Piperonal attenuates visceral adiposity in mice fed a high-fat diet: potential involvement of the adenylate cyclase–protein kinase A dependent pathway
Colour Online: See the article online to view Figs. 1–5 in colour.
Abstract
Scope
Piperonal is an aromatic compound found in vanilla and has a floral odor resembling vanillin. This study was aimed to test whether piperonal attenuates visceral adiposity induced by a high-fat diet (HFD) in mice and to explore the underlying molecular mechanisms.
Methods and results
Male C57BL/6N mice were fed a normal diet, HFD, or 0.05% piperonal-supplemented HFD (PSD) for 10 weeks. PSD-fed mice showed attenuation of body weight gain, total visceral fat pad weights, and plasma lipid levels compared to HFD-fed mice. Piperonal supplementation of the HFD increased the mRNA expression of certain isotypes of adenylate cyclase (Adcy) and protein kinase A (PKA) in the white adipose tissue (WAT) of mice. The adipogenesis-related genes were downregulated, whereas fatty acid oxidation- and thermogenesis-related genes were upregulated in the WAT of PSD-fed mice compared to those in HFD-fed mice. Piperonal directly activated Adcy by decreasing the Km for its substrate (ATP) in plasma membranes prepared from the WAT of mice. Furthermore, piperonal-induced inhibition of adipocyte differentiation and elevation of Adcy and PKA activities in 3T3-L1 cells were abrogated by an Adcy inhibitor.
Conclusion
The anti-adipogenic effect of piperonal in mice fed the high-fat diet appears to be associated with increased Adcy–PKA signaling in WAT.
Abbreviations
-
- Adcy
-
- Adenylate cyclase
-
- ALT
-
- alanine transaminase
-
- AMPK
-
- AMP-activated protein kinase
-
- aP2
-
- adipocyte fatty acid-binding protein
-
- AST
-
- aspartate aminotransferase
-
- ATP
-
- adenosine triphosphate
-
- cAMP
-
- cyclic adenosine monophosphate
-
- CEBPα
-
- CCAT/enhancer-binding protein α
-
- CPT
-
- carnitine palmitoyl transferase
-
- CREB
-
- cAMP-responsive element-binding protein
-
- FAS
-
- fatty acid synthase
-
- FBS
-
- fetal bovine serum
-
- FDA
-
- Food and Drug Administration
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- HFD
-
- high-fat diet
-
- HSL
-
- hormone-sensitive lipase
-
- mTOR
-
- mammalian target of rapamycin
-
- ND
-
- normal diet
-
- NOAEL
-
- no observed adverse effect level
-
- PGC-1α
-
- proliferator-activated receptor γ 2 coactivator 1 alpha
-
- PKA
-
- protein kinase A
-
- PPARγ2
-
- proliferator-activated receptor gamma 2
-
- PSD
-
- 0.05% piperonal-supplemented diet
-
- RAR
-
- retinoic acid receptor
-
- Rheb
-
- Ras homolog enriched in brain
-
- RXR
-
- retinoid X receptor
-
- TFAM
-
- transcription factor A, mitochondrial
-
- TSC1
-
- tuberous sclerosis 1
-
- UCP1
-
- uncoupling protein 1
-
- WAT
-
- white adipose tissue
1 Introduction
Obesity is a chronic disease of energy imbalance, where a long-term excess of energy intake over expenditure leads to the loading of that extra energy as triglycerides in white adipose tissues (WATs) 1. Increased fat mass is associated with recruitment and activation of T lymphocytes and macrophages in WAT 2, in turn resulting in increased expression of proinflammatory cytokines in WAT and systemic overabundance of inflammatory cytokines sooner or later. In obese subjects, the ability of WAT to accommodate surplus triglycerides can be exhausted, leading to the abnormal accumulation of triglycerides in other tissues. Elevated cellular triglyceride content in muscle and liver under “metaflammation” circumstances is associated with physiological dysfunction of those tissues and may be a primary contributing factor for the development of insulin resistance and nonalcoholic fatty liver disease 3. To date, pharmacological approaches to the management of obesity have used two distinct strategies: either targeting of satiety centers in the brain or reduction of the efficiency of fat absorption in the gastrointestinal tract 1. In the meantime, targeting the WAT lipid metabolism has also become an alternative strategy for treatment of the metabolic conditions associated with obesity.
