Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells
Abstract
Scope
Hydroxytyrosol (HT) is the major phenolic compound in virgin olive oil (VOO) in free and conjugated forms that may exert health benefits against atherosclerosis. The native form of HT is undetectable in plasma due to an extensive first pass phase II metabolism. Therefore, it is necessary to find strategies to obtain HT metabolites and to demonstrate their protective role against the endothelial dysfunction.
Methods and results
Biosynthesis of the main plasmatic HT metabolites was performed through Caco-2 cells. The bioactivity of HT and the mixture of metabolites was tested at physiological concentrations (1, 2, 5, and 10 μM) in human aortic endothelial cells (HAEC) co-incubated with TNF-α (10 ng/mL) for 18 and 24 h. After the incubations, cells and media were analyzed to test possible deconjugation of metabolites or conjugation of HT. Both HT and metabolites significantly reduced the secretion of E-selectin, P-selectin, ICAM-1, and VCAM-1, but only HT metabolites further reduced MCP-1 at 24 h. HT underwent a conjugation process after incubation leading to its main metabolites in a dose-dependent manner.
Conclusion
Physiological HT metabolites, synthetized for the first time by using an intestinal cell model, might be responsible in part for the protection against endothelial dysfunction.
Abbreviations
-
- ANOVA
-
- one-way analysis of variance
-
- ATCC
-
- American type culture collection
-
- BSA
-
- bovine serum albumin
-
- CM
-
- complete medium
-
- CV
-
- coefficients of variation
-
- CVD
-
- cardiovascular disease
-
- DMEM
-
- Dulbecco's modified eagle's medium
-
- ELISA
-
- enzyme-linked immunosorbent assays
-
- ESI
-
- electrospray ionization
-
- GlucHT
-
- hydroxytyrosol glucoronide
-
- glucHVac
-
- homovanillic acid glucuronide
-
- glucHVAlc
-
- homovanillic alcohol glucuronide
-
- HAEC
-
- human aortic endothelial cells
-
- HBSS
-
- Hanks’ balanced salt solution
-
- HSS
-
- high strength silica
-
- HT
-
- hydroxytyrosol
-
- HUVEC
-
- human umbilical vein endothelial cell
-
- HVac
-
- homovanillic acid
-
- HVAlc
-
- homovanillic alcohol
-
- ICAM-1
-
- intercellular cell adhesion molecule-1
-
- LDH
-
- lactate dehydrogenase
-
- LPS
-
- lipopolysaccharide
-
- LSD
-
- Fisher's least significant difference
-
- MCP-1
-
- monocyte chemotactic protein-1
-
- MD
-
- mediterranean diet
-
- MTT
-
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
-
- OO
-
- olive oil
-
- SD
-
- standard deviation
-
- SEM
-
- standard error of the mean
-
- μSPE
-
- micro-elution solid-phase extraction
-
- SPSS
-
- statistical package for the social sciences
-
- SRM
-
- selected reaction monitoring
-
- sulfHT
-
- hydroxytyrosol sulfate
-
- sulfHVac
-
- homovanillic acid sulfate
-
- sulfHVAlc
-
- homovanillic alcohol sulfate
-
- TEER
-
- transepithelial electrical resistance
-
- TNF-α
-
- tumor necrosis factor-alpha
-
- TQD
-
- triple quadrupole detector
-
- VCAM-1
-
- vascular cell adhesion molecule-1
-
- VOO
-
- virgin olive oil
1 Introduction
Virgin olive oil (VOO) a key component of the Mediterranean Diet (MD) has shown to contribute to cardiovascular disease (CVD) prevention. Part of this effect has been associated to VOO phenolic content 1, 2. Results from the PREDIMED study, the first randomized clinical trial designed to assess the beneficial effects of the MD on the primary prevention of CVD, reported that the MD supplemented with VOO has a dual effect on the prevention of CVD, reducing classical cardiovascular risk factors and also having an intense anti-inflammatory effect through the downregulation of endothelial dysfunction markers related to atherosclerosis, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), E- and P-selectin 3.
Hydroxytyrosol (HT) is the major phenolic compound in VOO in both free and conjugated form, mainly as oleuropein aglycone structures commonly named secoiridoids. HT has shown a wide range of biological functions, such as antioxidant, anticancer, anti-inflammatory, and neuroprotective activities, as well as having beneficial effects on the cardiovascular system 4, 5. The potential health benefits of HT have stimulated intense research on the bioavailability and metabolism of VOO phenolic compounds, a requisite to support their potential benefits for human health. Previous studies have shown that VOO phenolic compounds undergo rapid hydrolysis under gastric and intestinal alkaline conditions resulting in significant increases in the amount of free HT entering the small intestine 6, 7. Thus, HT becomes the major phenolic compound absorbed from the intestinal tract, which is further subjected to an extensive first pass metabolism, both in the intestinal epithelium and the liver, leading to the formation of phase II metabolites, mainly sulfate, methyl, and methyl–sulfate conjugates and glucuronides of HT 8-11.
Consequently, after the consumption of normal doses of VOO (less than 50 g/day) the native form of HT is not detected in plasma and the potential health benefits of VOO phenolic compounds could be either attributed to the biological activity of phase II metabolites or to free HT generated at cellular level by enzymatic deconjugation. So, it becomes essential to obtain the plasmatic HT metabolites, test them in cell-based experimental models at physiological concentrations and examine a possible deconjugation process of these metabolites at the cellular level.
