Detection of novel metabolites of flaxseed lignans in vitro and in vivo
Abstract
Scope
This study aimed to improve the knowledge of secoisolariciresinol diglucoside (SDG) transformation by human gut microbiota.
Methods and results
SDG-supplemented microbiota cultures were inoculated with the feces of five subjects. The same volunteers received a flaxseed supplement for 7 days. SDG metabolites in cultures, feces, and urine were monitored by LC-ESI-QTOF and LC-DAD. In all cultures, SDG was deglycosylated to secoisolariciresinol (SECO) within 12 h. SECO underwent successive dehydroxylations and demethylations yielding enterodiol (4–18% conversion) and enterolactone (0.2–6%) after 24 h. Novel intermediates related to SECO, matairesinol (MATA), and anhydrosecoisolariciresinol (AHS) were identified in fecal cultures. These metabolites were also found after flaxseed consumption in feces and urine (in approximate amounts between 0.01–47.03 μg/g and 0.01–13.49 μg/mL, respectively) in their native form and/or modified by phase II human enzymes (glucuronide, sulfate and sulfoglucuronide conjugates).
Conclusions
Derivatives of MATA and AHS are described for the first time as intermediates of SDG biotransformation by intestinal bacteria, providing a more comprehensive knowledge of lignan intestinal metabolism. The transformations observed in vitro seem to occur in vivo as well. The detection in urine of SDG intermediates indicates their gut absorption, opening new perspectives on the study of their systemic biological effects.
Abbreviations
-
- AHS
-
- anhydrosecoisolariciresinol
-
- ED
-
- enterodiol
-
- EF
-
- enterofuran
-
- EL
-
- enterolactone
-
- SDG
-
- secoisolariciresinol diglucoside
-
- SECO
-
- secoisolariciresinol
-
- SMG
-
- secoisolariciresinol glucoside
1 Introduction
Secoisolariciresinol diglucoside (SDG) is one of the most common lignans occurring in western diet, being present in berries, legumes, cereals, and especially in flaxseed 1-3. The dietary intake of lignans is recognized to exert several positive effects on human health, including antioxidative, antiproliferative, and oestrogenic-like properties, and being protective against breast and prostate cancer, cardiovascular diseases, and diabetes 4-8. Most of the biological activities of dietary SDG depend upon bacterial transformations occurring in the colon, since food-associated SDG is poorly absorbed and reaches the colon undigested. Here, the resident microbiota releases the aglycone secoisolariciresinol (SECO) and transforms it into the enterolignans enterodiol (ED) and enterolactone (EL) that present improved bioavailability and potent biological activity, especially as phytoestrogens (Fig. 1) 9-11.

Bacterial transformation of SDG takes place through a complex interaction between dominant and subdominant intestinal groups within the gut microbiota 12, 13. The removal of the glucose moieties from SDG, the first step of the transformation, is performed by strains producing β-glucosidases. Bacteria hydrolyzing SDG belong to Bacteroidales and Clostridiales (e.g. Bacteroides spp., Clostridium cocleatum, and C. ramosum), even though active bacteria were also described within other bacterial groups 14, 15. In particular, strains of Bifidobacterium, especially belonging to the species B. pseudocatenulatum, hydrolysed SDG and other glyco-conjugated polyphenols with high efficiency, this ability being regarded as an asset for innovative probiotic strains 16-18. The release of SECO is followed by demethylation and dehydroxylation reactions yielding ED 19-21. The intestinal bacteria capable of performing SECO demethylation (e.g. Blautia producta and Eubacterium limosum) and dehydroxylation (e.g. Eggerthella lenta and C. scindens) belong to Clostridiales 19-21. Also final reduction of ED to EL with the closure of the lactone ring is performed by subdominant populations of Clostridiales, among which only Lactonifactor longoviformis has been identified as EL-producing species 14, 20.
Many studies have reported that the dietary intake of flaxseed, one of the major sources of SDG, increases the concentration of ED and EL in blood, urine, and feces, in dose- and time-dependent mode 22-27. However, most of these studies focused only on matairesinol (MATA), ED, and EL and little is known about intermediate metabolites and their bioavailability 19, 28. In order to improve the knowledge of SDG metabolism, the present study aimed to (i) identify and quantify the metabolites produced in vitro by the human gut microbiota of five healthy subjects, (ii) evaluate in vitro the efficacy of a potential probiotic strain of B. pseudocatenulatum able to hydrolyze SDG, and (iii) identify in vivo the lignan metabolites detected in vitro and their possible conjugates, analyzing urine and fecal samples of the same volunteers after ingestion of flaxseed.
2 Materials and methods
2.1 Chemicals
All the chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA) unless otherwise stated. SDG was purchased from Acinopeptide (Chengdu, China) and dissolved in water at the concentration of 50 mM. Stock solutions of SECO, ED, and EL were prepared in methanol at the concentration of 3 mM. Appropriate volumes of the stock solutions were utilized as supplements for bioconversion experiments and to create calibration curves for HPLC analysis. Organic Flaxseed for dietary supplementation were purchased from Ki Group (Turin, Italy) and were grounded with a kitchen jug blender. Eluents for chromatography were purchased from Panreac (Barcelona, Spain).
