 1). We have previously showed the potential anti-diabetic effect of cocoa intake in a pre-diabetic state14 and the current study extends these findings to demonstrate that cocoa is also able to improve glucose metabolism in fully diabetic ZDF rats. In contrast, T-Cho, HDL-Cho, LDL-Cho, and TG levels were significantly elevated in both ZDF-C and ZDF-Co rats in comparison to the ZL group, indicating that cocoa diet was unable to improve lipid profile in 20-weeks old diabetic ZDF rats (Table 1).
| ZL | ZDF-C | ZDF-Co | |
|---|---|---|---|
| Body weight [g] | 329 ± 4a | 444 ± 8b | 400 ± 12c |
| Food intake [g] | 1223 ± 61a | 1839 ± 74b | 1918 ± 107b |
| Glycemia [mm] | 4.80 ± 0,50a | 13.20 ± 0.40b | 6.50 ± 0.60c |
| Insulinemia [ng mL−1] | 0.40 ± 0,02a | 4.36 ± 0.50b | 1.14 ± 0.22c |
| HbA1c [%] | 4.38 ± 0.19a | 10.40 ± 1.58b | 6.09 ± 0.79c |
| AUC [mmol L−1 min−1] | 1803 ± 96a | 4044 ± 608b | 3049 ± 339c |
| HOMA IR | 2.60 ± 0.20a | 90.96 ± 14.26b | 12.22 ± 1.36c |
| Cholesterol [mmol L−1] | 2.16 ± 0.26a | 6.29 ± 0.41b | 6.56 ± 0.52b |
| HDL [mmol L−1] | 2.27 ± 0.22a | 2.85 ± 0.37b | 3.08 ± 0.32b |
| LDL [mmol L−1] | 0.92 ± 0.09a | 2.28 ± 0.33b | 3.08 ± 0.32b |
| Triglycerides [mmol L−1] | 0.39 ± 0.09a | 2.74 ± 0.24b | 2.87 ± 0.31b |
- Data represent the means ± SD of 6–8 animals. a,b,cMeans in a row without a common letter differ, p < 0.05.
T2D is associated with functional and structural damages to the arterial wall that result in macrovascular complications and cardiovascular disease.2 Several interventional studies strongly suggest a potential role of cocoa intake in the maintenance of optimal vascular condition in healthy individuals and in situations of cardiovascular risk such as hypercholesterolemia and hypertension9 or during normal aging.15 Consequently, in the present study we evaluated the potential protective effect of cocoa on vascular stiffness and remodeling in a situation of high cardiovascular risk such as diabetes. To this end, hemodynamic parameters like arterial pressure and heart rate as well as markers of arterial stiffness like pulse pressure (PP) and augmentation index (AI), were evaluated in diabetic animals (Figure 1A–D). We observed a significant increase in systolic, diastolic, and mean systemic arterial pressure as well as PP in ZDF-C rats, indicating arterial stiffness which is consistent with the literature on experimental and clinical T2D.16 PP is a widely accessible indicator of arterial stiffness and its increase predicts a higher risk of cardiovascular events.17 ZDF-C rats also showed decreased heart rate as compared with ZL. Interestingly, all these effects were significantly abolished by cocoa intake. Furthermore, as shown in Figure 1E, in non-diabetic ZL rats, the peak pressure of the forward wave was smaller than the reflected wave, which is often referred as Murgo type C wave, characteristic of young and healthy subjects, and therefore the augmentation index was negative. In contrast, ZDF-C rats showed increased reflected wave (Murgo type A wave), characteristic of old and hypercholesterolemic subjects18 with positive augmentation index, suggestive again of increased stiffness. The shape of the pressure wave in ZDF-Co rats returned to the profile of ZL in most rats showing this group a near zero average augmentation index. Altogether, our results indicate that cocoa intake prevented increased blood pressure and aortic stiffness in ZDF diabetic animals. This vascular protective effect of cocoa has also been demonstrated in similar experimental models of cardiovascular risk such as hypertension19 and supported several small interventional studies suggesting that cocoa may also have therapeutic potential in preventing cardiovascular complications in diabetic patients.20
