The Efficacy of Nanoemulsion-Based Delivery to Improve Vitamin D Absorption: Comparison of In Vitro and In Vivo Studies
Abstract
Scope
Vitamin D (VD) is a fat-soluble vitamin that has a wide range of skeletal and non-skeletal functions. Although it can be synthesized through sun exposure and obtained from fortified foods, VD inadequacy is epidemic worldwide. Therefore innovative strategies are necessary for improving VD status. The present study examined VD absorption via nanoscale delivery systems.
Methods and results
We examine the physical characteristics and in vitro bioaccessibility of cholecalciferol (VD3) in nanoemulsion using a simulated gastrointestinal tract system. To evaluate the in vivo bioavailability, we orally administer three groups of mice with VD3 nanoemulsion, VD3 coarse emulsion, or vehicle nanoemulsion without VD3, and the serum 25(OH)D3 is measured using radioactive immunoassay. The nanoemulsion-based delivery system increases the in vitro bioaccessibility by 3.94-folds (p < 0.05), as indicated by the concentration of vitamin D3 in micelles. Our animal study shows that, when compared to the vehicle group, the coarse emulsion numerically increases the serum 25(OH)D3 by 36%, whereas the nanoemulsion statistically significantly increases the serum 25(OH)D3 by 73% (p < 0.01).
Conclusion
Our findings indicate that a nanoemulsion-based delivery system is a promising approach to improve VD bioavailability, and further studies are warranted to determine its efficacy in humans.
1 Introduction
Vitamin D (VD) is a fat-soluble vitamin that has a wide range of skeletal and non-skeletal functions. In addition to its importance in calcium absorption and bone health,1, 2 deficiency of VD has been associated with increased risks of a wide range of diseases, including cancers, cardiovascular diseases, immune, and inflammatory diseases.3 Although people can get VD through sunlight exposure and by consuming VD-fortified foods, about one-third of the population in the US is still at the risk of VD inadequacy (i.e., 30–49 nmol L–1 in blood of 25(OH)D3) or deficiency (i.e., <30 nmol L–1).4, 5 About one billion people worldwide have been estimated to have VD insufficiency.3 More importantly, even with the wide application of food fortification, VD deficiency is still increasing.5-8
Unlimitedly increasing the fortification level of VD in foods to combat VD deficiency may not be a feasible solution, since the benefits of most fat-soluble nutrients and dietary bioactive components follow a “U” shape—with risks at both low and high levels of consumption. Therefore, to improve VD status cannot be through unlimitedly increasing the supplemental level, otherwise it may put a subgroup of the population at a risk of exposure to high level of VD. Indeed, it has been estimated that around 1% of population in US may be at a possibly harmful level (>125 nmol L–1).5 Thus, improving the oral VD bioavailability and reducing the variation of VD absorption, rather than simply increasing the supplemental level, should be a better strategy to improve VD status for public health.
The bioavailability of VD is typically relatively low because its strong hydrophobicity leads to a low solubility in aqueous fluids, such as those in the gastrointestinal tract (GIT). Therefore, VD is often delivered in oil-in-water emulsion that is specifically designed to enhance its bioaccessibility by improving its solubility and the formation of mixed micelles.9, 10 Nanotechnology has witnessed a rapid growth in recent decades,11 and along with the emergence of nanotechnology, the utilization of nanoemulsion (d < 200 nm) over conventional coarse emulsion (d > 200 nm) as delivery vehicles for lipophilic nutrients and bioactives have received substantial attention in nutrition and food industry.12, 13 There are a number of physiochemical advantages associated with dietary nanoparticles, such as greater stability, lesser aggregation, and higher optical clarity.12, 14 Moreover, lipid nanoparticles tend to be digested more rapidly within the gastrointestinal tract because of its smaller dimension and large surface area.15
A significant number of in vitro studies have shown that reducing lipid nanoparticle size increases the bioaccessibility of hydrophobic components, e.g., curcumin16 and carotenoid,17 but in vivo studies are limited and some inconsistent findings from in vitro and in vivo studies have been reported.18 The present study aimed to determine the influence of nanoemulsion, comparing to a conventional coarse emulsion,19 on VD absorption using both in vitro and in vivo models. We examined the relative bioavailability, the improvement of VD absorption, as well as the influence on the expression of VD metabolically-related genes.