Modulation of adenylate cyclases (Adcys) via stimulatory G protein-coupled receptors leads to an increase in cyclic adenosine monophosphate (cAMP) concentration and the subsequent activation of protein kinase A (PKA), a ubiquitous cellular multiunit kinase that phosphorylates serine and threonine residues of downstream effector proteins 4. PKA stimulation is known to increase phosphorylation of AMP-activated protein kinase (AMPK), hormone-sensitive lipase (HSL), and cAMP-responsive element-binding protein (CREB) in WAT 5-7. AMPK activation inhibits adipocyte differentiation and blocks the expression of late adipogenic markers such as fatty acid synthase (FAS) and transcription factors such as proliferator-activated receptor gamma 2 (PPARγ2) and CCAT/enhancer-binding protein α (CEBPα), thereby inhibiting adipogenesis 5. HSL phosphorylation releases free fatty acids from intracellular lipid stores, which are then transformed into acyl-CoA and enter the fatty acid oxidation pathway through carnitine palmitoyl transferase (CPT)-1, the rate-limiting enzyme that controls the transfer of cytosolic fatty acids into the mitochondria for β-oxidation 6. Last, CREB activation promotes transcription of the genes involved in thermogenesis, such as proliferator-activated receptor γ 2 coactivator 1 alpha (PGC-1α) and uncoupling protein 1 (UCP1), resulting in increased thermogenesis in adipocytes 7, 8. Therefore, the Adcy/PKA-dependent pathway plays a key role in the regulation of triglyceride storage or mobilization in WAT.
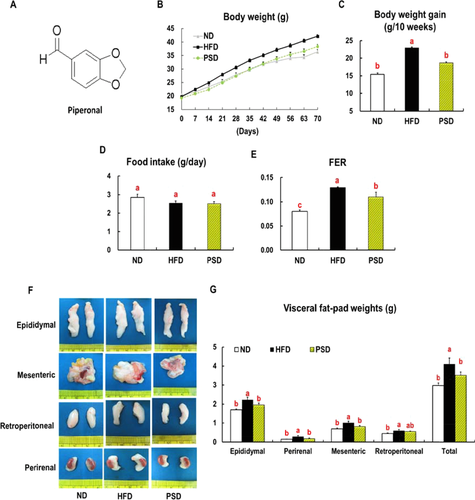
Piperonal (C8H6O3) is an aromatic compound (Fig. 1A) that naturally occurs in various plants, such as dill, vanilla, violet flowers, and black pepper 9. The richest natural source of piperonal is Vanilla planifolia pods, which, when dried, contain 10.3 mg of piperonal per gram 10. Piperonal has a floral odor commonly described as resembling vanillin or cherry; for this reason, piperonal has been used as a flavoring ingredient in confectionary, beverage, and bakery industries 11. Patients who receive olfactory stimuli with piperonal show a significant decrease in anxiety and distress during magnetic resonance imaging 12. In addition, piperonal is known to inhibit the growth of several foodborne bacterial pathogens, including Yersinia enterocolitica, Listeria monocytogenes, and Salmonella typhimurium 13. Our earlier work revealed that piperonal improves hepatic lipid accumulation and insulin resistance in mice fed a high-fat diet (HFD) via activation of the adiponectin–AMPK pathway 14. In the present study, we aimed to determine whether piperonal attenuates visceral adiposity in mice with diet-induced obesity and to explore the possible mechanisms underlying the lipid and/or energy metabolism in WAT.
2 Materials and methods
2.1 Animals and diets
Eighteen C57BL/6N mice (5 weeks of age) were purchased from Orient Bio, Gyeonggi-do, Korea. The mice were housed at a pathogen-free facility at 21°C ± 2.0°C, with 50% ± 5% relative humidity and a 12-h light/dark cycle. After a 1-week acclimation period, the mice were randomly distributed into three experimental groups (n = 6 each): normal diet (ND) group, HFD group, and 0.05% piperonal-supplemented HFD (PSD) group. The ND group was fed the purified diet based on the AIN-76 rodent diet composition. The HFD was identical to the ND except for the addition of 200 g fat/kg (170 g of lard plus 30 g of corn oil) and 1% cholesterol. The PSD group was fed the HFD diet supplemented with 0.05% of piperonal (w/w). The experimental diets were provided ad libitum for 10 weeks in the form of pellets. Food intake of the mice was recorded daily, and their body weights were measured weekly during the feeding period. At the end of the experimental period, the animals were anesthetized after 6-h fasting. Blood was collected from the anesthetized mice, and the plasma samples were prepared by centrifuging the blood at 2000 × g for 15 min at 4°C, with subsequent storage at −80°C. The visceral fat pads were harvested, weighed, and stored at −80°C until further use. All animal experiments were performed in accordance with the Korea Food and Drug Administration (FDA) guidelines. All the experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Yonsei Laboratory Animal Research Center (Permit no. 2010-0039).