The physiological significance of most of the published articles on polyphenols bioactivity has been questioned as they still describe the effects of molecules that are only present in planta or in food. Nevertheless, detailed studies applying plasmatic and microbial metabolites of phenolic compounds are starting to establish reasonable mechanisms through which these physiological metabolites may exert beneficial effects 12. In the case of VOO phenolics, a previous study tested the in vitro radical scavenging capacity of some of the HT metabolites observing that homovanillic alcohol (HVAlc) and the 3-O-glucuronide conjugate appeared to be active in terms of antioxidant activity 13. However, further observations suggested that none of the glucuronide conjugates of HT displayed antioxidant activity to protect LDL against oxidation 14.
Regarding the antiatherogenic effects, only HT in its native forms has been tested on preclinical experimental models describing its protective effects against atherosclerosis 15, 16 and no data have been published in relation to its biological metabolites. As reported by Valls et al., these metabolites could be involved in reducing the endothelial dysfunction in humans 17. However, the mechanisms have not been demonstrated.
Endothelial dysfunction is involved in the early stages of atherosclerosis and is a key process in the development of CVD 18. Briefly, the endothelial dysfunction in the early stages of atherosclerosis includes rolling (mediated by E- and P-selectin) and firm adhesion (mediated by VCAM-1 and ICAM-1) of blood leukocytes to the activated endothelial monolayer and directed migration (mediated by the monocyte chemotactic protein-1; MCP-1) of the bound leukocytes into the intima 18, 19. So the endothelial dysfunction biomarkers E-selectin, P-selectin, VCAM-1, ICAM-1, and MCP-1 were selected in the present study as they have been considered as useful predictive biomarkers of atherosclerosis 20. The surface and soluble expression of these molecules is greatly increased in atherosclerotic lesions as a result of stimulation by pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α). This cytokine was used in the present study as a stressor molecule for assessing the underlying mechanisms as it induces the release and surface expression of cell adhesion molecules 21, 22.
So, the main aim of the present study was to optimize the use of Caco-2 cell line as an alternative approach to obtain biological metabolites of HT previously detected in human plasma, and to evaluate their in vitro endothelial protective effect compared to HT alone at physiological concentrations 10.
2 Materials and methods
2.1 Solvents and reagents
HT (99.51% of purity, synthetic) was provided by Seprox Biotech (Madrid, Spain). Catechol, as the internal standard, (IS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydroxytyrosol-3-O-sulfate was custom-synthesized by Toronto Research Chemicals Inc. (Toronto, ON, Canada). Methanol (HPLC-grade), acetonitrile (HPLC-grade), ethanol, and dimethyl sulfoxide (DMSO) were purchased from Scharlab (Barcelona, Spain) and orthophosphoric acid (85%) from Panreac (Barcelona, Spain). Milli-Q water was obtained from a Milli-Q water purification system (Millipore Corp., Medford, MA, USA).
2.2 Caco-2 cell culture treatments to obtain HT metabolites
Caco-2 cell line was purchased from ATCC (American Type Culture Collection, US). Cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) high glucose (HyClone, Thermo scientific, Logan, Utah) supplemented with 10% of fetal bovine serum (Sigma-Aldrich, Madrid, Spain), 1% L-glutamine (HyClone, Thermo Scientific, Logan, UT, USA), 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; HyClone, Thermo Scientific), penicillin and streptomycin (100 mg/mL) (Gibco, Spain). Cells were cultured at 37°C, in a 5% CO2 humidified incubator. Media culture was replaced every 2 days and phosphate-buffered saline (PBS; HyClone, Thermo Scientific) was used to wash the cells. In preliminary experiments, the stability of HT was evaluated over time (6, 12, 24, and 48 h) with DMEM or Hanks’ Balanced Salt Solution (HBSS; HyClone, Thermo Scientific) media and finally, HBSS was chosen to perform all the experiments due to a better stability of HT over the time in these media.
In order to select the optimal cell treatment to obtain HT metabolites, T75 flask and transwell inserts (both from Corning Costar, Netherlands) were tested as two different culture conditions (Fig. 1A). On the one hand, Caco-2 cells were seeded in T75 flasks at a density of 6.6 × 104 cells/mL and their HT conjugation rate was evaluated at two differentiation states: three days after seeding (A treatment) or after 21 days of culture (B treatment). On the other hand, Caco-2 cells were seeded in the apical side of transwell inserts at a density of 4.6 × 104 cells/mL and cultured for 21 days (C treatment). D treatment consisted of 18 days of Caco-2 culture (4.6 × 104 cells/mL) in the apical side of transwell inserts and, after 18 days, an additional co-culture of Caco-2 cells was seeded in the basolateral side (5 × 104 cells/mL) for three more days, making a total of 21 days. Monolayer formation in transwell inserts was evaluated by transepithelial electrical resistance (TEER) measurement with a voltohmmeter (Millipore Corp.) selecting cells with TEER > 250 Ω/ cm2. For all treatments (A, B, C, and D), the initial concentration of HT in the culture medium was 100 μM (HT stock solution 100 mM in methanol) and three exposure times were tested (6, 12, and 24 h). To preempt cytotoxicity, methanol concentrations never exceeded 0.1% (v/v) in culture media. Finally, supernatants of T75 flasks (A and B treatments) and the sum of the apical and basolateral supernatants from transwell inserts (C and D treatments) were collected. Prior to chromatographic analysis (see Section 2.4), HT and its metabolites were extracted from the cell media using micro-elution solid-phase extraction (μSPE) plates with OASIS HLB μElution Plates 30 μm (Waters Corp., Milford, MA, USA) according to the previous study 23.