2 Experimental Section
2.1 Nanoemulsion Fabrication
To prepare oil-in-water nanoemulsion, cholecalciferol (VD3), the general supplemental form of VD, was purchased from Sigma–Aldrich (St. Louis, MO, USA, with a purity ≥ 98%). Corn oil was purchased from a food supplier (Mazola, ACH Food Companies, Inc., Memphis, TN). Quillaja saponin (Q-Naturale 200V) was kindly donated by Ingredion Inc. (Westchester, IL). All chemicals used were of analytical grade. Double distilled water was used to prepare all solutions and emulsions. A high-speed mixer (M133/1281-0, Biospec Products, Inc. Bartlesville, OK, USA) and high-pressure homogenizer (Microfluidics M110L, Newton, MA, USA) were used to make nanoemulsions. We prepared VD3-loaded oil-in-water nano- and coarse emulsions using the published procedures.19 Refer to the Supporting information Methods file for more details for the nanoemulsion fabrication.
2.2 The Simulated Gastrointestinal Tract System for the Determination of In Vitro Bioaccessibility
An in vitro gastrointestinal tract (GIT) system consisting of mouth, stomach, and small intestine phases was used to mimic the potential gastrointestinal conditions at each stage. Mucin, pepsin, bile salts, lipase, mono-, and di-basic sodium phosphates were purchased from Sigma–Aldrich. This model is based on previous studies for the development of standardized in vitro GIT models with slight modifications.20, 21 The samples were diluted fivefold in sodium phosphate buffer prior to passing them through the in vitro GIT system. Details for the in vitro GIT system and the determination of the in vitro bioaccessibility was described in the Supporting information Methods file.
2.3 Particle Characterization
The physicochemical properties of the initial emulsions, as well as solutions after exposure to in vitro digestion phases (mouth, stomach, and small intestine), were characterized in terms of the particle diameter and ζ-potential. The particle diameters of the samples were measured using a laser diffraction particle size analyzer (LS 13 320, Beckman Coulter, Brea, CA, USA). The particle size of each sample is reported as the surface-weighted mean diameter (d32). The particle charge (ζ-potentials) was measured using a particle electrophoresis instrument (Zetasizer Nano ZS, Malvern Instruments, Malvern, England). Samples were diluted in 10 mm phosphate buffer solution at the proper pH (corresponding to the initial, mouth, stomach, or small intestine conditions) to avoid multiple scattering.
2.4 Animal Study
An animal study was conducted to investigate the in vivo bioavailability of VD3 in nano- or coarse emulsions. The protocol (2013-0070) was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts, Amherst. 15 C57BL/6 mice (8-week-old) were purchased from Jackson Laboratory, Bar Harbor, ME, and housed in our vivarium (1–2 animals per cage) with a 12-h-light/-dark cycle and with free access to a standard AIN93M diet containing 1000 IU VD3 kg–1 diet.22 According to a previous study,23 the serum 25(OH)VD3, a standard blood biomarker for VD status,24 is sensitive to VD consumption up to 20 000 IU kg–1 diet. We therefore supplemented an extra 100 ug L–1 VD via oral administration of the emulsions, which was diluted from the original emulsion (100 mg L–1) and is equivalent to an extra 4000 IU kg–1 of VD supplementation in diet (1 ug = 40 IU) based on an assumption that animals consume an averagely equal amount of food and water (≈5 g of food and ≈5 mL of water for an adult mouse).25 The administration was implemented by replacing the water bottle with the bottle containing VD coarse or nano-emulsions. The intake and stability of the emulsions were monitored during the feeding period and the consumptions were not different among the three groups. Therefore, the VD concentrations for the three groups—the vehicle, the VD coarse emulsion, and the VD nanoemulsion group—were equivalent to 1000, 5000, and 5000 IU kg–1 of diet respectively. Our in vitro study showed that the nanoemulsion could increase the bioaccessibility by approximately fourfolds, and thus the nanoemulsion group might receive a concentration equivalent to 20 000 IU kg–1 diet via conventional administration, which is still within the sensitive dose response of serum 25(OH)VD3.23
2.5 Biochemical and Molecular Analysis
2.5.1 Serum 25(OH)VD3 Assay
After 3 days of feeding, animals were euthanized by CO2 asphyxiation followed by cervical dislocation and exsanguination by cardiac puncture. The blood was collected and serum 25(OH)VD3 was measured by radioimmunoassay at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University by radioactive immunoassay using the kit from DiaSorin (DiaSorin, Stillwater, MN, USA).
