Dairy Protein Supplementation Modulates the Human Skeletal Muscle microRNA Response to Lower Limb Immobilization
Abstract
Limb immobilization results in a rapid loss of muscle size and strength. The resultant alterations in signaling pathways governing myogenesis, catabolism, and mitochondrial biogenesis are likely to include posttranscriptional regulation mediated by altered microRNAs (miRNAs). Given that protein ingestion exerts an anabolic action and may act as a countermeasure to mitigate muscle loss with immobilization, it is important to examine miRNA in this context. The objective of the study is therefore to characterize the vastus lateralis miRNA response to 14 days of disuse in males (45–60 years) randomized to receive supplementation with 20 g d−1 of dairy protein (n = 12) or isocaloric carbohydrate placebo (n = 13). Biopsies are collected before and after a 2-week immobilization period. Of the 24 miRNAs previously identified in myogenic regulation, seven (miR-133a, −206, −15a, −451a, −126, −208b, and let-7e) are increased with immobilization irrespective of group; five (miR-16, −494, let-7a, −7c, and 7d) increased only in the carbohydrate group; and eight (miR-1, −486, −23a, −23b, −26a, −148b, let-7b, and −7g) are divergently expressed between groups (suppressed with protein). The ability of protein supplementation to differentially regulate miRNAs involved in key muscle regulatory pathways following short-term limb immobilization reflects potential protective function in mitigating muscle loss during limb immobilization.
1 Introduction
Skeletal muscle atrophy occurs rapidly in response to periods of disuse such as bedrest,1, 2 limb immobilization, or spaceflight.3 The maintenance of muscle mass is dependent on the dynamic balance between the synthesis (muscle protein synthesis [MPS]) and degradation (muscle protein breakdown [MPB]) of myofibrillar proteins. Muscle disuse results in net protein degradation promoting a loss of muscle contractile proteins, contributing to reductions in muscle mass, oxidative capacity, and contractile function.4-7 Periods of disuse may arise due to illness or injury requiring episodes of bedrest, limb immobilization, or reduced ambulation.8, 9 Experimental models of disuse atrophy include bedrest10, 11 and limb immobilization,12, 13 which involve the unloading of a specific joint replicating what occurs due to bone fractures or impact injuries. A less extreme intervention is step reduction, which in part mimics the reduced mobility characteristics associated with aging.14 Collectively, these models of disuse have been shown to lead to reductions in myofiber size and shifts in fiber types,15 reductions in oxidative capacity due to altered mitochondrial content via downregulation of mitochondrial biogenesis,16, 17 changes in myogenic satellite cell proliferative activity,18, 19 and increased caspase-3 dependent apoptotic signaling mechanisms.20, 21
The molecular mechanisms underpinning the adaptive changes in muscle phenotype with immobilization are undoubtedly complex. One possible mechanism is the role exerted by microRNAs (miRNAs), which impact on many pathways involved in the regulation of skeletal muscle size and function. miRNAs are short noncoding RNAs transcribed within the intronic sequences of genes.22 miRNAs promote transcript degradation, thereby exerting regulatory control over transcriptional processes.23, 24 Previous evidence suggests a widely published set of ≈30 miRNAs as being intricately involved in the regulation of myogenesis, satellite cell mediated repair, fiber type, and hypertrophy (miR-1, −133a, −133b, −206, 208a, −208b, −486, and −499a).25-27 The complete complement of miRNAs involved in skeletal muscle regulation is not yet known, although a consistent number of them have been shown to respond to various exercise and feeding stimuli across multiple animal and human trials.28-31 A number of these miRNAs have been experimentally validated to exert impact on atrophy (miR-23a/b),10, 32, 33 angiogenesis (miR-126),34, 35 regulation of cell cycle (miR-15a, −16, and −451a),36, 37 reactive oxygen species production (miR-1 and 133a),38 glucose and insulin responsiveness (miR-21 and −148b),39, 40 and mitochondrial biogenesis (miR-26a, miR-494).41, 42 Additionally, let-7 miRNAs are reported as having roles in myogenic stem cell meditated repair43, 44 and inflammation and apoptotic signaling45, 46 within ageing skeletal muscle.