After the selection of 21-day cultured cells in T75 flasks with 24 h of HT incubation (B treatment) as the optimal treatment (see Section 3.2), different HT concentrations (10, 15, 20, 25, 50, and 100 μM) in the culture medium were assayed in order to test the effect of the HT concentration on the transformation yield of HT into its metabolites.
2.3 Purification of HT metabolites from Caco-2 culture medium
Once the B treatment (21-day cultured cells in T75 flasks) incubated with 100 μM HT for 24 h was selected as the optimal treatment for obtaining HT metabolites, the μSPE plates were replaced by SPE cartridges with a higher sorbent size (OASIS HLB Elution Plates 6 g, 35 cc; Waters, Milford, MA, USA) to obtain major amounts of HT metabolites. The conditioning of the SPE cartridges was done by adding sequentially 35 mL of methanol and 40 mL of milli-Q water acidified at pH 2 with acetic acid (Fig. 1B). Extractions were done by loading 50 mL of Caco-2 supernatant, which had been previously mixed with 50 mL of phosphoric acid at 4%. The loaded cartridges were washed with 30 mL of milli-Q water and 20 mL of methanol at 5%. Finally, the retained phenolic compounds were eluted using 60 mL of methanol at 100%. This elution solvent was evaporated to dryness under a nitrogen stream in an evaporating unit at 30ºC (PIERCE Model 18780, IL, USA). In order to obtain a more concentrated solution, after evaporation, eluates from several cartridges were collected and consecutively evaporated in the same tube. The residue was reconstituted with 1 mL of methanol. The extract was filtered through a 0.22 μm nylon syringe filter (Teknokroma, Barcelona, Spain) and transferred to the autosampler vial before the quantification of HT metabolites by liquid chromatography. This stock solution of HT metabolites was used to study their effectivity on endothelial dysfunction model in HAEC.
2.4 Chromatographic analysis of HT metabolites
HT metabolites were analyzed by AcQuity UPLCTM coupled to PDA detector and a triple quadrupole detector (TQD)TM mass spectrometer (Waters, Milford). The analytical column was a High Strength Silica (HSS) T3 column (100 × 2.1 mm, 1.8 μm). During the analysis, the column was kept at 30ºC and the flow rate was 0.3 mL/min using 0.2% acetic acid as solvent A and methanol as solvent B. The elution started at 3% of eluent B and was linearly increased to 15% of eluent B in 6 min. Then, it was linearly increased to 70% in 8 min and further increased to 100% in 3 min. Then, it was returned to the initial conditions in 1 min, and the re-equilibration time was 2 min. The injection volume was 2.5 μL.
A TQD mass spectrometer was used as the detector system. Ionization was done by electrospray (ESI) in the negative mode and the data were collected in the selected reaction monitoring (SRM) mode. The ionization source parameters were capillary voltage of 3 KV, source temperature of 150ºC, and desolvation temperature of 400ºC with a flow rate of 800 L/h. Nitrogen (99% purity, N2LCMS nitrogen generator, Claind, Como, Italy) and argon (≥99.99% purity, Aphagaz, Madrid, Spain) were used as the cone and collision gases, respectively. Two transitions were acquired for HT and the metabolites generated, one for quantification and a second for confirmation purposes. The optimized SRM conditions are presented in Supporting Information Table 1. The software used was MassLynx 4.1. HT and HT-3-O-sulphate were quantified with their own calibration curves, and the other HT metabolites were tentatively quantified by using the calibration curve of HT. The calibration curves were constructed by using cellular medium free of HT metabolites.
2.5 HAEC cell culture for testing HT and HT metabolites activity
HAEC (Cascade Biologics™, Portland, OR, USA) at the fifth passage were seeded on Nunclon™ Δ surface 12-well plates at a density of approximately 44 × 103 of viable cells/mL (1 mL/well). For the first 24 h, cells were maintained in complete cell culture medium (CM) composed of M-200 medium supplemented with 2% (v/v) low-serum growth supplement, 10 mg/mL gentamicin, 0.25 mg/ml amphotericin B (purchased from Gibco by Life Technologies, Madrid, Spain), 100 U/mL penicillin and 100 mg/mL of streptomycin (from Biowest-Labclinics, Barcelona, Spain). The cells were grown to confluence at 37°C in a humidified incubator (Heracell 150; Madrid, Spain) with an atmosphere containing 5% CO2. The medium was replenished every 2 days with fresh CM. Viewed under an IMT2 microscope (Olympus, Barcelona, Spain), confluent monolayers displayed a typical monolayer phenotype of quiescent endothelial cells after 5 days in culture.
Experiments of time and dose-response were conducted in order to establish the final conditions of TNF-α as a stimulant. Stock solutions used for the experiments were: 5 mM of HT (commercial) dissolved in sterile H2O and 10 mM of the purified solution of HT metabolites dissolved in methanol (Panreac, Madrid, Spain). Appropriate dilutions were prepared until the final concentrations to be tested in the culture media (1, 2, 5, and 10 μM) were obtained. To preempt cytotoxicity, methanol concentrations never exceeded 0.1% (v/v) in culture media. Then, HAEC were co-incubated with HT or HT metabolites at 1, 2, 5, and 10 μM and TNF-α (10 ng/mL; Calbiochem, Darmstadt, Germany), the respective vehicle control alone (sterile H2O for HT or absolute methanol for HT metabolites) or TNF-α alone (10 ng/mL) for 18 or 24h. Additional experiments were performed to test HT at lower concentrations (<1 μM) equivalent to 9% of the residual HT remaining in the HT metabolites mixture at the concentrations tested (0.09, 0.18, 0.45, and 0.9 μM).