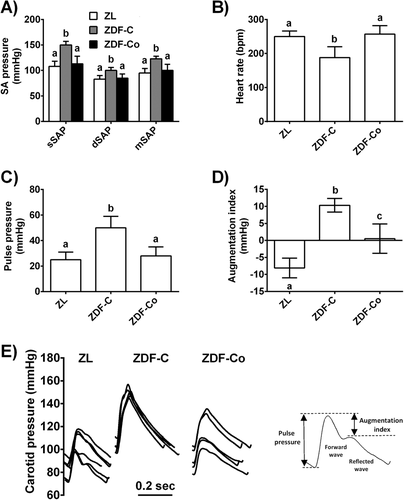
Since arterial stiffness usually precedes endothelial dysfunction, we also evaluated the response to vasodilators in isolated aorta rings. Nevertheless, there were no differences in the concentration–response curves for the vasodilator responses to the endothelium-dependent (acetylcholine) or -independent (sodium nitroprusside) agonists between the three groups (Figure S1A,B, Supporting Information), indicating that endothelial function was unaffected in ZDF diabetic rats. As previously shown,21 moderate hyperglycaemia can be enough to induce adverse structural changes in the vasculature but more severe hyperglycaemia is essential to cause endothelial dysfunction. Consequently, a longer period of diabetes seems to be necessary to induce endothelial dysfunction in ZDF animals.
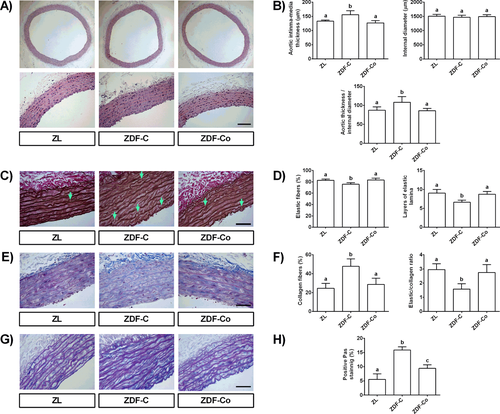
Increased stiffness results from alterations in the composition and structure of the arterial wall; therefore, we next investigated vascular remodeling in thoracic aortas of ZL and ZDF animals. Several mechanisms are involved in this process including hyperplasia of the arterial intima and media, changes in vascular collagen, and elastin or arterial calcifications.22 Accordingly, ZDF-C diabetic rats exhibited a significant increase in aortic medial thickness and in the ratio between aortic thickness and the internal diameter (Figure 2A,B), indicating intima hyperplasia and vascular smooth muscle cell hypertrophy.22 More importantly, the elastin content in aortas from ZDF-C animals was significantly reduced (Figure 2C,D) while collagen accumulation increased, resulting in prominently lower elastin to collagen ratio (E/C) in the media (Figure 2E,F). Especially, this decrease represented the decline in arterial compliance and advance in arterial stiffness.23 Furthermore, the layered architecture of elastic lamellae was found to be disrupted and fragmented (black arrows) and the loss of interlaminar elastic fibres within the aortic media layer resulted in leaving gaps partially filled with collagen and proteoglycans that were significantly increased in ZDF-C diabetic rats (Figure 2G,H). Importantly, all these adverse structural modifications induced by diabetes were fully prevented in diabetic rats fed with cocoa, supporting the protective effect in arterial stiffness observed in ZDF-Co rats. Altogether, these interesting novel findings reveal the beneficial effect of cocoa intake in improving vascular structural changes and remodeling in ZDF rats. Although, interventional studies evaluating the consumption of cocoa products in T2D patients are very limited, results from some small human studies have also showed a protective effect of cocoa intake on vascular function.20 Therefore, additional clinical trials in individuals with diabetes are needed in order to clarify the potential of cocoa to reduce the risk of vascular complications in diabetics.
