Complementary phenol-enriched olive oil improves HDL characteristics in hypercholesterolemic subjects. A randomized, double-blind, crossover, controlled trial. The VOHF study
Abstract
Scope
Consumption of olive oil (OO) phenolic compounds (PCs) has beneficial effects on lipid profile. HDL functionality is currently considered to be a more important issue than its circulating quantity. Our aim was to assess whether functional virgin olive oils (FVOOs), one enriched with its own PC (500 ppm; FVOO) and another with OOPC (250 ppm) plus additional complementary PCs from thyme (250 ppm) (total: 500 ppm; FVOOT (functional virgin olive oil with thyme)), could improve HDL functionality related properties versus a virgin OO control (80 ppm; VOO).
Methods and results
In a randomized, double-blind, crossover, controlled trial, 33 hypercholesterolemic volunteers received 25 mL/day of VOO, FVOO, and FVOOT during 3 wk. HDL cholesterol increased 5.74% (p < 0.05) versus its baseline after the FVOOT consumption in the participants without hypolipidemic medication. We detected, after FVOOT consumption, an increase in HDL2-subclass (34.45, SD = 6.38) versus VOO intake (32.73, SD = 6.71). An increment in esterified cholesterol/free cholesterol and phospholipids/free cholesterol in HDL was observed after FVOOT consumption (1.73, SD = 0.56; 5.44, SD = 1.39) compared with VOO intervention (1.53, SD = 0.35; 4.97, SD = 0.81) and FVOO intervention (1.50, SD = 0.33; 4.97, SD = 0.81). Accordingly, lecithin-cholesterol acyltransferase mass increased after FVOOT consumption (1228 μg/mL, SD = 130), compared with VOO consumption (1160 μg/mL, SD = 144). An improvement in HDL oxidative-status was reflected after FVOOT consumption versus its baseline, given an increment in the paraoxonase activity (118 × 103 U/L, SD = 24).
Conclusion
FVOOT improves HDL-subclass distribution and composition, and metabolism/antioxidant enzyme activities. FVOOT could be a useful dietary tool in the management of high cardiovascular risk patients.
Abbreviations
-
- ApoA-I
-
- apolipoprotein A-I
-
- CETP
-
- cholesteryl-ester transfer protein
-
- CHD
-
- coronary hearh disease
-
- DHR
-
- dihydrorhodamine 123
-
- EC
-
- esterified cholesterol
-
- FC
-
- free cholesterol
-
- FVOO
-
- functional virgin olive oil
-
- FVOOT
-
- functional virgin olive oil with thyme
-
- HDL-C
-
- HDL-cholesterol
-
- LCAT
-
- lecithin-cholesterol acyltransferase
-
- OO
-
- olive oil
-
- PA
-
- physical activity
-
- PAF-AH
-
- platelet-activating factor acetylhydrolase
-
- PC
-
- phenolic compound
-
- PL
-
- phospholipids
-
- PON
-
- paraoxonase/arylesterase
-
- TC
-
- total cholesterol
-
- TG
-
- triglyceride
-
- VOO
-
- virgin olive oil
1 Introduction
Olive oil (OO) phenolic compounds (PCs) exert antioxidant, anti-inflammatory properties, and chemoprotective activity in experimental studies 1. Moreover, OOPCs induce favorable changes in lipid profile, improve endothelial function, modify the hemostasis, and have antithrombotic properties in humans 2-4. Data from human studies show that OOPCs are protective against risk factors for coronary heart disease (CHD), particularly in individuals with oxidative stress 5-7. HDL cholesterol (HDL-C) levels are inversely and independently related with cardiovascular disease 8. Low levels of HDL-C are the most characteristic lipid feature in individuals with premature CHD 9. Currently, pharmacological or natural agents, which can increase HDL-C levels, are being considered as key factors for future therapies 10. Despite significant increases in HDL-C concentration through nicotinic acid, and cholesteryl-ester transport protein (CETP) inhibitor, these studies have been discontinued due to ineffectiveness or increased risk of mortality 11-13. The unexpected association of torcetrapib, an agent that increases plasma HDL-C and also cardiovascular mortality, has led not only to the discontinuation of further trials involving this drug 13, but also to considering the functional quality of HDL as being a more important issue than its circulating quantity. In addition, Voight et al. recently reported in a Mendelian randomization study that an increase in HDL-C concentration does not imply a reduction in the risk of suffering a myocardial infarction 14.