Dairy protein ingestion has well established roles in stimulating MPS and inhibiting MPB.47, 48 Protein consumption has been an effective countermeasure to mitigate disuse-induced losses in muscle mass, function, and MPS rates following acute disuse events in several studies.1, 49, 50 Others, however, have demonstrated protein supplementation as having no protective effect on muscle mass and function following acute immobilization events.7, 51 The muscular miRNA response to acute protein consumption has also been explored.52, 53 However, the ability of protein consumption to alter miRNA expression profiles during periods of disuse is unknown. Evidence indicating differential expression of miRNAs in individuals with smaller weaker versus larger stronger muscles suggests the potential role of miRNAs in regulating muscle function.24 The aim of the current study was to analyze the regulation of miRNAs previously identified as having actions within skeletal muscle important for the maintenance of mass, fiber composition, oxidative capacity, and apoptosis—in response to a 2-week unilateral lower limb immobilization as described by Mitchell et. al.7 Further, the actions of dairy protein supplementation, relative to an isocaloric carbohydrate supplement, to modulate the muscle miRNA expression were examined.
2 Experimental Section
2.1 Participants
Twenty-five healthy middle-aged men between the ages of 45 and 60 were recruited from local newspapers. The participants were free from metabolic conditions such as diabetes, musculoskeletal impairments, and had no history of heart disease or cancer, nor a family history of blood clots. Further, current smokers were also excluded from the study. Written consent was obtained before the study began with approval from the Northern Health and Disability Ethics Committee (New Zealand) (14/NTA/146). This paper reports secondary outcomes of a clinical trial7 registered at Australian New Zealand Clinical Trials Registry as ACTRN12615000454572. Twenty-five of the original 30 participants from the original study7 were included in this study as there was insufficient remaining muscle tissue from five participants.
2.2 Supplementation and Immobilization
One week prior to the commencement of immobilization and throughout the 2 weeks of immobilization, participants were provided with controlled breakfast and dinner along with dietary advice for their mid-day meal. The targeted protein intake was 1 g of protein kg−1 d−1 as this amount has been demonstrated to be sufficient to maintain muscle mass in ambulatory men in the upper end of the age range used in the present study.54 Participants in both groups slightly exceeded their mid-day protein intake targets and thus consumed of 1.1 g kg−1 d−1 of protein during the baseline and immobilization phase exclusive of the supplemental beverages, while achieving energy balance throughout the study.7 Participants were randomized to receive daily dietary supplementation with 20 g of milk protein concentrate, where the amino acid composition have been previously reported55 (n = 12, 51.4 ± 3.8 years and 28.2 ± 2.9 kg m−2) (Mean ± SD) or an isocaloric carbohydrate placebo (n = 13, 48.7 ± 2.7 years and 28.3 ± 3.3 kg m−2). Carbohydrate was chosen as a placebo in order to match the energy content of the protein without simulating the anabolic mTORC1 pathway.56 Both supplements were artificially vanilla flavored and sweetened to match taste as closely as possible. The powdered supplements were mixed in ≈150 mL of water and consumed with breakfast as this meal traditionally contains less protein than afternoon or evening meals.57 The total protein intake in the protein group was 1.3 g kg−1 d−1 while the placebo group continued to consume 1.1 g kg−1 d−1 of protein.7 As previously described,7 participants were subjected to a 14-day period of unilateral immobilization of the knee joint on a randomly selected limb at an angle of 60° using a rigid knee brace (DonJoy, IROM, Vista, CA). Participants were provided a set of crutches and asked to avoid any weight bearing on the immobilized leg. The brace was removed and readjusted every 2 days by an investigator who sealed the brace with signed tape to ensure protocol compliance.7, 58
2.3 Muscle Biopsy Sampling
Muscle biopsies (≈100 mg) were collected following an overnight fast from the vastus lateralis muscle under local anesthesia (1% Xylocaine) using a Bergström needle modification of manual suction. The pre-immobilization biopsy was collected from the nonimmobilized leg and the post biopsy was taken from the immobilized leg. Biopsies were snap frozen in liquid nitrogen and stored at −80 °C until further analyses.
2.4 miRNA Isolation/qPCR
Total RNA was extracted from ≈20 mg of muscle tissue using the AllPrep DNA/RNA/miRNA Universal Kit (QIAGEN GmbH, Hilden, Germany) following the manufacturer's instructions as previously described.24 A total of 10 ng of input RNA was used for cDNA synthesis using TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher Scientific, Carlsbad, CA, USA), miR abundance were measured by RT-PCR on a QuantStudio 6 (Thermo Fisher Scientific, Carlsbad, CA, USA) using Applied Biosystems Fast Advanced Master Mix (Thermo Fisher Scientific, Carlsbad, CA).