2.2 Biochemical analyses
Plasma concentrations of triglycerides, total cholesterol, free fatty acids, LDL-VLDL cholesterol, HDL-cholesterol, glucose, and activities of alanine transaminase (ALT) and aspartate aminotransferase (AST) were measured using commercial kits (Bio-Clinical System, Gyeonggi-do, South Korea). Plasma levels of adiponectin, tumor necrosis factor-alpha (TNF-α), interlukin-6 (IL-6) and leptin were measured by means of a commercially available mouse ELISA kit (Millipore, MA, USA).
2.3 An Adcy enzymatic assay
The plasma membranes from frozen epididymal adipose tissues of C57BL/6N mice (16 weeks of age) were extracted using HEPES-sucrose buffer (20 mM HEPES, pH 7.4, 4 mM EDTA, 250 mM sucrose, two tablets of a protein inhibitor, 1 mM sodium orthovanadate, and 1% of Triton X-100). The homogenates were centrifuged at 2000 × g for 5 min at 4°C, and the supernatants were centrifuged at 12 000 × g for 1.5 h at 4°C to obtain a membrane preparation. The total protein concentration in membrane fraction extracts was determined by the Bradford assay.
The cAMP assay was performed using the cAMP ELISA kit from Enzo Life Sciences International, Inc. (Plymouth Metting, PA, USA). Fifty microliters of an Adcy reaction mixture (100 mM Tris-acetate, pH 7.4, 20 mM KCl, 10 mM MgCl2, 20 mM phosphoenolpyruvate, 100 μg/mg pyruvate kinase, increasing concentrations of ATP, 20 μM guanosine triphosphate, 2 mM dithiothreitol, 0.4 mM bovine serum albumin, and 0.1 mM 3-isobutyl-1-methylxanthine) was added into microcentrifuge tubes in duplicate and maintained at 4°C. Enzymatic activities were determined using 125 μg of a membrane protein in the presence of piperonal or forskolin for 30 min at 37°C. The reaction was stopped by heating at 95°C for 5 min. The supernatant was stored after centrifugation at 10 000 × g for 5 min at 4°C. cAMP production rates were recorded in picomoles of cAMP produced per milligram of the membrane protein per minute.
2.4 Isolation of total RNA and semiquantitative RT-PCR
Total RNA was isolated from the epididymal adipose tissue of each mouse with the TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). RNA concentration was measured using a spectrophotometer, and the RNA was converted to cDNA using a reverse transcriptase (Invitrogen). Supporting Information Table 1 shows the forward (F) and reverse (R) primer sequences. The cDNA was denatured at 94°C for 10 min, followed by 30–35 cycles of PCR (94°C for 30 s, 55°C for 30 s, 72°C for 1 min). Finally, 4 μL of each PCR reaction mixture was mixed with 1 μL of 6 × loading buffer and then loaded onto a 2% agarose gel containing ethidium bromide. The mRNA levels of all genes were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control.
2.5 Western blot analysis
The epididymal adipose tissues of each mouse were homogenized in extraction buffer consisting of 100 mM Tris-HCl, pH 7.4, 5 mM EDTA, 50 mM sodium pyrophosphate, 50 mM NaF, 100 mM orthovanadate, 1% Triton X-100, 1 mM phenylmethanesulfonyl fluoride, 2 μg/mL aprotinin, 1 μg/mL pepstatin A, and 1 μg/mL leupeptin. The tissue homogenates were centrifuged at 1300 × g for 20 min at 4°C, and the supernatants were collected and stored at −80°C. The protein concentrations of tissue extracts were measured by the Bradford assay (Bio-Rad, Hercules, CA, USA). Lysate samples containing 40 μg of protein were subjected to SDS-PAGE and transferred onto nitrocellulose membranes (Amersham, Buckinghamshire, UK). The samples were incubated overnight with primary antibodies (diluted 1:1000) at 4°C. Western blotting was conducted using antibodies against AMPK, phospho-AMPK (Thr172), HSL, phospho-HSL (Ser563), CREB, and phospho-CREB (Ser133), tuberous sclerosis 2 (TSC2) (Ser1387), mammalian target of rapamycin (mTOR) (Ser 2448), and ribosomal protein S6 kinase (S6K) (Thr389) (Cell Signaling Technology; Beverly, MA, USA). The membranes were incubated with an anti-rabbit IgG HRPsc-2004 secondary antibody (diluted 1:5000; Santa Cruz Biotechnology, Dallas, TX). Finally, immunoreactive signals were detected using the Chemiluminescent Detection System (Amersham) and quantified in the Quantity One software (Bio-Rad).