2.2 In vitro transformation of SDG by fecal microbiota
Bioreactor batch cultures of human gut microbiota were carried out in 200 mL of a complex medium for microbiota cultures 29, supplemented with 0.6 g/L cellobiose. Fresh feces of five healthy volunteers (three men and two women, aged 25–50 years), who had not been taking prebiotics and/or probiotics for 2 weeks and antibiotics for at least 6 months, collected after obtaining written informed consent, were utilized to prepare inocula for microbiota cultures. For each subject, the fecal sample was homogenized (5% w/v) with the cultural medium, under a 85% N2, 10% CO2, 5% H2 atmosphere in an anaerobic cabinet (Anaerobic System, Forma Scientific, Marietta, OH), then 40 mL of the suspension were inoculated into four bioreactors (Sixfors V3.01, Infors, Bottmingen, Swiss) each containing 160 mL of fresh sterile medium. Immediately after the inoculum, a sample was collected in order to measure the background level of lignan metabolites. Then the culture was supplemented with SDG to achieve 0.8 mM and incubated for 24 h at 37°C in anaerobiosis, with the pH maintained at 6.5 30. Samples were periodically collected from cultures and stored at −80°C until analyzed to determine the lignans.
In the probiotic-supplemented cultures, the bioreactor was coinoculated with the microbiota and with 6.0 × 107 cfu/mL of B. pseudocatenulatum WC 0401. The strain was taken from our strain collection and was routinely cultured at 37°C in anaerobiosis in Lactobacilli MRS broth (BD Difco, Sparks, NV, USA) supplemented with 0.5 g/L l-cysteine HCl. The inoculum for bioreactors was prepared according to the procedure described by Tomàs-Barberàn et al. 30, utilizing cultures grown for 16 h in MRS-cysteine, where glucose was substituted with cellobiose to induce β-glucosidase activity 16.
Four parallel cultures were run for each microbiota specimen, two in the presence and two in the absence of the probiotic supplement. Data herein reported are the means of the duplicates.
2.3 In vivo trial
The same five volunteers who provided the fecal samples for the in vitro experiment were enrolled in a dietary trial. The experimental protocol (i.e. the utilization of fecal and urine specimens and the dietary supplementation with grounded linseed) was approved by the institutional review board of the local research ethics committee (Comitato Etico Provinciale, Azienda Policlinico di Modena, Italy) and was given the reference number 124-15. The volunteers consumed a western diet specifically excluding lignan-rich food and, after a 7-day washout period, the diet was supplemented with 25 g/day of grounded flaxseed for 7 days. Samples of urine and feces were collected at the beginning of the dietary treatment and after the last flaxseed intake and immediately stored at −80°C until analyzed to determine the lignans.
2.4 Identification and analysis of SDG and its metabolites
After thawing, formic acid was added to fecal cultures and urine samples at the concentration of 0.5% v/v, in order to stabilize the lignan metabolites. Fecal samples were homogenized (10% w/v) in a mixture of methanol, water, and formic acid (80, 19.5, and 0.5% v/v, respectively) using an Ultra-Turrax homogenizer (Ika, Staufen, Germany) for 1 min at 24 000 rpm. All the samples were clarified by centrifugation (14 000 × g for 10 min at 4°C) and filtration through 0.22 μm filters. Urine and fecal samples were diluted 1:3 and 1:5, respectively, in water plus 0.5% formic acid, before being analyzed.
2.4.1 Identification of metabolites
Metabolites in microbiota cultures, feces, and urine were identified using an UPLC system (Agilent 1290 Infinity, Agilent Technologies, Waldbronn, Germany) coupled to a 6550 Accurate-Mass Q-TOF (Agilent Technologies) with an ESI interface with jet stream technology (Agilent Technologies), hereinafter referred to as LC-QTOF. Compounds were separated on a reverse phase Poroshell 120 EC-C18 column (3 × 100 mm, 2.7 μm; Agilent Technologies) operating at 30°C and a flow rate of 0.4 mL/min. The mobile phase was composed of water with 0.1% formic acid (Phase A) and ACN with 0.1% formic acid (Phase B), mixed with a program that allowed 5% phase B to reach 25% at 10 min, 40% at 20 min, 90% at 24 min, 5% at 25 min, and 5% till 30 min. The ESI operated as follows: gas temperature, 280°C; drying gas flow, 9 L/min; nebulizer pressure, 35 psi; sheath gas temperature, 400°C; sheath gas flow, 12 L/min. Spectra were acquired in the m/z range from 100 to 1100 in negative mode and fragmentor voltage was 150 V. Data were processed using the Mass Hunter Qualitative Analysis software (version B.06.00), which lists and rates possible molecular formula consistent with the accurate mass measurement and the true isotopic pattern. A target screening strategy was applied, searching for a list of more than 60 possible lignan metabolites in the different samples (fecal cultures, urine, and feces). Screening was based on mass filtering at the exact mass using narrow mass extraction window (0.01 m/z). Besides, targeted MS/MS analysis provided additional information to achieve a reliable compound identification. MS/MS product ion spectra were collected at a m/z 50–800 range using a retention time window of 1 min, a collision energy of 20 V (for conjugated compounds) and 30 V (for aglycones), and acquisition rate of 4 spectra/s.
2.4.2 Quantitative analysis
The most intense compounds detected in fecal cultures were quantified using UV detection. Quantitative analyses was performed using an HPLC system (1200 Series, Agilent Technologies) equipped with a photodiode-array detector and a single quadrupole mass spectrometer detector in series (6120 Quadrupole, Agilent Technologies), hereinafter referred to as LC-DAD. Chromatographic conditions were the same as above, but using 0.5 mL/min of a mobile phase composed of 0.5% v/v formic acid in water (solvent A) and ACN (solvent B) with a gradient that allowed 5% phase B to reach 25% at 12 min, 40% at 25 min, 90% at 30 min. MS in SIM mode and negative mode was used to confirm the identification. Optimum conditions for the detection of the different compounds in SIM mode were set up with direct infusion of the standards (50 μM). Optimal ESI parameters using nitrogen as nebulizer gas were: capillary voltage, 3500 V; drying gas flow, 10 L/min; nebulizer pressure, 40 psi; and drying T, 300°C. Optimal fragmentor voltages were 200 V for SDG, 140 V for SECO, and 150 V for ED and EL. Compounds were quantified in UV at 280 nm. Calibration curves from 0.5 to 500 μM (three replicates) were obtained for SDG, SECO, ED, and EL at 280 nm. Metabolites in urine and feces could not be quantified using UV detectors due to its low sensitivity. ED, EL, SECO, and SDG were accurately quantified by MS (LC-QTOF) using calibration curves with their own standards (linearity until 2 μM).