Target miRNAs were miR −1-3p, −15a-5p, −16-5p, −21-5p, −23a-5p, −23b-5p, −26a-5p, −126-3p, −133a-3p, −133b, −148b-3p, −206, −208a-3p, −208b-3p, −451a, −486-5p, −494-3p, −499a-3p, let-7a-5p, let-7b-5p, let-7c-5p, let-7d-5p, let-7e-5p, and let-7g-5p (Table 1). The geometric mean of three reference miRNAs24, 59 was used for normalization based on miRNAs that showed the least variation among the current sample set (miR-320a, miR-423-5p, and miR-186-5p). The 2−ΔΔCT method was used to compare differences in raw miRNA expression.60
| miR | ID number |
|---|---|
| miR-1-3p | 477820_mir |
| miR-15a-5p | 477858_mir |
| miR-16-5p | 477860_mir |
| mir-21-5p | 477975_mir |
| miR-23a-3p | 478532_mir |
| miR-23b-3p | 478602_mir |
| mir-26a-5p | 477995_mir |
| miR-126-3p | 477887_mir |
| miR-133a-3p | 478511_mir |
| mir-133b | 480871_mir |
| miR-148b-3p | 477806_mir |
| miR-206 | 477968_mir |
| miR-208a-3p | 477819_mir |
| miR-208b-3p | 477806_mir |
| miR-451a | 477968_mir |
| miR-486-5p | 478128_mir |
| mir-494-3p | 478135_mir |
| miR-499a-3p | 478948_mir |
| let-7a-5p | 478575_mir |
| let-7b-5p | 478576_mir |
| let-7c-5p | 478577_mir |
| let-7d-5p | 478439_mir |
| let-7e-5p | 478579_mir |
| let-7g-5p | 478580_mir |
| HK miRs | |
| miR-186-5p | 477940_mir |
| miR-191-5p | 477952_mir |
| miR-320a | 478594_mir |
| miR-361-5p | 478056_mir |
| miR-423-5p | 478090_mir |
2.5 mRNA qPCR
As per Ref. [7], RNA was converted to cDNA and gene expression of PAX7, atrogenes (MuRF1/TRIM63 and Atrogin-1/FBXO32), and SOX6 was analyzed. For normalization, the geometric mean of stable reference genes C1orf43, CHMP2A, EMC7, and VCP was used.7 The 2−ΔΔCT method was used to compare difference in raw gene expression.60 Sequences of primers used for this analysis are shown in Table 2.
| Gene | Sequence |
|---|---|
| PAX 7 (Forward) | CCTTTGGAAGTGTCCACCCC |
| PAX 7 (Reverse) | TCGCCCATTGATGAAGACCC |
| ATROGIN1 (Forward) | GCAGCTGAACAACATTCAGATCAC |
| ATROGIN1 (Reverse) | CAGCCTCTGCATGATGTTCAGT |
| MuRF1 (Forward) | CCTGAGAGCCATTGACTTTGG |
| MuRF1 (Reverse) | CTTCCCTTCTGTGGACTCTTCCT |
| Sox6 (Forward) | GCAAGAACAGATTGCGAGAC |
| Sox6 (Reverse) | AATTGGGATCATGAGCGGAGG |
2.6 Statistical Analysis
Two way repeated measures analysis of variance with time as a within subject factor and group as a between subject factor was conducted using SigmaPlot for Windows version 12.1 (Systat 218 Software Inc., San Jose, USA) to determine differences in miRNA expression. Where appropriate, group and time differences were assessed using Holm–Sidak post-hoc tests. Linear stepwise regression was conducted using SPSS for Windows Version 24 (IBM Corp. USA), in order to determine which miRNAs best explained changes in mRNA for which they are putative regulators. Data are shown as mean ± SEM. Statistical significance was accepted at p < 0.05.
3 Results
3.1 miRNAs Modulated by Immobilization Irrespective of Supplement
miR-133a, −206, −15a, −451a, and −126 increased in both the protein (p = 0.047, p = 0.008, p = 0.043, p = 0.044, and p = 0.048 respectively) and placebo fed (p = 0.049, p = 0.025, p = 0.031, p = 0.029, and p = 0.037 respectively) groups following immobilization. miR-208b demonstrated a main effect of time, indicating increased expression irrespective of supplement group (p = 0.026) with no increases following immobilization with either protein (p = 0.078) or placebo supplementation (p = 0.089) (Figure 1). miR133b and −208a showed nonsignificant trends to increase (p = 0.085 and p = 0.093, respectively) following immobilization. miR-499a and miR-21 did not change with immobilization (p = 0.478 and p = 0.855) (data not shown).