2.6 Cell culture
Mouse embryo 3T3-L1 fibroblast cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM (Hyclone, Logan, UT) containing 10% of bovine calf serum (Gibco-BRL, Grand Island, NY) and penicillin–streptomycin (Hyclone, Logan, UT) at 37°C in a humidified incubator at 5% CO2. For the differentiation assay, preadipocytes were seeded in 12-well plates and cultured until 2 days postconfluence. After the 3T3-L1 cells became confluent, the medium was replaced with DMEM consisting of 10% of fetal bovine serum (FBS; Gibco-BRL), 1% of penicillin–streptomycin, 1 μg/mL insulin, 1 μM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), and 0.5 mM isobutylxanthine (Sigma-Aldrich) with or without piperonal (Sigma-Aldrich), SQ22,536 (an Adcy inhibitor, Sigma-Aldrich) and H89 (a PKA Inhibitor, Sigma Aldrich). The cells were then maintained in DMEM containing 10% of FBS with 1 μg/mL insulin for another 2 days, followed by culturing with DMEM containing 10% of FBS for additional 4 days with or without piperonal and SQ22,536.
2.7 Oil red O staining
After 8 days of 3T3-L1 differentiation, the medium was removed from the wells. The cells were washed twice with 1 × PBS and fixed in 10% formaldehyde for 1 h. The fixed cells were stained with a filtered Oil red O solution (0.5% Oil red O in isopropanol) for 30 min at room temperature and washed with distilled water. After washing and complete drying, we added 500 μL of isopropanol to each well, followed by incubation at room temperature for 10 min to release Oil Red O from lipid staining. The extraction solution was transferred to another 96-well plate, which was subjected to OD measurement at a wavelength of 490 nm using a microplate reader (Versa Max). The images of stained cells were captured by means of an Olympus microscope (Tokyo, Japan).
2.8 A PKA enzymatic assay
3T3-L1 cells were lysed in 200 μL of lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, pH 7.4), and then centrifuged at 2000 × g for 15 min at 4°C. The supernatant was stored at −80°C until use. The samples were added into the appropriate wells, followed by addition of ATP to start the reaction. The reaction was terminated and a specific phospho-substrate antibody was added into the wells. After the addition of an IgG HRP-conjugated secondary antibody, color development was initiated by addition of tetramethylbenzidine (the substrate). The color development was stopped with a stop solution, and intensity of the color was measured on a microplate reader at 450 nm. The kinase assay was performed using the PKA Assay Kit (Kamiya Biomedical Company, Tokyo, Japan).
2.9 Statistical analysis
The results on body weight gain, visceral fat pad weights, and plasma biochemical data are presented as mean ± SEM of six mice per group. RT-PCR and western blot data are shown as mean ± SEM of three independent experiments (n = 2 per experiment) for each group. Data were subjected to one-way analysis of variance (Duncan's multiple range test) in the SPSS software (version 12.0). In all statistical tests, p values less than or equal to 0.05 were assumed to denote statistical significance.
3 Results
3.1 Piperonal attenuates visceral adiposity and improves plasma lipid levels
Mice fed the PSD for 10 weeks showed significant reductions in final body weight (−10%), body weight gain (−19%), and food efficiency ratio (−15%) as compared with HFD-fed mice, without changes in food intake (Fig. 1B–E). The total visceral (epididymal, perirenal, mesenteric, and retroperitoneal) fat pad weights were significantly lower in the PSD-fed mice than in the HFD-fed mice (Fig. 1F). The PSD-fed mice had lower plasma levels of triglycerides (−21%), total cholesterol (−14%), LDL-VLDL cholesterol (−18%), free fatty acids (−17%) and glucose (−26%) than did HFD-fed mice (Table 1). Piperonal supplementation of the HFD resulted in significantly elevated HDL-cholesterol (+3%), adiponectin (+20%) and decreased leptin (−25%), TNF-α (−22%), and IL-6 (−28%), levels and ALT (−15%) and AST (−27%) activity in the plasma of mice in the plasma of mice (Table 1).