Due to the absence of available standards for most of the intermediates and for phase II metabolites and the difficulty to synthesize them, an accurate quantification of these compounds with LC-DAD or LC-QTOF was not possible. In order to estimate the levels of the detected metabolites or at least to establish their order of magnitude, a quantification using standard curves of compounds with the most similar structure was applied.
3 Results
3.1 Identification of SDG metabolites in microbiota cultures
3.1.1 Metabolite stability
The stability of lignan metabolites was first evaluated by LC-DAD in the final time-point of SDG-supplemented microbiota cultures of one of the subjects, comparing samples supplemented of 0.5% v/v formic acid with untreated samples. The metabolites disappeared over time in untreated samples kept at room temperature and in an air atmosphere (i.e. the normal HPLC analysis conditions), while addition of formic acid prevented their degradation (Supporting Information Fig. 1). Thus, all the samples from cultures, feces, and urine were stabilized with formic acid after thawing to enable quantification and identification of all the SDG-derived compounds.
3.1.2 Metabolite identification
A reliable identification of the metabolites was obtained by means of LC-QTOF, which provided their mass and molecular formula, and from their MS/MS fragmentation pattern. The targeted approach enabled the identification with high score (≥94) and low error (≤3) of a total 17 metabolites in fecal cultures (Table 1, Fig. 1), all presenting a UV spectrum similar to that of SECO.
| Compound identification | RT, LC-DAD | RT, LC-QTOF | m/z | Score | Error | Molecular formula | MS/MS fragments | |
|---|---|---|---|---|---|---|---|---|
| (min) | (min) | experimental | ||||||
| 1 | SDG | 10.68 | 8.16 | 685.2724 | 98.4 | −1.31 | C32H46O16 | 523, 361 |
| 2 | SMG | 12.23 | 9.61 | 523.2195 | 97.3 | −2.01 | C26H36O11 | 361 |
| 3 | SECO | 14.20 | 11.58 | 361.1655 | 98.11 | −2.08 | C20H26O6 | 346, 331, 313, 298, 179, 165 |
| 4 | Demethyl-SECO | 12.65 | 10.14/11.19 | 347.1510 | 94.22 | −1.92 | C19H24O6 | 332, 317, 299, 179, 165 |
| 5 | Demethyl-dehydroxy-SECO | 15.47 | 12.70/13.71 | 331.1546 | 99.5 | 0.95 | C19H24O5 | 316, 301, 283, 268, 179, 165 |
| 6 | Didemethyl-SECO or dihydroxy-ED | 10.91 | 8.64 | 333.1349 | 98.34 | −1.17 | C18H22O6 | 315, 303, 285, 181, 135 |
| 7 | Didemethyl-dehydroxy-SECO or hydroxy-ED | 13.66 | 11.04/12.89 | 317.1401 | 96.96 | −1.35 | C18H22O5 | 299, 287, 269, 239, 179, 165 |
| 8 | Demethyl-MATA | 16.55 | 13.80/14.55 | 343.1188 | 99.7 | −0.01 | C19H20O6 | 328, 299, 284, |
| 9 | Demethyl-dehydroxy-MATA | 19.96 | 17.07/17.72 | 327.1233 | 97.45 | 1.22 | C19H20O5 | 312, 283, 269 |
| 10 | Didemethyl-MATA or dihydroxy-EL | 13.86 | 11.50 | 329.1035 | 98.46 | −0.93 | C18H18O6 | 285, 267, 207, 187, 161, 138 |
| 11 | Didemethyl-dehydroxy-MATA or hydroxy-EL | 16.83 | 14.19/14.32 | 313.1079 | 99.64 | 0.95 | C18H18O5 | 269, 251, 199, 165, 136 |
| 12 | Demethyl-AHS | 18.47 | 16.44 | 329.1395 | 99.63 | 0.59 | C19H22O5 | 314, 281,131,107 |
| 14 | Didemethyl-AHS | 14.68 | 12.11 | 315.1235 | 99.65 | 0.70 | C18H20O5 | 297, 267, 161, 146 |
| 15 | Didemethyl-dehydroxy-AHS | 17.58 | 14.94 | 299.1288 | 99.87 | 0.34 | C18H20O4 | 281, 267, 251,131,107 |
| 16 | ED | 16.48 | 13.58 | 301.1444 | 99.28 | 0.60 | C18H22O4 | 283, 271, 253, 241 |
| 17 | EL | 20.53 | 17.54 | 297.1135 | 99.23 | −0.56 | C18H18O4 | 253, 189, 165 |
- Reliable identification was deduced from mass and molecular formula and from MS/MS fragmentation pattern.