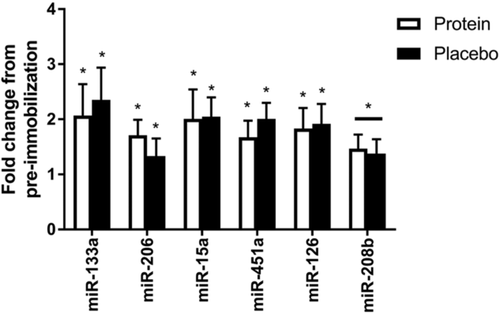
3.2 miRNAs Differentially Expressed with Protein Supplementation During Immobilization
miR-1, −486, − 23a, −23b, −26a, and −148b showed group by time interaction effects, with lower expression in the protein group compared to the placebo group following immobilization (p = 0.003, p = 0.019, p < 0.001, p = 0.002, p = 0.021, and p = 0.035, respectively) (Figure 2). miR-486, −23a, −23b, −26a, −148b, −16, and −494 demonstrated increased abundance from their respective baselines only in the placebo group (p = 0.007, p = 0.004, p = 0.002, p = 0.004, p < 0.001, p = 0.008, and p = 0.022, respectively). miR-16 and −494 displayed nonsignificant trends to be differentially expressed between groups (p = 0.064 and p = 0.06, respectively) following immobilization.

3.3 Let-7 Family Members Modulated with Protein and/or Immobilization
Let-7b and −7g demonstrated group by time interaction effects (p = 0.01 and p = 0.003, respectively) with lower abundance in the protein group compared to the placebo group following immobilization. Let-7a, −7c, −7d, and −7g showed increased abundance from their respective baselines only in the placebo group (p = 0.04, p = 0.022, p = 0.015, and p = 0.002, respectively) while remaining unchanged in the protein group (p = 0.151, p = 0.422, p = 0.162, and p = 0.803, respectively). Let-7e was increased following immobilization in both the protein (p = 0.047) and the placebo fed groups (p = 0.049) (Figure 3).
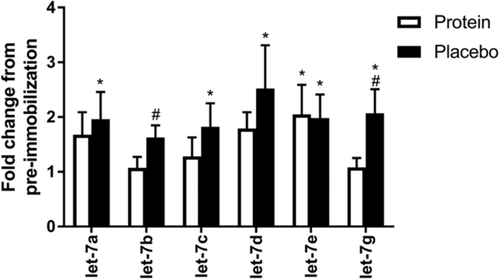
3.4 Relationship Between miRNA and mRNA
Based on previous literature, 11 miRNAs (miR-133a, −206, −486, −1, −23a, −23b, 26a, −148b, let-7a, −7b, and −7e) were identified as putative regulators of PAX7 abundance, however, using a stepwise regression model only fold change in let-7a expression was found to relate to the fold change in PAX7 abundance (r = 0.434 and p = 0.030) (Figure 4A). From the literature, four miRNAs (miR-23a, −23b, −26a, and −148b) were identified as putative regulators of MuRF1 and Atrogin-1, however, only miR-23a was negatively related to Atrogin-1 (r = −0.476 and p = 0.019) (Figure 4B), and miR-26a was positively related to MuRF1(r = 0.589 and p = 0.004) (Figure 4C). While 3 miRNAs (miR-208a, −208b, and −499a) are thought to regulate SOX6 expression, only miR-208b had a positive correlation with SOX6 (r = 0.778 and p < 0.001) (Figure 4D).