2.5.2 Quantitative Real-Time PCR
From the harvested tissue samples (small intestinal epithelial mucosa, liver and kidney), total RNA was isolated with TRIzol using the manufacturer's protocol (Life technologies, Grand Island, NY). The concentrations of total RNA were determined spectrophotometrically (NanoDrop Lite, ThermoFisher Scientific, Waltham, MA). cDNA was synthesized from 1 μg of total RNA using the protocol from Takara's PrimeScript RT reagent kit (Takara Bio USA, Inc, CA, USA). Real-time PCR was performed on the ViiA™ 7 Real-Time PCR System (Applied Biosystems, Foster City, CA) utilizing the following thermal cycling conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 60 s. Primer sequences were listed in Supporting information Table S1.
2.6 Statistics
Data are expressed as means ± SEM. Data analysis was performed using SAS (Version 9.4, SAS Institute, Cary, NC). Comparisons between groups were made using ANOVA. Associations between variables were assessed by Pearson's correlation. Bartlett's Test was used for assessing homogeneity of variation. A multivariate approach was used for the repeated measures of the free fatty acid release. Statistical significance was accepted when p < 0.05 or, when multiple comparison conducted, a false discovery rate cutoff of q < 0.2 was used. For the gene expression data analysis, the expression of each gene was normalized to the housekeeping gene GAPDH (Cttarget gene-CtGAPDH). Statistical analyses were performed based on ΔCt, and the relative gene expression were reported as 2−ΔΔCt, where ΔΔCt = ΔCtExperiment-ΔCtControl.
3 Results and Discussion
3.1 Physical Characteristics of Particles Across the Simulated GIT Phases
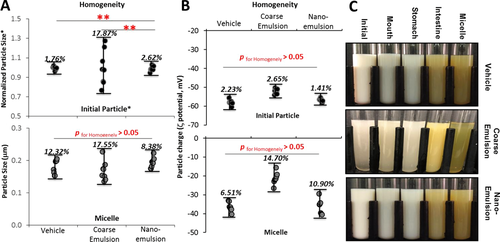
As expected, for the initial emulsions, the mean particle diameter of the coarse emulsion was ≈20-fold higher (p < 0.01) than that of the nanoemulsion groups. The particle sizes for the nanoemulsions with or without cholecalciferol were not different from each other (Figure 1), which indicated that incorporation of the oil-soluble VD3 did not interfere with the formation of nanoparticles. The homogeneity of the particle size in the initial phases was significantly higher for the nanoemulsion than that for the coarse emulsion (p < 0.01), as indicated by the smaller coefficient of variation (Figure 3A). The natural surfactant (Q-Naturale) used in this study has previously shown to be a highly effective emulsifier: (i) it rapidly adsorbs to oil droplet surfaces during homogenization, thereby leading to small initial droplets; and (ii) it forms a protective coating around the droplet surfaces that inhibits coalescence and flocculation through a combination of steric and electrostatic repulsion.26 There was a slight increase in the mean particle diameters of the nanoemulsions after incubation in the simulated saliva and gastric fluids (Figure 1), which is consistent with the previous report.19 The mucin in the simulated saliva may promote bridging, whereas the low pH and high ionic strength in the gastric fluids may alter the colloidal interactions between the oil droplets.27 There was an appreciable reduction in the particle size after they were incubated in the small intestine phase (Figure 1). These changes may have occurred for a number of reasons, such as lipid digestion, mixed micelle formation, and generation of insoluble sediments.19 The variation of particle sizes in the mixed micelle phase for the coarse emulsion remained higher when comparing to the nanoemulsion groups though it did not reach statistical significance (Figure 3A).