3.2 Piperonal prevents the HFD-induced downregulation of Adcy and PKA genes in WAT
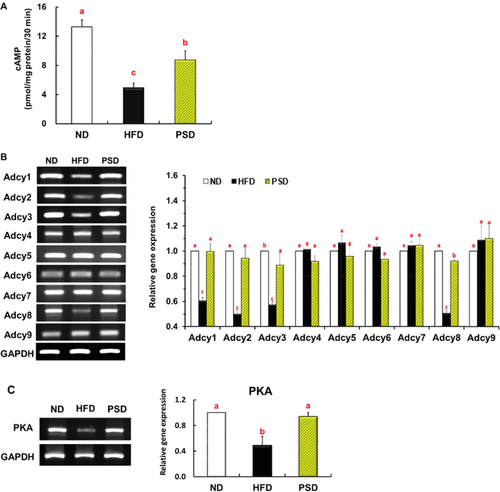
We found that mice fed the PSD exhibited increased Adcy activity in the epididymal adipose tissue of mice compared to the HFD group. (Fig. 2A). The nine isoforms of Adcy (Adcy1 through Adcy9) were all expressed in the visceral adipose tissue of mice. The mRNA expression profile of Adcy isoforms in the WAT of HFD-fed mice differed from that in ND-fed mice, with a significant decrease in the expression of Adcy1, Adcy2, Adcy3, and Adcy8. Piperonal prevented these HFD-induced reductions in Adcy1, Adcy2, Adcy3, and Adcy8 expression, without affecting the expression of the other isoforms (Fig. 2B). Similarly, the downregulation of the PKA gene induced by the HFD in the WAT of mice was completely reversed by piperonal supplementation (Fig. 2C).
3.3 Piperonal prevents the HFD-induced modulation of PKA-related signaling molecules in WAT
The immunoblot results indicated that piperonal supplementation of the HFD significantly prevented the HFD-induced downregulation of phospho-AMPK, phospho-HSL, and phospho-CREB levels: the downstream targets of PKA in the visceral adipose tissues of mice (Fig. 3A). The protein levels levels of tuberous sclerosis 2 (TSC2), which was significantly downregulated by the HFD as compared to the ND, was upregulated back by piperonal supplementation (Fig. 3B). The HFD-induced upregulation of the signaling molecules downstream of TSC2, such as phospho-mTOR, the ribosomal protein S6 kinase (S6K), adipogenic transcription factors (PPAR-γ and CEBPα), and their target genes, FAS and adipocyte fatty acid-binding protein (aP2), was significantly prevented for all the above genes in visceral adipose tissue by piperonal supplementation (Fig. 3C). Piperonal significantly increased the mRNA levels of CPT1, molecules involved in mitochondrial fatty acid oxidation (Fig. 3D), and thermogenic genes, such as PGC-1α, retinoid X receptor (RXR), retinoic acid receptor (RAR), UCP1, PR domain containing 16 (PRDM16), and transcription factor A, mitochondrial (TFAM) (Fig. 3E) in the visceral fat tissues of mice fed the HFD. Piperonal significantly increased and decreased, respectively, the mRNA expression of adiponectin and leptin, in the epididymal adipose tissues of mice fed the HFD (Fig. 3F).

3.4 Piperonal attenuates adipogenic differentiation of 3T3-L1 cells through Adcy
To test our hypothesis that piperonal may act as an allosteric activator of Adcy that is known to promote cAMP production from ATP, the concentration-response activity was measured using the plasma membranes prepared from epididymal adipose tissue of the mice. Piperonal caused a concentration-dependent cAMP accumulation after a 30-min stimulation, yielding a characteristic sigmoidal curve with an EC50 value of 12.1 μM and a maximal response at ∼100 μM (Fig. 4A). Only this maximal effective dose (100 μM) was used in subsequent experiments to determine the effects of piperonal on the kinetic properties of Adcy in the presence of an increasing concentration of the substrate, ATP. EC50 for ATP-dependent cAMP was significantly reduced in the piperonal-treated membrane extracts as compared with untreated extracts. The magnitude of the piperonal-induced decrease in Km for ATP was similar to that observed in membranes treated with forskolin, a direct activator of Adcy, indicating that the interaction with piperonal affected the function of Adcy in the plasma membranes of WAT in a manner similar to forskolin-induced activation (Km = 2.1 mM for piperonal and Km = 1.7 mM for forskolin versus Km = 3.1 mM for control; Fig. 4B).