4 Discussion
Following short-term lower limb immobilization with either protein or placebo supplementation, this study analyzed the expression of 24 miRNAs previously demonstrated as having roles in regulatory aspects of muscle physiology. It was demonstrated that 18 miRNAs exhibited increased abundances following the intervention in the placebo group, with protein supplementation attenuating this increase in 13 miRNAs. Previously, the reduction in muscle size, knee extension and flexion torque, and synthesis rate of mitochondrial proteins following immobilization has been reported in the same cohort.7 However, these data demonstrated that protein supplementation exerted no detectible impact on muscle mass or function. While our previous report did not include change in muscle fiber size or populations, others suggest reductions in muscle fiber size and changes in fiber populations function following disuse.15, 61
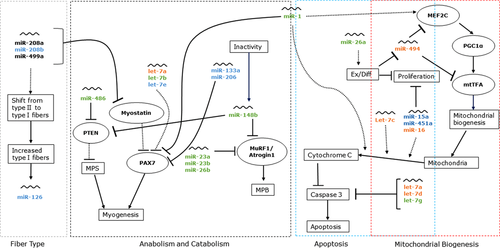
miR-133a, −206, −486, −1, −23a, −23b, 26a, −148b, let-7a, −7b, and −7e are all thought to regulate satellite cell dependent myogenesis via direct or indirect PAX7 inhibition.10, 26, 27, 32, 33 Currently, miR-133a, −206, and let-7e were elevated following immobilization irrespective of supplement. The increases in miR-133a, −206, and let-7e expressions suggest that immobilization inhibits myogenesis and remodeling via satellite cell activity (Figure 5). Reduced satellite cell activity has been observed in sedentary individuals, elderly, and disuse models where muscle mass and function are impaired.62, 63 miR-486, −23a, −23b, 26a, −148b, let-7a, and −7b were differently expressed following immobilization, increasing only in the placebo group, a change that was attenuated with protein supplementation. Differential expression of miR-1, −486, and let-7b between the protein and placebo groups suggests a potential role for protein supplementation to reduce inhibition of myogenesis following immobilization,64, 65 while increased miR-26a is thought to promote cell differentiation and proliferation in response to stress.42 Alterations in the expression of these 11 miRNAs were compared to PAX7 mRNA changes in a stepwise regression model, which demonstrated only let-7a to be related to PAX7 abundance indicating its potential importance for regulating PAX7 during disuse.
Presently we report increased expression of miR-486 and −148b post-immobilization in the placebo, but not the protein group. Both miR-486 and −148b are implicated in promoting Akt signaling via inhibition of PTEN and have the potential to regulate the mTOR pathway downstream of Akt. Long-term MPS was not different between groups in the present cohort7 and disuse-induced atrophy in humans has been found to not alter basal Akt signaling.66 While it is unlikely these miRNAs are acting to regulate mTOR, their upregulation in the placebo group is possibly a result of insulin-dependent activation of Akt signaling26, 67 due to carbohydrate consumption rather than immobilization per se.
miR-23a, −23b, and −148b inhibit catabolism via repression of MuRF1, Atrogin 1, and SMAD proteins.32, 33, 40, 68 Currently we report elevated miR-23a, −23b, −26a, and −148b with placebo consumption, an increase that was attenuated with protein ingestion. miR-148b has previously been shown to increase with reduced physical activity in rats and also following one month of detraining in active men.40 We have previously demonstrated elevated expression of both MuRF1 and Atrogin1 after immobilization irrespective of protein intake.7 While elevated miR-23a currently is inconsistent with previous findings that demonstrate miR-23a was less abundant in mouse cells where MuRF1 was upregulated,69 the presence of a negative relationship between Atrogin-1 and miR-23a supports critical regulation of this mRNA. We also demonstrate a positive relationship between MuRF1 mRNA and miR-26a expression supporting the role of this miRNA in the regulation of catabolic genes. The positive direction of this relationship is unexpected, however, and along with the upregulation of miRNAs, which suppress catabolism (miR-23a, −23b, −26a, and −148b) may signify either involvement of negative feedback, dysregulation of catabolic signaling mechanisms during disuse, or involvement of other gene targets.
miR-208a, −208b, and −499a are encoded within the introns of slow twitch myosin heavy chain genes and are known to inhibit SOX6 activity.70 SOX6 functions to maintain a fast twitch fiber phenotype, suggesting increases in these miRs may promote fast-to-slow fiber shifts.25, 71 Currently miR-208b was increased with immobilization irrespective of group, while miR-208a and −499a were unchanged. However in rat following 28 days of hind limb suspension, declines in miR-499 and −208b (60 and 40%, respectively) with corresponding ≈2.2-fold increases in SOX6 expression were evident.71 Currently of these three miRNAs, just miR-208b demonstrated a positive relationship with SOX6 expression indicating potential negative feedback. The difference between species and atrophy models15 are likely to have contributed to the observed disconnect in perceived fiber type shifts.