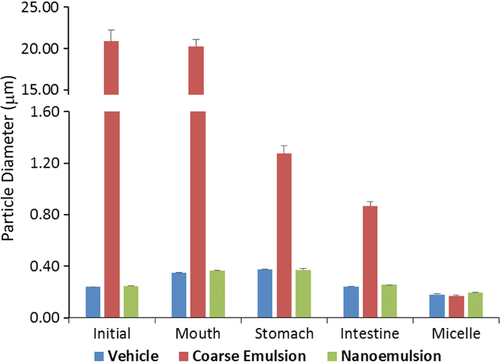
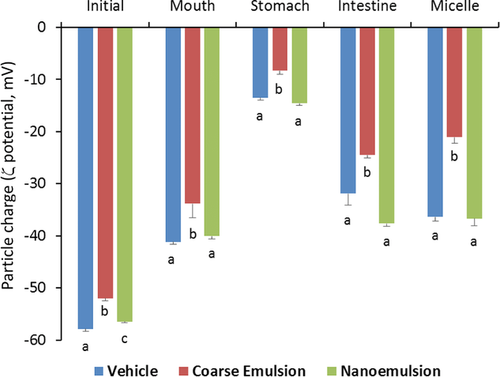
The electrical charge (ζ-potential) on the particles was measured after each stage of the GIT model to provide some information about changes in interfacial characteristics (Figure 2). In all the phases (initial, mouth, stomach, intestine, and micelle), the magnitude of the negative charges for particles in the coarse emulsion was significantly less than those particles in nanoemulsions with or without cholecalciferol (p < 0.05). The pattern of the negative charge changes across all the phases was similar for all three groups, which is in agreement with previous studies.19 The magnitude of the negative charges on the particles decreased after exposure to simulated mouth saliva and gastric fluids. After exposure to the simulated small intestinal solution, the magnitudes of the negative charge on all emulsions increased appreciably. Unlike the particle size, the coefficients of variations for the electronic charges were not statistically different among the three groups (Figure 3B). The digital photographs of the three groups across all phases are shown in Figure 3C, with a minimal difference for the coarse emulsion. In particular, the coarse emulsions appeared less opaque than the nanoemulsions, which was due to the lower degree of light scattering for the larger droplets. In addition, there was evidence of a thin layer of droplets at the top of the coarse emulsions, which can be attributed to the increase in the creaming velocity with increasing droplet size.
3.2 In Vitro Vitamin D Bioaccessibility
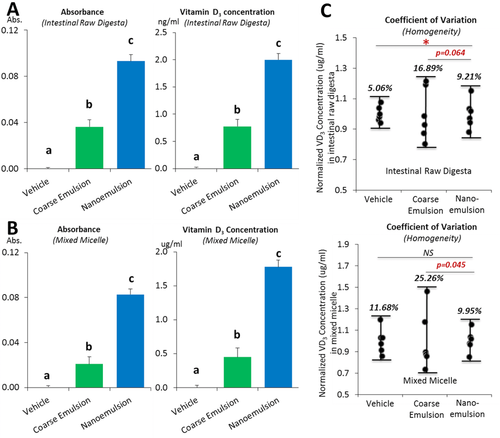
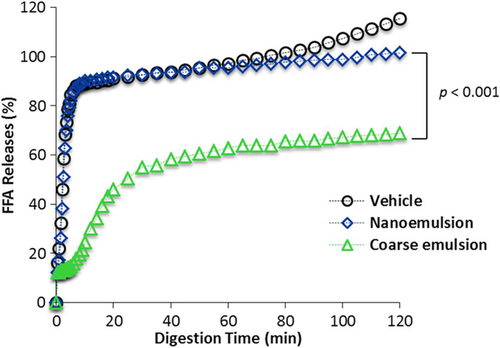
The VD3 concentrations in the intestinal total digesta and mixed micelle were measured after the small intestinal phase using solvent extraction and UV–Vis spectrophotometry. The absorbance was normalized to the empty vehicle control group, and the relative VD bioaccessibility was calculated by dividing the VD concentration in the nanoemulsion by the VD concentration in the coarse emulsion. The VD3 concentration in the total intestinal digesta of the VD nanoemulsion group is 2.58-fold higher (p < 0.01) than the coarse emulsion group (Figure 4A), suggesting that there was less cholecalciferol degradation or other losses in the GIT when the VD was encapsulated in the nanoemulsion. The VD3 concentration in the mixed micelle solution of the nanoemulsion group is 3.94-fold higher (p < 0.01) than the conventional coarse emulsion group (Figure 4B), suggesting that nanoemulsification improved the incorporation of cholecalciferol into the mixed micelles. As a result, there would be a higher level of VD available for absorption by intestinal epithelial cells. The homogeneity test showed that the coefficients of variation for the VD3 concentration in the intestinal raw digesta (16.89 vs 9.21%, p = 0.064) and mixed micelle (25.26 vs 9.95%, p = 0. 045) of the VD coarse emulsion group were higher than that of the nanoemulsion group (Figure 4C). The homogeneity data of the bioaccessibility were consistent with the data of the particle size (Figure 3A). For the free fatty acid release (Figure 5), there was a rapid release for the nanoemulsions (both in the presence and absence of VD) throughout the first 10 min, followed by a slow release at longer incubation times, and all lipids were digested at the end of the 120 min incubation. However, the release of free fatty acids for the VD coarse emulsion was significantly slower than that for the nanoemulsions (p < 0.01), and at the end of 120 min incubation, there were only 69% of the lipids were digested for the coarse emulsion. The observed differences in the rate and extent of lipid digestion can be attributed to differences in the specific surface areas of the systems. Nanoemulsions have a much higher surface area per unit amount of lipid than coarse emulsions, and so there is more room for lipase molecules to adsorb and hydrolyze the triacyglycerols.