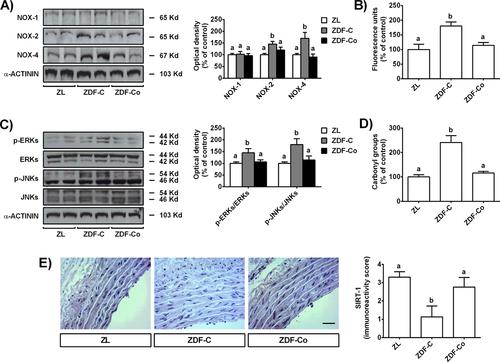
It is widely accepted that oxidative stress plays a central role in the pathogenesis of vascular stiffening and remodeling and vascular oxidative stress has been consistently demonstrated in experimental models and patients with diabetes.24, 25 There are several sources of ROS in the vascular wall including the NOXs family, enzymes of the mitochondrial respiratory chain or xanthine oxidase. However, high glucose and diabetic conditions are known to increase ROS production mainly via activation of NOXs, especially NOX-1, NOX-2, and NOX-4.26 According to that, we found that diabetic ZDF-C rats presented increased NOX-2 and NOX-4 levels and ROS generation in aorta tissues (Figure 3A,B). However, cocoa intake significantly down-regulated NOX-2 and NOX-4 protein expressions and decreased vascular ROS levels in diabetic animals. Likewise, the activation of signaling pathways closely related to oxidative damage, such as ERKs and JNKs, were totally prevented in arteries from ZDF-Co rats (Figure 3C). As a result, arterial oxidative injury, as indicated by increased ROS, NOX-2, and NOX-4 and protein carbonyl levels (Figure 3D), was noted in diabetic ZDF-C rats but not in those fed with cocoa (ZDF-Co). Notably, diabetic ZDF-C rats also showed a significant decrease in the levels of sirtuin-1 (SIRT-1) (Figure 3E). SIRT-1 has been shown to protect against ROS-mediated oxidative damage in arteries by suppressing NADPH oxidase activation.6 In fact, inhibition of SIRT-1 significantly increased vascular superoxide production and enhanced NADPH oxidase activity and mRNA expression of NOX-4 in aortic rings.6 Interestingly, we showed here that cocoa intake strongly prevented SIRT-1 depletion induced by diabetes, probably contributing to the down-regulation of NOX-2 and NOX-4 levels in the aorta of ZDF animals. These findings are consistent with previous in vitro studies showing that one of the main cocoa flavanols, epicatechin (EC), and the cocoa colonic metabolite 3,4-dihydroxyphenylacetic acid prevented the activation of NOX-4 and the decrease of SIRT-1 levels induced by a high glucose challenge in renal tubular cells.27 More importantly, it has been recently showed that EC prevented SIRT-1 decreases induced by aging and reverses the loss of vascular function both in aortas from aged animals and in aged bovine coronary artery endothelial cells.28 Altogether, the present results demonstrate that cocoa intake prevents the vascular oxidative injury induced by diabetes. Since cocoa reduced the levels of NOXs and ROS and prevented SIRT-1 depletion induced by diabetes, we suggest that this could be one of the pathways that contribute to decrease oxidative stress damage in arteries from ZDF rats. This antioxidant effect of cocoa may explain the improvement in blood pressure, aortic remodeling, and insulin sensitivity found in the diabetic animals.29 Likewise, the demonstrated anti-diabetic effects of cocoa flavanols12 can directly reduce hyperglycaemia and improve glucose tolerance, also contributing to reduce the risk of vascular damage.