After co-incubation, supernatants were collected and stored at –20°C for batched measurements of the soluble forms of the selected cell adhesion molecules (VCAM-1/CD106, E-selectin/CD62E, P-selectin/CD62P and ICAM-1/CD54) or chemokine (MCP-1/CCL2) and for the activity of the lactate dehydrogenase (LDH) released. Cells were lysed with NaOH 0.5 M and stored at –80°C for measuring total cellular protein amount in order to adjust cell adhesion molecules and chemokine measurements.
In order to study the possible deconjugation of HT metabolites or conjugation of HT during the incubation with HAEC cells, the metabolite pattern in the cells and also in the culture media at the end of the incubation (24 h) was performed according to the same chromatographic method described in Section 2.4.
2.6 Cytotoxicity assays
The cytotoxicity effect of HT (25-200 μM) on Caco-2 cells was tested using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay 24. 200 000 Caco-2 cells/mL were seeded in 24-multiwell plates and incubated with DMEM. After 24 h, DMEM media were replaced with the same media enriched with different concentrations of the HT for 24 h at 37°C and 5% CO2. Cells were then washed twice with PBS. DMEM enriched in 0.5 mg/mL of MTT (Sigma-Aldrich, Madrid, España), was added to each well and incubated for 3 h. To determine the formazan production, once supernatants discarded, cells were lysed using an extraction solution composed of DMSO and ethanol at a concentration of 1:1. A colorimetric assay was performed at 570 nm optical density.
The cytotoxicity effect of HT or HT metabolites on HAEC was assessed with a Cytotoxicity Detection Kit LDH (Roche Applied Science, Mannheim, Germany) as previously described 25. For these experiments, TNF-α treatment was considered the maximum stress condition for the cells. The activity of the LDH released from the cells was measured in cell-free supernatants collected at the end of the experiment. Results are expressed as the mean optical density (OD: 492 nm) and standard deviation (SD; error bars) of LDH produced by the cells under each treatment condition.
2.7 Total cellular protein quantification
In order to adjust the soluble cell adhesion molecules (E-selectin, P-selectin, VCAM-1, and ICAM-1) and chemokine (MCP-1) total cellular protein concentration from cell lysates was determined using the Bradford protein assay from Bio-Rad with bovine serum albumin (BSA). A range of concentrations of BSA were used as the calibration standards for the protein assay. The absorbance was measured at 595 nm. The results were expressed in milligrams of total cellular protein per milliliter compared to the maximum stimulation with TNF-α (10 ng/mL).
2.8 Measurement of soluble cell adhesion molecules and chemokine protein secretion by HAECs stimulated with TNF-α
Luminex® Performance Assay was used to determine VCAM-1, E-selectin, ICAM-1, and P-selectin protein secretion and DuoSet® enzyme-linked immunosorbent assays (ELISA) was used to detect MCP-1/CCL2 chemokine protein secretion. Both protein detection kits were from R&D Systems (Vitro, Madrid, Spain) and the tests were conducted according to the manufacturer's protocol.
All experimental data were compared to the outcomes in the TNF-α-alone incubation, since this achieved the maximal secretion of soluble cell adhesion molecules or chemokine. A vehicle-alone control (sterile H2O for HT or absolute methanol for HT metabolites) was run in parallel and used as the control in the experiments. Results are expressed as the percentage of soluble cellular adhesion molecules or chemokine protein secretion adjusted by total cellular protein and standard error of the mean (SEM; error bars) compared to the maximum stimulation with TNF-α (10 ng/mL).
2.9 Statistical analyses
Unless otherwise stated, all experiments were performed at least twice and each incubation condition was set up in duplicate. One-way analysis of variance (ANOVA) followed by the Fisher's least significant difference (LSD) post-hoc test was used for multiple comparisons. A value of p < 0.05 was considered statistically significant.
A requisite for the analytical quality of the model was the control of several aspects involved in the cellular process and analytical performance of measurements. Thus, we evaluated the precision of the model by calculating the SD, SEM, and the coefficients of variation (CV). All the results were analyzed with the Statistical Package for the Social Sciences (SPSS) software (version 22.0).
3 Results
3.1 Cytotoxicity
Results from the Caco-2 MTT cytotoxicity assay showed that HT was not cytotoxic at doses from 25 to 150 μM (data not shown).
The cytotoxicity assay of the supernatants taken at the end of the study corresponding to the co-incubation of HT or HT metabolites at different concentrations (1, 2, 5, and 10 μM) and TNF-α (10 ng/mL) for different exposure times (18 and 24 h), measured as the activity of LDH release showed no difference compared to the TNF-α (10 ng/mL) treatment alone, confirming that HT and HT metabolites are not cytotoxic at the concentrations and times tested (Supporting Information Fig. 1). Lower concentrations of HT (<1 μM) did not show any toxicity (data not shown).