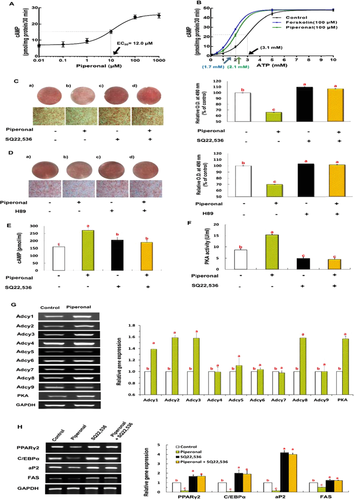
To confirm that piperonal exerts its anti-adipogenic effect through the direct activation of Adcy in the plasma membrane, we examined the lipid accumulation and regulation of the Adcy/PKA signaling cascade in 3T3-L1 cells in the presence or absence of a specific Adcy inhibitor, SQ22,536. Piperonal (100 μM) treatment significantly inhibited adipogenic differentiation of 3T3-L1 cells, whereas this positive effect of piperonal was attenuated by the inhibitors of Adcy (100 μM SQ22,536; Fig. 4C) and PKA (100 μM H89; Fig. 4D). 3T3-L1 cells treated with piperonal during differentiation showed significantly elevated Adcy and PKA activities as compared with untreated control cells, and these effects of piperonal were abrogated by the Adcy inhibitor (Fig. 4F and H). Furthermore, mRNA from 3T3-L1 cells treated with piperonal during differentiation showed significantly elevated expressions of Adcy isoforms (1, 2, 3, and 8) and PKA gene as also shown in the WAT of PSD mice (Fig. 4G). Similarly, piperonal-induced downregulation of adipogenic genes—such as adipogenic transcription factors (PPAR-γ and CEBPα) and their target genes (aP2 and FAS)—was almost abrogated by the Adcy inhibitor (Fig. 4H).
4 Discussion
The presently used dose of 0.05% piperonal [equivalent to 50 mg/(kg body weight)] in mice corresponds to approximately 4 mg/(kg body weight) in humans when calculated on the basis of normalization to the body surface area as recommended by Reagan-Shaw et al. 15. In our preliminary study, piperonal supplemented to the HFD at 0.01 and 0.05% levels for 28 days resulted in a dose-dependent reduction in the body weight of mice while, 0.1% showed no further reduction compared to 0.05% (data not shown). On the basis of these results, animals were fed 0.05% piperonal for a longer period in the present study. Piperonal has been granted the “generally recognized as safe” status by the Flavor and Extract Manufacturers Association and was approved by the FDA as a flavor ingredient. The FDA reported that piperonal is safe for human consumption at an acceptable daily intake of 2.5 mg/(kg body weight) 16. A no-observed-adverse-effect level (NOAEL) of 500 mg/(kg body weight) per day for piperonal in rats was reported elsewhere 16, and this level corresponds to approximately 81 mg/(kg body weight) in humans 15. Therefore, the dose used in the present study is approximately 60% higher than the level of acceptable daily intake of piperonal [2.5 mg/(kg body weight) in humans] but less than 5% of the NOAEL [corresponding to 81 mg/(kg body weight) in humans].
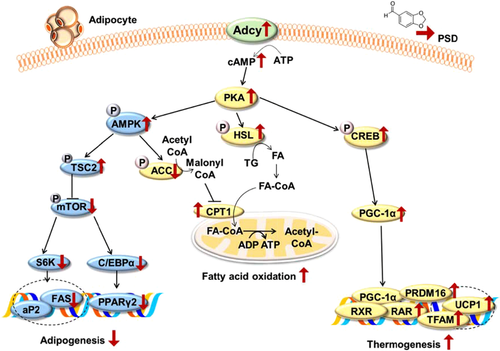
This study reveals an important novel function of piperonal in the protection against visceral adiposity and dyslipidemia in mice with diet-induced obesity. We found that piperonal reduced both visceral fat pad weights and plasma lipid levels in mice on the HFD (Table 1). Increased adiponectin levels in the plasma (Table 1) and adipose tissues (Fig. 3F) together with AMPK phosphorylation may regulate adipose tissue function and hyperlipidemia in PSD group. In addition, piperonal increased the protein levels of phospho-AMPK, phospho-HSL, and phospho-CREB in the WAT of mice fed the HFD (Fig. 3A). We hypothesized that these three key target molecules (Fig. 3A) are likely controlled by a common upstream regulator that affects multiple lipid metabolic pathways. Indeed, it is well known that the activation of PKA participates in the regulation of important metabolic processes or mechanisms, such as fatty acid oxidation, thermogenesis, and insulin sensitivity in the WAT and liver of mice 19, 20. In mice fed piperonal, increased expression of thermogenic genes such as PGC-1α, RXR, RAR, UCP1, PRDM16, and TFAM (Fig. 3E) may be responsible for the prevention of HFD-induced weight gain.