3.3 In Vivo Vitamin D Bioavailability
After observing the improvement of in vitro bioaccessibility, we further examined the in vivo bioavailability of VD in nanoemulsion or coarse emulsion using an animal study (Figure 6A). When compared to the vehicle control group that has a baseline of 1000 IU kg–1 of VD in the diet but without VD in the nanoemulsion, the supplementation of cholecalciferol (4000 IU kg–1 equivalent) via oral administration of coarse emulsion increased the serum 25(OH)VD3 level by 36.04% but did not reach a statistically significant degree (13.1 ± 0.09 vs 17.9 ± 2.82 ng mL–1, p > 0.05), whereas the supplementation of VD3 (4000 IU kg–1 equivalent) via nanoemulsion significantly increased the serum concentration of 25(OH)D3 by 73.10% (13.1 ± 0.09 vs 22.7 ± 1.10 ng mL–1, p < 0.01). By using the serum 25(OH)VD3, which is considered as the single best biomarker for blood VD status,24 this study established the correlation between in vitro and in vivo methods, which was not observed in our previous study where vitamin D2 was used as the biomarker.18 This study is consistent with evidence from previous studies which showed that the nanotechnology-based oral administration of dietary bioactive components (curcumin) or commonly used drugs (ibuprofen) were able to improve their biological functions (pancreatic cancer prevention up to tenfolds).28, 29 Further studies are necessary to determine the biological consequence of the improved VD bioavailability by nanoemulsion. A higher coefficient of variation of the in vivo bioavailability (Figure 6B) for the coarse emulsion group was consistent with that of in vitro bioaccessibility (Figure 4C) and particle size (Figure 3A), indicating that the nanoemulsification can help to decrease the heterogeneity of VD absorption.

3.4 The Expression of VD Metabolically-Related Genes
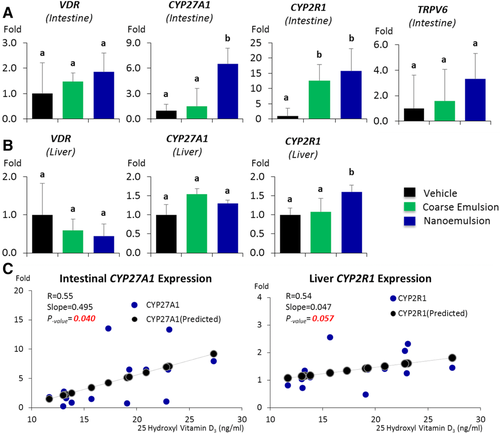
Using real-time PCR, we examined the expression of VDR, Cyp27A1, Cyp2R1, and Trpv6 in intestinal epithelial mucosa, VDR, Cyp27A1, and Cyp2R1 in liver, and VDR, Cyp27B1, and Cyp24A1 in kidney. These genes participate in VD metabolism and their expression is potentially inducible by VD intake. We observed a significant increase of intestinal Cyp27A1 expression in the VD nanoemulsion group when compared to the vehicle and coarse emulsion groups, a significant increase of the intestinal Cyp2R1 expression in the VD nanoemulsion and coarse emulsion groups when compared to the vehicle group (Figure 7A, p < 0.05), and a significant increase of liver Cyp2R1 expression in the VD nanoemulsion group when compared to the vehicle and coarse emulsion groups (Figure 7B, p < 0.05). No differences were observed for the genes examined in the kidney. The expression of intestinal Cyp27A1 (p = 0.04) and liver Cyp2R1 (p = 0.057) was correlated with the serum 25(OH)VD3 level (Figure 7C). In Fleet's study, a steady decrease for the Cyp27B1 expression was observed in the kidney.23 The nonsignificance for Cyp27B1 expression across the groups observed in this study might be attributed to the short feeding period in our study (3 days) compared to the rat (4 weeks) and mouse study (7 weeks) in Fleet's studies. Nevertheless, the increase of Cyp27A1 and Cyp2R1 in intestine or liver samples for the VD nanoemulsion or coarse emulsion groups, and correlations of their expressions with serum 25(OH)VD3 level demonstrated the response of these VD metabolically-related genes with the VD supplementation via nano- or coarse emulsion. Particularly, the statistically significant increases of intestinal Cyp27A1 expression and liver Cyp2R1 expression in the VD nanoemulsion group compared to the coarse emulsion group indicated an improvement of VD absorption by nanoemulsion. The gene expression data for all genes measured are shown in Supporting Information Table S1.