The evolution of SDG metabolites was monitored for 24 h during fecal batch fermentations inoculated with the gut microbiota of five healthy volunteers (Figs. 2 and 3). No lignan metabolite was detected in inoculated cultures before the addition of SDG. SDG (compound 1) and SECO (compound 3) were identified through their typical fragmentation profiles and were confirmed by comparison with the standards. The spectrum of secoisolariciresinol glucoside (SMG; compound 2, m/z 523.2195) was very similar to that of SDG and its main fragment at m/z 361 showed the loss of a glucose moiety from SDG (Table 1). In all the cultures SDG was rapidly hydrolyzed to SECO, with SMG as the intermediate. The release of the aglycone was complete in 4–12 h, depending on the volunteer. Further transformations of SECO occurred at different extent in the five cultures, originating diverse intermediates, products, and conversion yields. Four different derivatives of SECO (m/z 361.1656) lacking methyl and/or hydroxyl groups were found: compound 4 (m/z 347.1510) was tentatively identified as demethyl-SECO, compound 5 (m/z 331.1546) as demethyl-dehydroxy-SECO, compound 6 (m/z 333.1349) as didemethyl-SECO (dihydroxy-ED), and compound 7 (m/z 317.1401) as didemethyl-dehydroxy-SECO (hydroxy-ED). Similar fragmentation pattern was observed for this group of metabolites, with the loss of 15, 30, and 48 amu for SECO and demethyl derivatives and the loss of 18, 30, and 48 amu for didemethyl derivatives, while fragments 179 and 165 amu were common for all the derivatives (Table 1). Compounds 4, 5, and 7 presented two retention times in LC-QTOF, due to the presence of isomers differing in the position from which demethylation (3-O or 3′-O) and dehydroxylation (4 or 4′) occurred. It was not possible to determine the position of the lost methyl or hydroxyl residues with the available instrument. Compounds 12 (m/z 329.1395), 14 (m/z 315.1235), and 15 (m/z 299.1288) were identified as the anhydrous forms of 4, 6, and 7, respectively, similar in structure to anhydrosecoisolariciresinol (AHS). In particular, 12 was tentatively identified as demethyl-AHS, 14 as didemethyl-AHS, and 15 as didemethyl-dehydroxy-AHS. As observed for SECO derivatives, AHS derivatives showed similar losses in the fragmentation patterns. Compounds 16, and 17 were identified as, ED, and EL, respectively, by comparison with the standards and as confirmed by their fragmentation profile (Table 1).
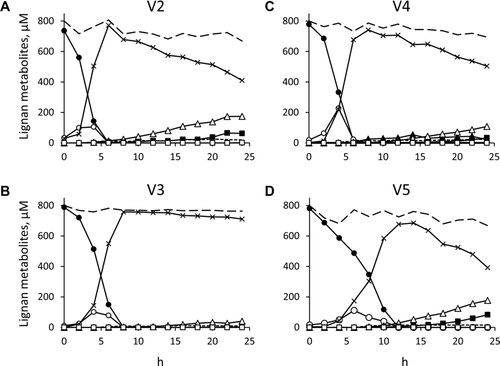
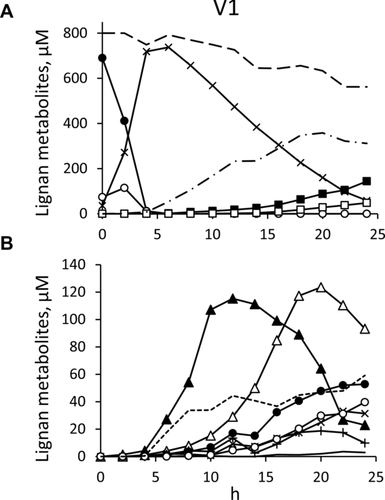
3.1.3 Differences between individuals
In the cultures inoculated with the microbiota of subject V3, most SECO remained untransformed after 24 h of incubation (711 μM), and a minor amount was transformed into 5, 12, and ED (41, 9, and 2 μM, respectively), while EL did not appear (Fig. 2B). Similar transformation profiles were observed for microbiota cultures of subjects V2, V4, and V5 (Fig. 2A, C, and D, respectively), although high variability was obtained in the amount and progression of the metabolites. In these cultures, the concentration of SECO progressively decreased whereas other metabolites gradually appeared, such as 4, 5, 12, ED, and traces of EL. Compound 4 was detected only in V4 cultures, where it increased during the first 14 h (up to 54 μM) and progressively decreased toward the end of the process. Compound 5 gradually accumulated throughout the incubation, reaching the highest concentration after 24 h of incubation (108–177 μM for V2, V4, and V5 cultures). Enterolignans appeared in V2, V4, and V5 cultures, with ED increasing up to 34–83 μM after 24 h, and EL being always below 1.5 μM. Compound 12 occurred at a concentration that reached up to 15–22 μM.
The microbiota cultures of V1 behaved differently compared to the others, with a more complex profile of SDG metabolites (Fig. 3), which were generated in greater number and abundance, causing the almost complete disappearance of SECO at 24 h (Fig. 3A). The most abundant intermediates were 4 and 5. The former reached 115 μM in the first 12 h, then decreased to be nearly completely consumed, while the latter reached 124 μM after 20 h (Fig. 3B). While compound 4 decreased, other SECO derivatives, such as 6 and 7, were produced and peaked between 20 and 24 h, even though at lower concentration than 5.
Unlike the cultures of V2, V3, V4, and V5 microbiota, V1 yielded also derivatives of the dibenzyl-butyrolactone lignan MATA, obtained by dehydrogenation of SECO. Compounds 8 (m/z 343.1188), 9 (m/z 327.1233), 10 (m/z 329.1035), and 11 (m/z 313.1079) were identified as demethyl-MATA, demethyl-dehydroxy-MATA, didemethyl-MATA (dihydroxy-EL), and didemethyl-dehydroxy-MATA (hydroxy-EL), respectively (Table 1). They were the lactones obtained by dehydrogenation of the corresponding diols 4, 5, 6, and 7. In MS/MS experiments, specific losses were observed in demethyl (−15 and −44 amu) and didemethyl (−44 and −62 amu) derivatives. As in the case of SECO derivatives, the removals of methyl and hydroxyl groups from different position yielded different isomers of 8, 9, and 11, but the instrument did not allow their unambiguous identification. MATA and its derivatives were precursors of the EL, which showed the same loss (−44 amu) in the fragmentation pattern. Intermediates 10, 11, and 8 achieved the highest concentrations at 24 h (53, 40, and 3 μM, respectively) while 9 appeared in traces only at 24 h. Among AHS derivatives, 12 was the most abundant (44 μM), being produced in the first 12 h and remaining nearly constant until the end of the experiment. Compounds 14 and 15 appeared at 24 h at low concentrations (9 and 4 μM, respectively). Finally, in V1 cultures the enterolignans ED and EL progressively accumulated since 12 h and reached their maximum at 24 h (144 and 48 μM, respectively).