3.2 Effect of Caco-2 cell pretreatment on HT metabolite production
Table 1 shows the metabolism yield after exposing HT (100 μM) to different Caco-2 cell pretreatment models (A, B, C, and D) at different exposure times (6, 12, and 24 h). Comparing the T75 flask and transwell treatments (A and B versus C and D, respectively), the results showed that HT was much less stable in transwell inserts and also showed less metabolic activity compared to cells cultured in T75 flasks. Regarding the different exposure times, the highest metabolic transformation was obtained after 24 h. Longer exposure times were tested, but more than 24 h did not increase the metabolism yield and the total amount of metabolites obtained decreased with the time, probably due to a degradation process.
| Concentration (μM) | 6 h | 12 h | 24 h | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T75 FLASK | TRANSWELL | T75 FLASK | TRANSWELL | T75 FLASK | TRANSWELL | |||||||
| A | B | C | D | A | B | C | D | A | B | C | D | |
| HT* | 76.10 ± 10.60a) | 66.47 ± 10.47b) | 41.87 ± 10.25c) | 23.52 ± 9.81c) | 45.15 ± 15.71a) | 39.20 ± 5.24b) | 9.29 ± 3.04c) | 13.90 ± 1.53c) | 39.91 ± 1.32a) | 6.98 ± 0.20b) | 5.18 ± 3.77b) | 5.25 ± 0.33b) |
| sulfHT** | 0.11 ± 0.00a) | 10.77 ± 0.88b) | 10.08 ± 3.02b) | 10.37 ± 3.30b) | 0.63 ± 0.77a) | 27.73 ± 3.57b) | 758 ± 2.62c) | 7.27 ± 3.58c) | 2.33 ± 0.83a) | 63.42 ± 9.23b) | 8.00± 2.44c) | 7.99 ± 1.33c) |
| 4-GlucHT* | nd | 0.19 ± 0.04 | 1.03 ± 0.63 | 1.08 ± 0.72 | nd | 0.48 ± 0.19 | 0.62 ± 0.40 | 0.62 ± 0.35 | 0.13 ± 0.03a) | 0.60 ± 0.13b) | 0.64 ± 0.43b) | 0.76 ± 0.39b) |
| 3-GlucHT* | nd | 0.06 ± 0.03 | 0.03 ± 0.01 | 0.04 ± 0.02 | nd | 0.10 ± 0.11a) | 0.01 ± 0.01b) | 0.02 ± 0.01b) | 0.02 ± 0.01a) | 0.31 ± 0.06b) | 0.02 ± 0.01a) | 0.03 ± 0.00b) |
| sulfHVac* | nq | nq | 0.08 ± 0.02 | 0.07 ± 0.04 | nq | nq | 0.06 ± 0.03 | 0.07 ± 0.06 | nq | 0.06 ± 0.04 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| glucHVac* | nd | nd | 0.02 ± 0.02 0 | 0.03 ± 0.04 | nd | nd | 0.04 ± 0.02 | 0.03 ± 0.01 | nd | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 |
| sulfHVAlc* | 0.03 ± 0.00a) | 0.28 ± 0.02b) | 0.03 ± 0.00a) | 0.05 ±0.02a) | 0.02 ± 0.00 | nq | 0.03 ± 0.02 | 0.02 ±0.02 | 0.02 ± 0.02a) | 2.27 ± 0.48b) | 0.01 ± 0.00a) | 0.02 ± 0.02a) |
| glucHVAlc* | nq | 0.11 ± 0.02* | nq | nq | nq | 0.35 ± 0.08 | nq | nq | nq | 0.29 ± 0.16 | nq | nq |
- A: Three-day cultured cells in T75 flasks.
- B: Twenty-one-day cultured cells in T75 flasks.
- C: Twenty-one-day cultured cells in the apical side of transwell inserts.
- D: Eighteen-day cultured cells in the apical side plus 3-day co-cultured cells in the basolateral side of transwell inserts (21 days in total).
- For treatments, C and D values were the sum of apical and basolateral sides. The initial concentration of HT in culture media for all the experiments was 100 μM. Based on the yield transformation of HT into phase II metabolites, the differentiated cells in flasks (B treatment) after 24 h of HT exposure were selected as the best approach.
- HT: hydroxytyrosol; sulfHT: hydroxytyrosol sulfate; 4-GlucHT: hydroxytyrosol 4’ glucuronide; 3-GlucHT: hydroxytyrosol 3’ glucuronide; sulfHVac: homovanillic acid sulfate; glucHVac: homovanillic acid glucuronide; sulfHVAlc: homovanillic alcohol sulfate; glucHVAlc: homovanillic alcohol glucuronide. n.d.: not detected; n.q.: not quantified
- Different superscript letters (a,b,c) mean significant differences between treatments (A,B, C, and D) in the same time tested for each metabolite.
- One-way analysis of variance (ANOVA) followed by the Fisher's least significant difference (LSD) posthoc test was used for multiple comparisons (p < 0.05).
- *Quantified using the calibration curve of HT.
- **Quantified using the calibration curve of HT-3-O-sulfate.