Previously, we reported a significant decrease in mitochondrial protein synthesis following immobilization.7 miR-494 inhibits crucial regulators of mitochondrial biogenesis MEF2C and mtTFA.41 Immobilization increased miR-494 expression, while protein supplementation attenuated this increase, suggesting protein supplementation may have supported the maintenance of mitochondrial signaling pathways. Caspase 3 and cytochrome C are key mitochondrial proteins responsible for apoptotic regulation and are downregulated with immobilization-induced atrophy.9, 72 Together, miR-1, −15a, −16, −451a, and let-7c regulate cell cycle function via inhibition of cytochrome C, differentially from let−7a, −7g, and −7d which directly inhibit caspase 3. Immobilization elevated miR-15a and −451a irrespective of group, while miR-1, −16, and let-7c were increased with placebo but not protein ingestion suggesting divergent caspase 3 dependent apoptosis in the placebo compared to the protein group (Figure 5). miR-15a, −16, −1, and let-7c indirectly promote cytochrome C inhibition of caspase 3, thereby attenuating apoptosis. Increased abundance of let-7a and −7g in the placebo group may indicate a negative feedback mechanism preventing caspase 3 activity. Together, these findings demonstrate a potential protective role for protein supplementation on muscle metabolism via the reduced inhibition of mitochondrial biogenesis and apoptotic pathways compared to the placebo group.
Rezen et al. demonstrated 15 of 152 miRNAs were significantly altered with 10 days of bedrest,10 while Rullman et al.11 reported 19 of 455 miRNAs were regulated following 21 days of bedrest in hypoxic conditions. Both reports employed non-targeted analytical approaches (nanostring and array, respectively) that potentially identify lowly expressed miRNAs with minor physiological roles rather than previously established key miRNAs that target mRNAs in pathways known to be dysregulated during atrophy. Currently, ten of the 15 miRNAs reported to be decreased in Rezen et al.10 were shown to be increased following immobilization, while protein supplementation attenuated the increases in let-7a, −7b, −7c, −7d, −7g, miR- 23a, −23b, and 148b. Yet our results are in part consistent with findings from Rullman et al.11 who also reported increases in miR-15 and let-7 family. Two rodent disuse studies73, 74 also report either reductions or no change in miR expression with a tendency to return to resting levels following reloading. The current brace based limb immobilization model is likely less severe and mechanistically distinct from bedrest or even hind limb suspensions models, which along with differences in techniques used to detect miRNA could explain discrepancies between the present study and previous work. For example, miR-133a was reduced with bedrest, whereas it increased in the present model.11, 75 Furthermore, there remains conflicting evidence regarding the ability of protein ingestion to attenuate disuse atrophy1, 7, 50, 51, 76, 77 with the previously reported analysis from this current study failing to identify an effect of protein supplementation.7
While no phenotype differences were shown between supplement groups,7 divergent miRNA expression may reflect differential regulation of signaling pathways involved in muscle function. Therefore, the lack of phenotype differences is a limitation in understanding the mechanisms that underpin the differential miRNA expression. It is possible, however, that had the immobilization event been longer or the dose of protein larger, phenotype type differences may have become evident. From the current findings, it is evident that 2 weeks of lower limb immobilization is sufficient to alter basal expression of muscle regulatory miRNAs. Supplementation with 20 g of dairy protein in middle-aged men with matched diets appears to attenuate changes in expression of some miRNAs during limb immobilization, indicative of differential regulation of the biochemical signaling pathways involved in muscle maintenance.
Abbreviations
-
- miRNA
-
- microRNA
-
- MPS
-
- muscle protein synthesis
-
- MPB
-
- muscle protein breakdown
Acknowledgments
The authors acknowledge vital help from Anja Blix and Karl L. Filipsson in the smooth completion of the study.
C.J.M., R.F.D., and D.C-S. received financial support from the New Zealand Primary Growth Partnership (PGP) post-farm gate program, funded by Fonterra Co-operative Group Ltd and the NZ Ministry for Primary Industries (MPI), to conduct this study. S.D.P. is the Fonterra Chair in Human Nutrition, University of Auckland; A.C.F. is a current employee of Fonterra Co-operative Group Ltd. The other authors declare no competing financial interests.
The study was designed by C.J.M., D.C-S., S.D.P., and A.C.F. Performed experiments: R.F.D., N.Z., and V.C.F. Sample Collection: R.F.D., C.J.M., B.R.D., and J.F.M. Analyzed data: R.F.D. Critically evaluated and contributed to the manuscript: R.F.D., J.F.M., S.D.P., C.J.M., and D.C-S. C.J.M. is responsible for the final content of the manuscript.
Conflict of Interest
The authors declare no conflict of interest.