3.1.4 Effects of supplementing cultures with B. pseudocatenulatum WC 0401
The microbiota cultures that were supplemented with 6.0 × 107 cfu/mL of B. pseudocatenulatum WC 0401 did not exhibit relevant differences in terms of both the pattern and the succession of SDG metabolites, compared with the corresponding nonsupplemented cultures (data not shown). The major differences were observed for cultures inoculated with the microbiota from V3 and V5, where hydrolysis of SDG into SECO was more efficient in presence of B. pseudocatenulatum WC 0401. SDG was depleted 2 h ahead, and the peak of SECO was anticipated as well. As a whole, also in V3 and V5 fermentations, the presence of B. pseudocatenulatum WC 0401 did not affect the fate of SECO, the generation of the other bioconversion intermediates, and the production of ED and EL.
3.2 SDG metabolites in feces and urine
3.2.1 Metabolite identification
In order to test the presence in vivo of the SDG metabolites detected in vitro, feces and urine samples of the same five volunteers who offered inoculum for microbiota cultures were analyzed at the beginning and at the end of a 7-day dietary supplementation of flaxseed. LC-QTOF led to the identification of a panel of different molecules in fecal samples and urine (Tables 2 and 3). They were tentatively identified on the basis of their m/z, molecular formula, and fragmentation pattern. The molecular formula was obtained with high score (>94) and low error (<2.75 ppm). The approach followed in this study was mainly qualitative due to the absence of available standards for most of the compounds and the lack of 24-h urine and feces samples. However, those metabolites for which authentic standards were available were accurately quantitated. The content of the remaining metabolites (mainly conjugated metabolites and novel intermediates) was indirectly estimated understanding that the results obtained were not accurate and subjected to some error. Ranges of concentrations for the five volunteers are shown in Tables 2 and 3. The metabolic pattern was very similar among the volunteers, both in feces and urine, with only few variations (Table 4).
| Compound identification | RT, LC-QTOF | m/z | Score | Error | Molecular | MS/MS fragment | Quantitation (μg/g)a | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (min) | experimental | formula | 0 day | 7 days | ||||||
| 1 | SDG | 8.163 | 685.2704 | 77.07 | 1.21 | C32H46O16 | 523, 361 | — | traces–0.55 | a |
| 2 | SMG | 9.610 | 523.2176 | 83.11 | 2.21 | C26H36O11 | 361 | — | 0.19–0.89 | a |
| 3 | SECO | 11.588 | 361.1656 | 97.00 | 0.89 | C20H26O6 | 346, 269, 179, 165 | — | 1.13–2.67 | b |
| 13 | Demethyl-dehydroxy-AHS | 20.570/21.519 | 313.1438 | 95.93 | 2.75 | C19H22O4 | 281, 253, 174, 147, 131, 107 | — | 0.01–0.65 | d |
| 15 | Didemethyl-dehydroxy-AHS | 14.978 | 299.1287 | 99.28 | 0.53 | C18H20O4 | 281, 251, 173, 149, 131, 107 | — | 0.04–1.06 | d |
| 16 | ED | 13.588 | 301.1445 | 99.29 | 0.13 | C18H22O4 | 283, 271, 253, 241, 107 | 0.02–0.18 | 0.12–18.82 | c |
| 16s | ED sulfate | 11.013/11.318 | 381.1014 | 84.05 | −0.57 | C18H22O7S | 301, 253, 80 | — | 0–0.26 | c |
| 17 | EL | 17.543 | 297.1138 | 97.52 | −1.22 | C18H18O4 | 253, 189, 165, 121, 107 | 0.94–2.84 | 9.44–47.03 | d |
| 17s | EL sulfate | 13.306/13.532 | 377.0688 | 89.31 | 2.03 | C18H18O7S | 297, 253, 189, 80 | — | 0.10–0.43 | d |
- a SDG, SECO, ED, and EL were quantified with their own standards. The other compounds were approximately quantified according to the curve of SDG (a), SECO (b), ED (c), and EL (d).
- Reliable identification was based on the exact mass and molecular formula and from MS/MS fragmentation patterns. The range of concentrations found in the five volunteers is reported.