Significant differences (p < 0.05) were observed on the biotransformation of HT into its main metabolites between the two T75 flask models (A and B) at all time points studied (6, 12, and 24 h), observing that Caco-2 cells under B treatment (21-day cultured cells) had a significantly higher metabolic activity compared to A treatment (3-day cultured cells). These results confirmed that differentiated Caco-2 cells can exert a higher conjugation enzymatic activity (catechol-O-methyltransferase, UDP-glucuronosyltransferase, and sulfotransferase) compared to nondifferentiated cells and thus, they appeared to be a more appropriate approach to obtaining HT phase II metabolites. In the case of the transwell models, no significant differences were observed in the production of any HT metabolite when comparing C and D treatments. This could be attributed to the low enzymatic activity of the nondifferentiated co-cultured cells over the 3 days used in D treatment.
Based on the biotransformation yield of HT into phase II metabolites, the differentiated cells in T75 flasks (B treatment) after 24 h of HT exposure were selected as the best approach to obtaining HT metabolites with the lowest concentration of residual free HT in the culture medium. Apart from being more efficient in terms of metabolic activity, T75 flasks are much cheaper, easier to handle, and have a bigger surface compared to transwell inserts, which allows higher amounts of metabolites to be obtained.
Moreover, different HT concentrations in the culture medium were assayed in order to test the effect of the initial HT concentration on its transformation yield into phase II metabolites. Results showed that the metabolic transformation was similar in all concentrations tested (10, 15, 20, 25, 50, 100 μM) (Supporting Information Table 2). Based on these results and considering that after the incubation of 100 μM HT a major amount of HT metabolites was obtained, 100 μM HT was selected to produce the stock solution of HT metabolites.
3.3 Effect of HT and HT metabolites on E-selectin, P-selectin, VCAM-1, ICAM-1, and MCP-1 protein secretion
After 18 h of co-incubation, significant reductions were observed with HT at all tested doses in E-selectin levels (p<0.05), with reductions ranging from 32.7 to 45.9% compared to TNF-α alone. HT metabolites significantly reduced E-selectin protein secretion by 36.6% (p<0.05) only at 10 μM (Supporting Information Table 3 and Fig. 2).
After 24 h of co-incubation, the reduction trend observed at 18 h was confirmed. HT reduced E-selectin, P-selectin, VCAM-1, and ICAM-1 at all tested doses (1, 2, 5, and 10 μM) compared to TNF-α alone (p<0.05) but the reductions did not follow a dose-dependent response (Fig. 2). HT metabolites reduced E-selectin, P-selectin, VCAM-1, and ICAM-1 at 2, 5, and 10 μM and additionally, they also reduced MCP-1 significantly at 5 and 10 μM compared to TNF-α alone. In this case, the molecules were reduced in a dose-dependent manner (p < 0.05; Fig. 2).
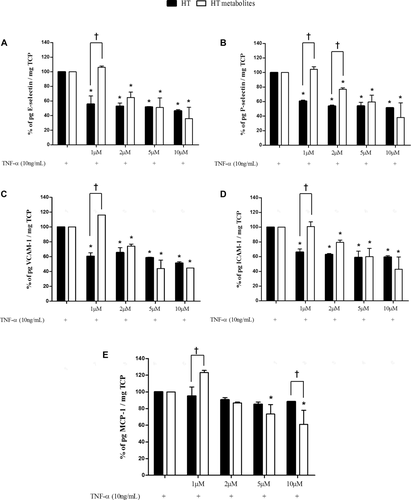
It is noteworthy that at 10 μM, HT metabolites were more effective than HT at reducing E-selectin, P-selectin, VCAM-1, ICAM-1, and MCP-1 with respective reductions of 62.4, 61.9, 55.4, 57.0, and 39.0% compared to HT (53.6, 48.6, 48.7, 40.6, and 11.7 %, respectively (Supporting Information Fig. 2 and Table 3). However, these reductions in HT metabolites compared to HT were only significant for the MCP-1 molecule.
To exclude the effects of the residual HT present in the HT metabolite mixture, lower concentrations of HT (0.9, 0.45, 0.18, and 0.09 μM) equivalent to the 9% of the tested doses of HT metabolites (10, 5, 2, and 1 μM, respectively) were tested (Fig. 3). Results showed that concentrations of 0.45 and 0.9 μM were effective in the reduction of VCAM-1, ICAM-1, P-selectin, and E-selectin indicating that the activity of the metabolite mixture could be due in part to the residual HT in the case of the highest doses (5 and 10 μM). However, when testing 0.18 μM HT any significant effect was observed in the inhibition of the adhesion molecules, whereas the concentration of 2 μM of the HT metabolites mixture exerted a significant effect in the reduction of all adhesion molecules. In addition, the reduction percentages observed after testing 0.45 and 0.9 μM of HT were 10% lower compared to their respective HT metabolites mixtures (10 and 5 μM). These results indicate that HT metabolites are in part responsible for the attenuation of the inflammation-induced expression of adhesion molecules and could probably exert a synergistic effect together with HT. In the case of the chemokine MCP-1, lower concentration assays confirmed that HT has no effect on reducing MCP-1 at any concentration.
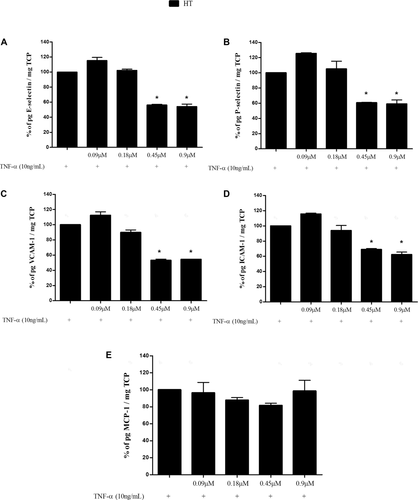
Vehicle control condition for HT (sterile H2O) or HT metabolites (absolute methanol) are not shown due to their low values of E-selectin, P-selectin, VCAM-1, ICAM-1, and MCP-1 protein secretion concentrations. They were not within the limits of detection of the techniques used for determining these molecules in this study, so they were assumed as practically zero.