Diabetic milieu not only increases ROS generation but also attenuates the levels of intracellular antioxidants and compromises the antioxidant defence enzymes in aortas. Accordingly, we found that oxidative stress in arteries from ZDF-C rats was associated with decreased nuclear levels of p-Nrf2 (Figure 4A), a transcription factor that plays an important role in anti-oxidative responses by up-regulating multiple antioxidant components.30 Consequently, the antioxidant defense system was depleted in aortas from ZDF-C diabetic rats, as levels of metallothionein (MT) (Figure 4B) and the activity of antioxidant enzymes such as GPx and GR (Figure 4C) were considerably reduced. Once again, cocoa diet significantly prevented Nrf2 inactivation in aortas and their down-stream antioxidants were restored to the level of the non-diabetic control. MT is a potent antioxidant protein that has been reported to protect against diabetes-induced cardiovascular and renal pathogenic alterations in animal diabetic models.31 Moreover, decreased MT levels have been associated with aortic pathologic damages, including aortic remodeling and oxidative stress.31 Notably, our results are in concordance with those demonstrating that increased MT via up-regulation of Nrf2 plays a prominent role in the prevention of diabetic nephropathy and cardiomyopathy by the natural compound sulforaphane.32, 33 Taken together, these outcomes indicate that cocoa diet avoids diabetes-induced depletion of Nrf2 and their antioxidants enzymes, mainly MT, contributing to prevent oxidative stress and vascular injury in diabetic rats. Although the role of Nrf2 in diabetes is not fully understood, it has been indicated that decreased Nrf2 activity in arteries contributes to oxidative stress and favours vascular remodeling and dysfunction in diabetes.34 Accordingly, the increase of Nrf2 activity by several activators has been considered as a promising approach for preventing the development of vascular complications in diabetes. However, constitutive activation of Nrf2 has been also found in a variety of cancers;35 therefore, additional studies about the role of Nrf2 activation in specific tissues are necessary to better understand the multiple roles of Nrf2 in diabetes.

Finally, it is interesting to note that health promoting properties of cocoa are mainly attributed to its flavanol compounds (epicatechin and procyanidims) and methylxanthines (theobromine). More specifically, it has been suggested that epicatechin and procyanidims, respectively, are the main components responsible for the acute and long-term vascular effects of cocoa.36 On the contrary, the consumption of theobromine alone did not result in significant effects but ingested simultaneously with cocoa flavanols enhanced their vascular effects.37
4 Concluding Remarks
In conclusion, the present work demonstrates for the first time the protective effect of regular cocoa intake on vascular damage in a rat model of T2D and the likely mechanisms involved. The beneficial effects of cocoa include amelioration of glucose metabolism, improvement of hemodynamic status, and the prevention of aortic stiffening and remodeling in diabetes. At the molecular level, cocoa prevented SIRT-1 depletion induced by diabetes, down-regulated NOX-4 and NOX-2 levels, and reduced ROS generation in the arteries of diabetic animals. Likewise, the decreased levels of active Nrf2 and their associated antioxidants were also prevented by cocoa, collaborating in avoiding oxidative stress and vascular injury in diabetic rats. Overall, these findings indicate a novel mechanism to alleviate cardiovascular risk in the diabetic population and support the potential use of cocoa to protect or delay the progression of vascular complications in diabetes.
Acknowledgements
This work was supported by grants AGL 2015–67087-R and SAF2016-77222-R (MINECO/FEDER, UE) from the Spanish Ministry of Science and Innovation (MINECO). D.Á.-C. is a FPI fellow from the predoctoral program of MINECO (BES-2016-076721). D.Á.-C. carried out the experiments and analysed the data. M.E.L.-O. performed and interpreted the histological analysis. F.P.-V., D.M.-C., and B.B. developed and interpreted the hemodynamic and vascular reactivity studies. L.G. contributed to the critical revision of the manuscript. S.R. highly contributed to the conception and design of the study. M.A.M. conceived and designed the study and wrote the manuscript with significant contributions from all other authors.
Conflict of Interest
The authors declare no conflict of interest.