It is noteworthy that several improvements can be considered for future studies. First, this study demonstrated that the coarse emulsion solution was not as homogeneous as nanoemulsion. The relative high heterogeneity of the coarse emulsion, compared to the nanoemulsion, was reflected in the significantly larger coefficients of variations of the initial particle size, the in vitro bioaccessibility, and in vivo bioavailability. It should also be noted that a thin layer of creaming was observed in the coarse emulsions during the course of the study. The creaming made it difficult to ensure that VD was consumed completely and evenly, and this reflected in the higher heterogeneity of the in vivo bioavailability in the coarse emulsion group. In future studies, it will be important to overcome this problem, e.g., by converting the samples into powders or adding a thickening agent to inhibit creaming. Second, although the serum 25(OH)VD3 is responsive to a high VD supplementation level up to 20 000 IU kg–1,23 the magnitude of changes in serum 25(OH)VD3 at the equivalent 5000 IU kg–1 supplementation might not reflect that at other dosage, e.g., 1000 IU kg–1 for rodent diet. Therefore, studies with a series of dosage of VD supplementation are necessary in the future. Third, the feeding period only lasted for 3 days. Although the peak of serum 25(OH)VD3 level should be reached after 12–24 h following the feeding, but a steady level might request more time to establish. It might also take more time for animals to establish a steady state level of the expression of genes related to VD metabolism, and the influences of the nano-sized particles on pathophysiological properties, such as intestinal immune system and gut microbiome, might also not be established. A longer feeding period is recommended for future studies. Nevertheless, the present study clearly demonstrated that the nano-based delivery system improved the bioavailability and homogeneity of VD absorption.
4 Concluding Remarks
VD insufficiency has become epidemic in the United States, ≈32% of the population suffering from this condition.5 Lower VD status is particularly prevalent in populations with more pigmented skin, urban residences, and people living in areas with latitudes above 37 degrees in the US.4 In addition to well-established factors such as season, VD fortified food, and the use of VD supplementation that contribute to improve VD status, body fat (or BMI), and use of oral contraceptive pills are risk factors for VD inadequacy.30-32 With the concept that the health benefit of VD intake, similar to other fat-soluble nutrients, follows a “U” shape, with risks at both low and high levels,5 more practicable strategies, rather than unlimited increases of the supplemental level of VD, are necessary to improve VD status. In this study, we demonstrated that the nanoemulsification significantly improved VD absorption from both in vitro (3.94-folds) using a simulated GIT model and in vivo studies as indicated by the increased serum 25(OH)VD3. The VD nanoemulsion also reduced the variation of VD absorption as indicated by the significantly reduced coefficient of variation of various variables measured when compared to the coarse emulsion. The improvement of VD bioavailability and homogeneity of VD absorption via nanoemulsion would not only improve VD absorption but also reduce the risk for some individuals being exposing to access VD. Although several limitations exist in study as described above, the results demonstrated that the nanoemulsion has the potential to be utilized as a novel strategy to deliver VD in certain applications, and thereby help to improve VD status, which remains a critical public health issue in our society.5
Abbreviations
-
- GIT
-
- gastrointestinal tract
-
- VD
-
- vitamin D
-
- VD3
-
- cholecalciferol
Acknowledgments
A.S.K. and C.G. contributed equally to this work. The food technology section was completed by A.S.K. and C.E.G. in D.J.M.’s laboratory. The nutrition section was completed by C.G., A.S.K., and A.B. in Z.L.’s laboratory. C.G. contributed to a portion of the data analysis. Z.L. and D.J.M. wrote the manuscript with contributions from R.J.W.
This project is supported in part by the USDA/NIFA (2014-67017-21762), USDA/Hatch (MAS00454) and UMass Honors Research Grant.
Conflict of interest
The authors declare no conflict of interest.