3.4 Analysis of HT and metabolites in cells and culture media after incubation
The metabolite pattern in the cells and also in the medium at the end of the incubation was analyzed. Unexpectedly, results showed that after 24 h of incubation with HT, the native compound underwent a conjugation process leading to its main metabolites in a dose-dependent manner (Fig. 4), revealing that HAEC cells have conjugation enzymatic capabilities, mainly catechol-O-methyltransferase and sulfotransferase activity (Supporting Information Table 4). On the other hand, after incubating the HT metabolite mixture with HAEC cells, small amounts of free HT were detected in cells and HT did not increase in parallel to the tested dose, indicating that the metabolites were not hydrolysed in cells during the assay and probably HT was degraded during incubation. Results from culture media presented a very similar profile compared to cells (Fig. 4).

4 Discussion
The present study is among the first to obtain a mixture of physiological phase II metabolites of HT and demonstrating that they are able to exert an effect in the reduction of the endothelial dysfunction biomarkers in an in vitro endothelial cell model at plasmatic concentrations achievable following ingestion of olive oil 10. The remaining amount of unaltered HT in urine is very low (<1%) 8, 11 and undetectable in plasma after the consumption of nutritional amounts of VOO phenols and, therefore, the role of HT phase II metabolites in preventing atherosclerosis must be considered. However, in vitro studies with endothelial cell models carried out to date used HT 26, 27 rather than the majority forms that appear in human plasma.
So, in order to perform mechanistic studies, first, it is necessary to obtain the HT metabolites, which are not commercially available. For this proposal, in the present study Caco-2 cells were incubated with HT in order to produce a mixture of HT metabolites resembling the human plasma after the intake of virgin olive oil. Previous studies have used different approaches to obtaining phase II metabolites of phenolic compounds 28-31. The chemical synthesis approach could be considered the cheapest, but its main drawback is that most chemical procedures yield mixtures of α- and β–anomers along with side products 32. Biocatalysis-assisted synthesis using tissue homogenates or recombinant conjugating enzymes (glucuronosyltransferases and sulfotransferases) is an alternative strategy for preparing phenolic conjugates 33. However, enzymatic synthesis requires the use of either expensive enzymes, co-factors (uridine 5′-diphospho-glucuronic acid and adenosine-5′-phosphosulfate), or liver microsomes and the degree of conversion is also very low 28. Another alternative could be the use of biological samples to obtain phenolic phase II metabolites, which have been reported previously for the isolation of resveratrol glucuronide conjugates from human urine 30, thymol sulfate from rat urine 29, or catechin and quercetin from rat plasma 31. The main drawback of this approach is that it requires the performance of an in vivo study and the amounts obtained are very small.
In relation to VOO phenolic metabolites, a previous study attempted to synthetize HVAlc and the glucuronic forms of HT using porcine liver microsomes 34. In another study the potential hepatic metabolism of VOO phenolic compounds by HepG2 cell lines was tested, and the glucuronide, methylglucuronide, and methyl conjugates of HT were the main metabolites produced 35. In both studies no sulfate conjugates were produced, which could be attributed to the low sulfotransferase activity of HepG2 cell line and its very low/partial expression of cytochrome P-450 36. Our previous studies in humans indicate that sulfation constitutes the major pathway for HT metabolism in humans 9, 10, and up to date, any effective and simple strategy to obtain a pool of the real phase II circulating HT metabolites has been developed.
In the present study, we demonstrate that the use of Caco-2 cells as bioreactors for the generation of phase II metabolites of HT is a very appropriate tool given their availability, long life-span, stable phenotype, high metabolic efficiency, ease of handling, and the fact that they do not require the use of human volunteers or animals. Caco-2 cells, which are usually used as a model for studying the intestinal transport and metabolism of some dietary compounds 37, are obtained from human colon adenocarcinoma and spontaneously differentiate in a confluence culture as intestinal absorptive cells due to changes in their morphology 38. Once they are differentiated, Caco-2 cells exert phase II conjugate enzymes and cytochrome P-450 isoforms, and are useful for studying their metabolic activity and characterizing human circulating metabolites, as reported in previous studies in which the metabolic profile was determined after a polyphenol exposure 7, 37.
This approach also allowed to obtain a metabolic profile very similar to the human plasma after the consumption of nutritional amounts of VOO phenols described in our previous study 10. In this study the main metabolites detected in plasma after a sustained intake of 25 mL of VOO with high phenolic content (500 mg total phenolic compounds/ kg oil) were the sulfated forms of HT (79%), homovanillic alcohol sulfate (sulfHVAlc) (19%), and in a minor proportion the homovanillic acid sulfate (sulfHVac). The Caco-2 intestinal cell model allowed us obtaining a mixture in which the main metabolites were HTsulf (80%), sulfHVAlc (10%) and in a minor proportion, HT glucuronides and free HT (Fig. 5). So the obtained mixture of metabolites is very close to the previously observed human plasma profile, with a 90% of resemblance.