| Groups | ND | HFD | PSD |
|---|---|---|---|
| Triglyceride (mmol/L) | 0.59 ± 0.04c) | 0.90 ± 0.01a) | 0.71 ± 0.02b) |
| Total cholesterol (mmol/L) | 3.63 ± 0.15c) | 6.57 ± 0.13a) | 5.62 ± 0.31b) |
| LDL-VDL cholesterol (mmol/L) | 3.63 ± 0.12c) | 6.57 ± 0.14a) | 5.62 ± 0.34b) |
| HDL-cholesterol (mmol/L) | 1.34 ± 0.12a) | 1.05 ± 0.05b) | 1.09 ± 0.10c) |
| Free fatty acid (μEq/L) | 572 ± 42.8 | 868 ± 14.9 | 722 ± 63.6 |
| Glucose (mmol/L) | 7.10 ± 0.27b) | 9.39 ± 0.57a) | 6.88 ± 0.62b) |
| Adiponectin (ng/mL) | 25.8 ± 1.13a) | 12.9 ± 0.93c) | 17.1 ± 1.35b) |
| Leptin (ng/mL) | 11.2 ± 1.55c) | 29.1 ± 1.74a) | 16.1 ± 2.11b) |
| TNF-α (pg/mL) | 16.0 ± 0.92c) | 19.4 ± 1.63a) | 17.3 ± 2.76b) |
| IL-6 (pg/mL) | 19.7 ± 1.50c) | 58.9 ± 3.87a) | 29.8 ± 2.44b) |
| Alanine aminotransferase (IU/L) | 4.85 ± 0.96b) | 9.87 ± 0.93a) | 7.89 ± 1.23b) |
| Aspartate aminotransferase (IU/L) | 6.78 ± 0.66b) | 12.1 ± 1.03a) | 7.79 ± 0.58b) |
- Values are expressed as mean ± SEM, n = 6. Mean values labeled with different letters are statistically significantly different (p < 0.05).
In the present study, using plasma membranes prepared from epididymal adipose tissue of mice, we confirmed that piperonal supplemented to HFD increased the Adcy activity (Fig. 2A). We demonstrated that piperonal increases mRNA expression of PKA in the WAT of mice fed the HFD (Fig. 2B) and enhances the PKA activity in differentiated 3T3-L1 cells (Fig. 4E).It is well known that PKA is regulated by changes in intracellular cAMP levels, which are elevated after the activation of Adcy located in the plasma membrane of a cell 21. The cAMP–PKA signaling pathway is intimately involved in cell growth, differentiation, and apoptosis 22-26. Recently, several studies revealed that cAMP is associated with the regulation of adipose-tissue development and functions via modulation of gene expression related to adipogenesis 27-29. For example, the elevation of intracellular cAMP levels by incubation with forskolin (an Adcy activator) significantly suppresses adipogenesis judging by both the morphological phenotype and the expression levels of CEBPα and PPAR-γ in 3T3-L1 preadipocytes 30. In contrast, the inhibition of cAMP levels by SQ22,536, an Adcy inhibitor, promotes adipogenesis of 3T3-L1 cells 30. In the present study, using the plasma membranes prepared from mouse WAT, we confirmed that piperonal allosterically activates Adcy by decreasing Km for ATP and subsequently increasing cAMP production in a manner similar to forskolin (Fig. 4B). Next, our hypothesis—that this positive effect of piperonal (attenuation of visceral adiposity) may be mediated by Adcy activation—was proven in 3T3-L1 cells treated with an Adcy inhibitor during differentiation. We demonstrated that piperonal increases mRNA expression of Adcy isoforms and PKA in 3T3-L1 cells (Fig. 4G). Treatment of 3T3-L1 cells with an Adcy inhibitor (SQ22,536) and PKA inhibitor (H89) almost abrogated the piperonal-induced attenuation of adipocyte differentiation and elevation of Adcy and PKA activities (Fig. 4C–F). Compared to their HFD-fed counterparts, mice fed piperonal for 10 weeks exhibited increased adenylate cyclase activity in their epididymal adipose tissues (Fig. 2A). Kamienski and Casida 17 reported that following oral administration of piperonal to mice, 89% of the dose was recovered in the urine, the major metabolite being piperonylglycine. In rats, 93% of an oral dose was recovered in 24 hours in the urine, 71% as piperonylglycine and 20% as piperonylic acid 18. Based on this, we speculate that in addition to piperonal, its metabolites may also be responsible for observed anti-adipogenic effects and increased adenylate cyclase activity in vivo. A further study evaluating the adenylate cyclase activity evoked by piperonal metabolites is warranted.