- Symbols: s, sulfate; g, glucuronide; sg, sulfoglucuronide.
| Compound identification | RT, LC-QTOF | m/z | Score | Error | Molecular formula | MS/MS fragment | Quantitation (μg/mL)* | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (min) | Experimental | 0 day | 7 days | |||||||
| 3g | SECO glucuronide | 8.322 | 537.1978 | 99.59 | 0.09 | C26H34O12 | 361, 175, 113 | 0.21–0.36 | 0.36–7.67 | a |
| 3s | SECO sulfate | 9.034 | 441.1221 | 98.82 | 0.53 | C20H26O9S | 361, 165, 80 | 0.07–0.22 | 0.19–3.27 | a |
| 3sg | SECO sulfoglucuronide | 6.638 | 617.1540 | 95.00 | 0.74 | C26H34O15S | 537, 441, 361, 287, 255, 175, 113 | — | 0.04–0.42 | a |
| 5g | Demethyl-dehydroxy-SECO glucuronide | 9.057/9.735 | 507.1870 | 97.97 | 0.24 | C25H32O11 | 331, 175, 113, | — | 0.03–0.51 | b |
| 5s | Demethyl-dehydroxy-SECO sulfate | 9.927/10.232 | 411.1113 | 97.2 | 0.94 | C19H24O8S | 331, 283, 80 | — | 0.13–0.57 | b |
| 5sg | Demethyl-dehydroxy-SECO sulfoglucuronide | 7.870/8.932 | 587.1440 | 98.03 | −0.52 | C25H32O14S | 507, 411, 331, 255, 187, 113 | — | 0.01–0.08 | b |
| 9g | Demethyl-dehydroxy-MATA glucuronide | 11.784/12.055 | 503.1559 | 96.04 | −0.24 | C25H28O11 | 327, 175, 113 | — | 0.04–0.43 | c |
| 9s | Demethyl-dehydroxy-MATA sulfate | 12.620/12.869 | 407.0809 | 96.63 | −1.23 | C19H20O8S | 327, 283, 80 | 0–0.01 | 0.02–0.12 | c |
| 9sg | Demethyl-dehydroxy-MATA sulfoglucuronide | 9.351/9.577 | 583.1119 | 97.71 | 0.99 | C25H28O14S | 503, 407, 327, 270, 256, 113 | — | traces–0.06 | c |
| 11g | Didemethyl-dehydroxy-MATA glucuronide | 10.375/11.539 | 489.1395 | 97.14 | 1.03 | C24H26O11 | 313, 191, 175, 113 | 0.02–0.14 | 0.16–0.92 | c |
| 11s | Didemethyl-dehydroxy-MATA sulfate | 12.782 | 393.0651 | 98.71 | −0.31 | C18H18O8S | 313, 269, 191, 123, 80 | — | 0.02–0.21 | c |
| 11sg | Didemethyl-dehydroxy-MATA sulfoglucuronide | 9.437 | 569.0974 | 98.98 | −0.58 | C24H26O14S | 489, 393, 313, 255, 175, 113 | 0.01–0.03 | 0.05–0.24 | c |
| 15g | Didemethyl-dehydroxy-AHS glucuronide | 11.177 | 475.1612 | 98.57 | −0.26 | C24H28O10 | 299, 175, 113 | — | 0.02–0.64 | c |
| 15s | Didemethyl-dehydroxy-AHS sulfate | 11.747 | 379.0858 | 98.51 | −0.46 | C18H20O7S | 299, 281, 251, 173,80 | — | 0.02–0.16 | c |
| 15sg | Didemethyl-dehydroxy-AHS sulfoglucuronide | 9.215 | 555.1170 | 88.94 | −0.57 | C24H28O13S | 475, 379, 299, 255, 175 | — | 0.02–0.09 | c |
| 16 | ED | 13.588 | 301.1445 | 99.41 | −0.24 | C18H22O4 | 271, 253, 241, 131, 107 | — | 0.01–0.07 | b |
| 16g | ED glucuronide | 10.199/10.379 | 477.1758 | 97.84 | 1.38 | C24H30O10 | 301, 175, 113 | — | 2.21–7.77 | b |
| 16s | ED sulfate | 11.012 | 381.1025 | 94.15 | −2.31 | C18H22O7S | 301, 253, 80 | 0–0.06 | 3.29–6.96 | b |
| 16sg | ED sulfogluronide | 8.684 | 557.1346 | 95.22 | −1.49 | C24H30O13S | 477, 381, 301, 254, 175, 113 | — | 2.50–8.83 | b |
| 17 | EL | 17.555 | 297.1131 | 99.92 | 0.45 | C18H18O4 | 253, 189, 121, 107 | traces | 0.01–0.24 | c |
| 17g | EL glucuronide | 12.402 | 473.1447 | 98.39 | 1.1 | C24H26O10 | 297, 175, 113 | 0.12–1.87 | 1.92–13.49 | c |
| 17s | EL sulfate | 13.272/13.487 | 377.0699 | 98.00 | 0.31 | C18H18O7S | 297, 253, 189, 80 | 0.04–0.43 | 0.50–6.30 | c |
| 17sg | EL sulfoglucuronide | 10.018 | 553.1013 | 97.52 | 0.93 | C24H26O13S | 473, 377, 297, 255, 175, 113 | 0.01–0.03 | 0.02–0.73 | c |
- *ED and EL were quantified with their own standards. The other compounds were approximately quantified according to the curve of SECO (a), ED (b), and EL (c).
- Reliable identification was based on the exact mass and molecular formula and from MS/MS fragmentation patterns. The range of concentrations found in the five volunteers is reported.
- Symbols: s, sulfate; g, glucuronide; sg, sulfoglucuronide.