Results from the TNF-α stimulated HAEC cells indicated that after 24 h of incubation both HT metabolites and free HT at physiological concentrations (1 to 10 μM) were effective at reducing E-selectin, P-selectin, VCAM-1, and ICAM-1 molecules involved at the first stages of atherosclerosis. In a previous study the inhibition of endothelial activation by free HT was described in a human umbilical vein endothelial cell (HUVEC) model, observing that HT was able to reduce lipopolysaccharide (LPS)-stimulated expression of VCAM-1 at low micromolar concentrations 15. Another study observed reductions in E-selectin, VCAM-1, and ICAM-1 after HT exposure on HUVEC at doses of 5 and 25 μM of HT. 39. Regarding MCP-1, Richard N et al. also observed a significant reduction of MCP-1 after HT exposure (25 μM) on murine macrophages (RAW264.7 cells) 40. In the two latter studies, the effect of HT metabolites was not tested, so the physiological significance of the results is questionable.
After the HT treatments we also observed that the remaining amounts of free HT in the cells and media did no differ significantly among the tested doses (Fig. 4), which was in accordance with the observed effects in the modulation of the cell adhesion molecules that were also maintained regardless of the dose. However, when HT metabolites were incubated with HAEC cells, an increasing concentration of these metabolites was detected in the cells after the assay with a parallel dose-dependent inhibition. These data provide evidence of the potential effect of HT phase II metabolites in the reduction of the endothelial dysfunction biomarkers.
The residual amount of free HT in the obtained metabolite mixture represents the major difference with the plasma data. Therefore, in order to test the possible activity of the residual amounts of HT present in the metabolite mixtures, lower concentrations were tested. Results indicated that in the case of adhesion molecules (VCAM-1, ICAM-1, E-selectin, and P-selectin) the attenuation effect of the metabolite mixtures could be due to a large extent to the residual HT. However, in all cases, reductions of the metabolite mixtures were 10% greater compared to their corresponding amounts of residual HT, indicating that metabolites also contribute in the inhibition. The effect of the metabolites was confirmed when the dose of 2 μM HT metabolites was tested, observing a significant inhibition of all adhesion molecules secretion, whereas its corresponding percentage of residual HT did not show any effect. From these results it can be concluded that metabolites might be in part responsible for the attenuation of the inflammation-induced expression of adhesion molecules.
In the case of the chemokine MCP-1, HT did not show any effect at any tested dose, whereas the doses of 5 and 10 μM of HT metabolites presented a significant effect, indicating in this case that metabolites clearly seem to be active on the inhibition of the secretion of this chemokine. The specific effect of HT metabolites on reducing MCP-1 could also indicate that free HT and HT metabolites could act reducing the endothelial dysfunction through different endpoints and having complementary activities.
Unexpectedly, after the incubation assays with HT, an important amount of HT metabolites were detected in the cells and media, most of them sulfo and methylsulfo-conjugated (Fig. 4). Catechol-O-methyltransferase have been previously described in endothelial cells after incubating HUVEC with (–)-epicatechin, identifying the endothelial NADPH oxidase as a specific candidate target for the methylated forms 41. SULT1A1, the main sulfotransferase enzyme responsible for the detoxification of xenobiotics, has been found in many different human tissues, with the highest abundance found in the liver, but it has never been detected in human aortic endothelial cells. Thus, the data provided in the present work add value to the study suggesting that aortic sulfotransferases may play an important role in reducing local cellular concentrations of native forms of phenolic compounds.
Although phase II phenolic metabolites have been generally considered to be pharmacologically inactive and targets for excretion 42, our results indicate that the inhibition of the endothelial dysfunction could be partly due to the HT metabolites. The effect of flavonoids (quercetin and (+)-catechin) and their different conjugates on cell adhesion has been recently reviewed 43 and it was established that conjugation can significantly affect the activity of aglycones both reducing and increasing the effects. So, given that the effects of conjugation can differ greatly on cell adhesion, research on the biological activity has to be done on a case-by-case basis.
Our results also demonstrate that effects other than the reduction of ox-LDL and cholesterol, which have been demonstrated in humans 1, 44-46, may explain the anti-atherogenic action of VOO phenolic compounds. Two additional mechanisms involved in the vascular damage, platelet aggregation, and proliferation of smooth muscle cells, are also antagonized by the VOO phenolic compounds 16.
In conclusion, this is the first time that HT metabolites have been synthetized by using a differentiated Caco-2 cell line, obtaining a purified extract that resembles the human plasma HT metabolite profile after VOO intake. Results indicate that both free HT and HT metabolites are effective in the reduction of the endothelial dysfunction biomarkers but only HT metabolites reduce MCP-1 at physiological doses. Novel data are presented regarding the presence of xenobiotic detoxification enzymes in HAEC cells, which add value to the study suggesting that catechol-O-methyltransferase and sulfotransferases may play an important role in reducing local cellular concentrations of native forms of phenolic compounds in aortic endothelial cells. Our data suggest that HT metabolites might be responsible in part for the protection against endothelial dysfunction, and thus they are likely to contribute to the reduced risk in the early stages of atherosclerosis, which provide a clear future direction in olive oil phenols research.
Acknowledgments
This study was supported by the Spanish Ministry of Education and Science, The MEFOPC Project (AGL2012-40144-C03-03, AGL2012-40144-C03-02 and AGL2012-40144-C03-01) and by the University of Lleida through the M-C. López de las Hazas grant.
The authors have declared no conflict of interest.