Results from the EUROLIVE study showed an increase in HDL-C levels, and a decrease in in vivo lipid oxidative damage, in a dose-dependent manner with the phenolic content of the OO administered 15. Furthermore, we provided for the first time a first-level evidence of an HDL function enhancement by a virgin olive oil (VOO) in healthy humans 16. In addition, our group recently reported that OOPCs enhance cholesterol efflux related genes in pre/hypertensive subjects 17. In this sense, Helal et al. reported in a linear, nonrandomized, and noncontrolled trial that VOO consumption improved the capacity of HDL to mediate cholesterol efflux 18. Moreover, some animal and humans studies have reported that OO has effects on some parameters related to HDL functionality 19.
PC-rich foods, without increasing the amount of fat consumed, could have a dual action because antioxidants could also revert to pro-oxidants 20-22. Functional food with complementary antioxidants, according to their structure/activity relationship, could be a suitable option to obtain beneficial effects avoiding these harmful ones. Our aim was to test whether enriched VOOs (FVOOs (functional virgin olive oils); 500 ppm), one enriched with its own PC (FVOO) and another with them plus additional complementary PCs from thyme (FVOOT (functional virgin olive oil with thyme)), could improve properties related with a better low cardiovascular risk HDL profile, such as HDL size, metabolism, antioxidant status, and composition.
2 Materials and methods
2.1 OO preparation and characteristics
VOO with a low phenolic content (80 ppm) was used as a control condition and as a matrix of enrichment to prepare two FVOOs (500 ppm). FVOO (500 ppm) was enriched with its own PCs by addition of a phenol extract obtained from freeze-dried olive cake. FVOOT (500 ppm) was enriched with its own PC and complemented with thyme phenolics using a phenol extract obtained from a mixture of freeze-dried olive cake and dried thyme. Hence, FVOOT contained 50% of olive PC and 50% of thyme phenolics (Fig. 1). The PCs content is the main difference among the three OOs, being the fatty acids and fat-soluble micronutrients very similar among them. The FVOO presents the highest amount of hydroxytyrosol derivatives, whereas the FVOOT presents the highest amount of flavonoids, lignans, and it is the only OO with detectable monoterpenes. The procedure to obtain the phenolic extracts and enriched oils has been published 23. For the wash-out period, a common OO kindly provided by Borges Mediterranean Group was used. The total phenolic content of OOs was determined by Folin–Ciocalteu method 24. The phenolic profile of the OOs was analyzed by ultraperformance LC-MS/MS 25.
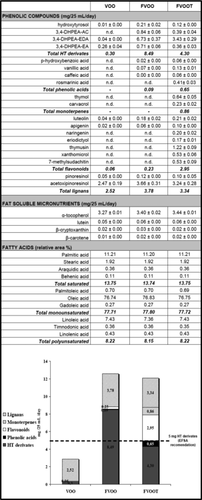
Chemical characterization of VOHF study olive oils. Values are expressed as means ± SD of mg/25 mL oil/day. The acidic composition is expressed as relative area percentage.
Abbreviations: VOO, virgin olive oil; FVOO, functional VOO enriched with its own phenolic compounds; FVOOT, functional VOO enriched with its own phenolic compounds plus additional complementary ones from thyme; 3,4-DHPEA-AC, 4-(acetoxyethyl)-1,2-dihydroxybenzene; 3,4-DHPEA-EDA, dialdehydic form of elenolic acid linked to hydroxytyrosol; 3,4-DHPEA-EA, oleuropein aglycone.