Adcy consists of a short and variable amino-terminus, followed by two repeats of a module composed of six transmembrane spans (M1 and M2) and two cytoplasmic domains of approximately 40 kDa each (C1 and C2). The crystal structure of the Adcy complex with an Adcy inhibitor or activator has revealed that the Adcy inhibitor binds to the C1 domain and undergoes the conformational change that is necessary for Adcy inactivation. By contrast, an Adcy activator, such as forskolin, binds to the N-terminal portion of the C1–C2 interface and increases cAMP synthesis at the C-terminal portion of the C1–C2 interface 31. Further studies are needed to elucidate the conformational interaction between piperonal and Adcy.
In the present study, using plasma membranes prepared from epididymal adipose tissue of mice, we confirmed that piperonal supplemented to HFD increased the Adcy activity (Fig. 2A). During the past decade, nine hormone-sensitive, membrane-bound Adcy isoforms (Adcy1 through Adcy9) have been identified, each with distinct regulation and expression patterns 32. Of note, the expression of Adcy1, Adcy2, Adcy3, and Adcy8 specifically responded to both HFD and piperonal supplementation in opposite directions, respectively, in the WAT of mice (Fig. 2B). Adcy1, Adcy2, and Adcy8 have been repeatedly reported to be involved in neuronal regulation of learning and memory 33, 34, whereas Adcy3 is known to be a crucial element of the odorant-induced signal transduction cascade and a pivotal player in axonal guidance during migration of dopaminergic neurons from the olfactory bulb toward the brain 35. Recently, it was reported that Adcy3 gene polymorphisms are associated with obesity in Chinese and Swedish populations 36, 37. This finding leads to an intriguing hypothesis that Adcy3 expressed in WAT may play an important role in the regulation of adiposity. Gene function studies showed that Adcy3 knockout mice have greater body weight, which is due primarily to greater fat mass as compared to the corresponding wild-type mice 38. On the other hand, transgenic mice overexpressing Adcy3 have significantly lower total body weights and fat mass as compared to wild-type mice 39. Furthermore, our recent work revealed that heterozygous Adcy3-null mice show increased visceral adiposity in the absence of hyperphagia and develop abnormal metabolic features such as impaired insulin sensitivity, dyslipidemia, and increased plasma levels of proinflammatory cytokines either on lab chow or on the HFD 40. These animal studies have highlighted the possibility that Adcy3 activators may be useful for the prevention or treatment of obesity and the associated metabolic complications. Nevertheless, further research is needed to determine which isoform of Adcy most likely contributes to the antiadipogenic effect of piperonal in mice with diet-induced obesity.
In conclusion, piperonal significantly improves visceral adiposity and dyslipidemia in mice fed the HFD, and these beneficial effects of piperonal are associated with downregulation of genes involved in adipogenesis and upregulation of genes involved in fatty acid oxidation and thermogenesis in WAT. It would be worthwhile to see if piperonal can also reverse visceral adiposity induced by a chronic high-fat diet. It was reported that Adcy is associated with muscle cell migration and adhesion in primary muscle cells 41, and attenuated hepatic insulin resistance and obesity in obese mice 42. In future studies, it will be intriguing to investigate the physiological roles of piperonal in other tissues. Although the effects of piperonal in humans have yet to be elucidated, our findings raise the possibility that piperonal may be useful for the prevention of obesity and the associated metabolic complications.
Acknowledgments
Songyi Chu, Vikram P. Narayan, and Taesun Park designed the experiments, researched and analyzed data, and wrote the manuscript. Mi-kyung Sung contributed to acquisition and interpretation of data and reviewed and edited the manuscript.
This work was supported by the National Research Foundation of Korea Grant funded by the Korea Government (NRF-2016R1A2B4016189), and SRC program (Center for Food & Nutritional Genomics: Grant no. 2015R1A5A6001906) of the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology.
The authors have declared no conflicts of interest.