| Feces | Urine | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | V1 | V2 | V4 | V5 | ||||||||||
| 0 day | 7 days | 0 day | 7 days | 0 day | 7 days | 0 day | 7 days | 0 day | 7 days | 0 day | 7 days | 0 day | 7 days | 0 day | 7 days | 0 day | 7 days | |
| 1 | + | + | + | + | + | |||||||||||||
| 2 | + | + | + | + | + | |||||||||||||
| 3 | + | + | + | + | + | |||||||||||||
| 3g | + | + | + | + | + | + | + | + | ||||||||||
| 3s | + | + | + | + | + | + | + | + | ||||||||||
| 3sg | + | + | + | + | ||||||||||||||
| 5g | + | + | + | + | ||||||||||||||
| 5s | + | + | + | + | ||||||||||||||
| 5sg | + | + | + | + | ||||||||||||||
| 9g | + | + | + | + | ||||||||||||||
| 9s | + | + | + | + | + | |||||||||||||
| 9sg | + | + | + | + | ||||||||||||||
| 11g | + | + | + | + | + | + | + | |||||||||||
| 11s | + | + | + | + | ||||||||||||||
| 11sg | + | + | + | + | + | + | + | + | ||||||||||
| 13 | + | + | + | + | + | |||||||||||||
| 15 | + | + | + | + | + | |||||||||||||
| 15g | + | + | + | + | ||||||||||||||
| 15s | + | + | + | + | ||||||||||||||
| 15sg | + | + | + | + | ||||||||||||||
| 16 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| 16g | + | + | + | + | + | |||||||||||||
| 16s | + | + | + | + | + | + | + | + | + | + | ||||||||
| 16sg | + | + | + | + | + | |||||||||||||
| 17 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||
| 17g | + | + | + | + | + | + | + | + | ||||||||||
| 17s | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| 17sg | + | + | + | + | + | + | + | + | ||||||||||
3.2.2 Lignan metabolites in feces
The main metabolites previously detected in fecal cultures, SDG, SMG, SECO, ED, and EL were also identified in the feces of the five volunteers (Table 2). At the beginning of the trial, only EL and ED occurred in the feces of the subjects. The former was found in the feces of all the volunteers (in a range between 0.02 and 0.18 μg/g), and the latter only in samples from V3, V4, and V5 (between 0.94 and 2.84 μg/g) but a consistent increase was observed in all the volunteers after 7 days of flaxseed consumption and their sulfate forms, absent at the beginning of the trial, were identified together with SDG, SMG, and SECO. Moreover, two intermediate metabolites derived from AHS, didemethyl-dehydroxy-AHS (15), and a compound identified as demethyl-dehydroxy-AHS (13), not detected in cultures, were found at final time in the feces of all volunteers. SDG, SECO, ED, and EL were accurately quantified with their own standards showing the highest concentrations in feces for EL (9.44–47.03 μg/g) followed by ED (0.12–18.82 μg/g). Assuming a similar MS response of aglycones and their sulfate conjugates, smaller amounts of 16s and 17s were observed compared to ED and EL. SECO was also present but at smaller concentrations (1.13–2.67 μg/g) and only traces of SDG were detected. The estimated levels of intermediate metabolites (13 and 15) were present in the same order of magnitude.
3.2.3 Lignan metabolites in urine
In urine samples, the glucuronide, sulfate, and sulfoglucuronide forms of SECO (3g, 3s, and 3sg), 3′-O-demethyl-4′-dehydroxy-SECO (5g, 5s, and 5sg), 3′-O-demethyl-4′-dehydroxy-MATA (9g, 9s, and 9sg), 4-hydroxy-EL (11g, 11s, and 11sg), 4′-dehydroxy-3-O,3′-O-didemethyl-AHS (15g, 15s, and 15sg), ED (16g, 16s, and 16sg), and EL (17g, 17s, and 17sg) were detected. The MS/MS spectrum of all the conjugates showed a fragment at m/z corresponding to the intact free form, with a loss of 176, 80, and 256 amu for glucuronides, sulfates, and sulfoglucuronides, respectively. Sulfoglucuronides also showed fragments corresponding to the loss of the sole glucuronide or sulfate moiety. Fragments at m/z 175 were observed in all glucuronide conjugates, m/z 80 in sulfates and m/z 255 in sulfoglucuronides.
At the beginning of the treatment, urines contained phase II conjugates of SECO (3g and 3s), MATA derivatives (9s, 11g, and 11sg), ED (16s, 16g, 16sg), and EL (17, 17g, 17s, and 17sg; Tables 3 and 4). Most of the metabolites were present in all the five volunteers although at different concentrations (Table 4). After 7 days of flaxseed consumption, all the glucuronide, sulfate, and sulfoglucuronide forms of SECO, 5, 9, 11, 15, ED, and EL, and the unconjugated ED and EL were found in all the urine samples (Tables 3 and 4). ED and EL were detected at low concentrations, between 0.01–0.07 μg/mL and 0.01–0.24 μg/mL, respectively. Higher amounts were observed for their respective conjugates, especially glucuronides (2.21–7.77 μg/mL and 1.92–13.49 μg/mL) and sulfates (3.29–6.96 μg/mL and 0.50–6.30 μg/mL), assuming a similar response in MS compared to the aglycones. Similar concentrations were observed for SECO conjugates. Quantities of glucuronide, sulfate, and sulfoglucuronide forms of the intermediate metabolites were estimated to be in the range of 0.01–0.92 μg/mL, although for an accurate quantitation their own standards should be used (Table 3). The estimated concentration of the compounds detected at t0 was always higher in the final samples.
4 Discussion
The fate of dietary lignans is affected by the composition of the gut microbiota and interindividual differences in the transformation pathway may occur. The present study aimed to provide a comprehensive description of bacterial transformation of the flaxseed lignan SDG, gaining information from both in vitro and in vivo experiments. SDG metabolites were monitored during the course of human gut microbiota cultures and, in parallel, the same subjects who donated the microbiota for in vitro processes received a dietary supplement of flaxseed in a pilot study aimed to determine fecal and urinary lignan metabolites.
The lignan compounds that were identified during the course microbiota cultures (1–12 and 14–17) and the metabolites occurring in the feces after flaxseed supplementation (1–3, 13, 15–17) were assembled in a comprehensive scheme of SDG transformation by intestinal bacteria (Fig. 1). The two glucose moieties were successively removed from SDG to yield SMG, and then SECO. Deglycosylation was not the limiting step of the biotransformation pathway, since the microbiota of all the subjects was efficient in releasing SECO, likely due the functional redundancy of gut bacteria able to hydrolyze polyphenol glyco-conjugates 20. Consistently, the utilization of a probiotic aiming at increasing the release of the aglycone is not expected to improve the bioavailability of enterolignans, since the presence of B. pseudocatenulatum WC 0401 in microbiota cultures increased the rate of SDG hydrolysis but did not affect the fate of SECO.
SECO can undergo demethylation and dehydroxylation reactions yielding intermediates 4, 5, 6, and 7 and ED, which is finally transformed into EL, as described in previous studies 19, 20. The data herein reported suggest that the closure of the lactone ring is not necessarily the last reaction to occur. Indeed, the lactones 8, 9, 10, and 11 are generated and are likely transformed through the same series of reactions, similarly yielding EL. Even though 10 has already been reported 13, intermediates 8, 9, and 11 are described for the first time in the present study. However, MATA was not detected in the present study, thus it is not possible to definitively assess that SECO itself is the subject of lactone closure. EL was already identified as the final product of MATA conversion by gut microbiota 31, 32, hence 8, 9, 10, and 11 are most likely the intermediates of this transformation as well. For the first time, it is shown that SECO is transformed also into the cyclic ethers 12, 13, 14, and 15, similar in structure to AHS. These intermediates can likely undergo the above-described demethylation and dehydroxylation reactions. It is noteworthy that final product enterofuran (EF), albeit not identified in this study, has been described as product of transformation of AHS by intestinal bacteria, via 12, 14, and 15 intermediates 33, and resulted as a minor product of SECO incubation with the gut microbiota 31. Dehydration of SECO to AHS could be obtained chemically by acid treatment 34 and the gut intestinal bacteria have never been described to perform this reaction. The experimental conditions and the treatment of the samples exclude that the AHS intermediates are artifacts, also considering that AHS itself was never detected in the present study, despite the relatively high concentration of SECO, in particular during microbiota cultures. Therefore, the bioconversion yielding 12 and/or the other AHS ethers still remains to be clarified and deserves deeper investigation. The detection for the first time of some metabolites could also be due to their instability in air conditions and at room temperature, which may have prevented their detection in previous works. The addition of formic acid may have allowed their stabilization and detection both in in vivo and in vitro samples.
From the qualitative point of view, the pattern of metabolites observed in feces and urine was very similar in the five subjects. The experimental design, not aimed to measure total daily excretion in feces and urine, and the lack of standards for many metabolites did not allow comparison of the amounts of diverse metabolites among different volunteers. However, the concentration of lignan metabolites in feces and urine was reasonably consistent with total daily excretion reported by previous intervention studies 24, 25, 27, 35. Furthermore, the increase of SDG metabolites that can be quantified by 10- to 100-fold in both urine and feces as a consequence of flaxseed administration, was in agreement with the increase of enterolignans reported in literature 13, 25, 27.
Regarding the enterolignans, based on studies with cultivable bacteria, EL-producing taxa are subdominant in the gut microbiota 14. All the five volunteers enrolled in the present study harbored functionally active lactone-producing microorganisms and, as a consequence of flaxseed supplementation, excreted EL and lactone intermediates with urine. In particular, also before flaxseed supplementation, EL occurred in all the fecal samples and, likewise ED, was present in both feces and urine in the native form and/or modified by phase II human enzymes. Interestingly, high interindividual differences were observed among the fecal cultures of the five subjects. In particular, the microbiota culture of V1 was the sole producing intermediates 8–11 and yielding EL in 24 h of incubation, while in the other cultures lactone intermediates did not appear and EL was produced in negligible amount toward the end of the incubation. The diverse efficiency in lactones production in vitro can be ascribed to the different survival and/or growth kinetics of lactone-producing strains under the culture conditions.
The intermediates 5, 9, 11, 13, 15 were found in their native form and/or modified by phase II human enzymes in the feces and urine of all the subjects at the end of the treatment, demonstrating that the proposed pathway of bacterial transformations of SECO through diols, lactones, and ethers occurred also in vivo and that intermediates were efficiently absorbed at colonic level. While intermediate 5 and 11 were previously described among metabolites occurring in urine of humans receiving flaxseed 19, 28, the occurrence of 9, 13, and 15 as a consequence of flaxseed administration is reported in the present study for the first time.
Most of the lignan metabolites occurring in feces were in their native form. Only ED and EL were present also as sulfates, according to the occurrence of enterohepatic circulation for these compounds 35, 36. In urine, the vast majority of metabolites occurred as sulfate, glucuronide, and sulfoglucuronide, while ED and EL were excreted also in the native form, in agreement with previous literature 36. SDG and intermediates 2 and 15, occurring in feces, were not found in urine under any form, suggesting that likely they were not absorbed through the colon. On the other hand, compounds 3, 5, 9, and 11 were not detected in feces, probably because they were rapidly metabolized by intestinal bacteria, and/or they are rapidly absorbed in the hindgut.
As a whole, the metabolic pathway leading quickly to lactone formation can compete with the one passing through diol metabolites, resulting in ED and EL production. Furthermore, the identification of novel metabolites and their detection in urine and feces open new perspectives toward detailed quantification studies and their investigation of their biological activity.
Acknowledgments
A.Q. was supported with a Ph.D. grant by Spinner 2013, European Union, Regione Emilia Romagna. A.A. and A.Q. carried out fermentation experiments; A.L. and S.R. managed the in vivo study; R.G.-V. carried out the chemical analysis; F.T.-B. and M.R. were responsible for the study design and manuscript preparation. All authors critically reviewed the manuscript and approved the final version submitted for publication.
The authors have declared no conflict of interest.