2.2 Study design
The VOHF (Virgin olive Oil and HDL Functionality) study was randomized, double-blind, crossover, controlled trial with 33 hypercholesterolemic volunteers (total cholesterol (TC) > 200 mg/dL); (19 men), aged 35–80. Exclusion criteria included the following: BMI > 35 kg/m2, smokers, athletes with high physical activity (PA; >3000 kcal/day), diabetes, multiple allergies, intestinal diseases, or other disease or condition that would worsen the adherence to the measurements or treatment. The study was conducted at IMIM-Hospital del Mar Medical Research Institute (Spain) from April to September 2012.
Subjects were randomized to one of three orders of administration of raw OOs (1-VOO, 2-FVOO, 3-FVOOT): Sequence 1-FVOO, FVOOT, VOO; Sequence 2-FVOOT, VOO, FVOO; Sequence 3-VOO, FVOO, FVOOT. In the crossover design, intervention periods were of 3 wk with an ingestion of 25 mL/day raw OO distributed along meals and preceded by 2-wk wash-out periods with a common OO.
To avoid an excessive intake of antioxidants and PCs during the clinical trial period, participants were advised to limit the consumption of polyphenol-rich food. PA was evaluated by a PA-Questionnaire at the baseline and at the end of the study. A set of portable containers with the corresponding 25 mL of OO for each day of consumption were delivered to the participants at the beginning of each OO administration period. The participants were instructed to return the containers to the center after the corresponding OO consumption period in order to register the amount of OO consumed in the period. Subjects with less than 80% of treatment adherence (≥5 full OO containers returned) were considered noncompliant volunteers for this treatment.
The present clinical trial was conducted in accordance with the Helsinki Declaration and the Good Clinical Practice for Trials on Medical Products in the European Community. All participants provided written informed consent, and the local institutional ethics committees approved the protocol (CEIC-IMAS 2009/3347/I). The protocol is registered with the International Standard Randomized Controlled Trial Register (www.controlled-trials.com;ISRCTN77500181) and followed CONSORT guidelines.
2.3 Dietary adherence
Twenty-four-hour urine was collected at the start of the study and before and after each treatment. Urine samples were stored at –80ºC prior to use. We measured urinary hydroxytyrosol sulfate and thymol sulfate as biomarkers of adherence to the type of OO ingested in urine by ultra-HPLC-ESI-MS/MS 26. A 3-day dietary record was administered to the participants at baseline and before and after each intervention period. A nutritionist personally advised participants to replace all types of habitually consumed raw fats with the OOs, and to limit their polyphenol-rich food consumption.
2.4 Systemic biomarkers analyses
Blood samples were collected in fasting state at least of 10 h at the start of the study and before and after each treatment. Plasma samples were obtained by centrifugation of whole blood directly after being drawn and were preserved at –80ºC until use. EDTA-plasma glucose, TC, and triglyceride (TG) levels were measured using standard enzymatic automated methods and, apolipoprotein A-I (ApoA-I) and apolipoprotein-B100 by immunoturbidimetry, in a PENTRA-400 autoanalyzer (ABX-Horiba Diagnostics, Montpellier, France). HDL-C was measured as a soluble HDL-C determined by an accelerator selective detergent method (ABX-Horiba Diagnostics). LDL-C was calculated by the Friedewald equation whenever TGs were less than 300 mg/dL. EDTA-plasma glutathione peroxidase activity was assessed through glutathione oxidation–reduction measured by a Paglia and Valentine method modification using cumene hydroperoxide as oxidant of glutathione (Ransel RS 505, Randox Laboratories, Crumlin, UK) 27. Enzymatic activity of CETP (MBL, Woburn, MA, USA) and mass concentration of lecithin-cholesterol acyltransferase (LCAT) (American Diagnostica GmbH, Pfungstadt, Germany) were analyzed in serum by fluorimetric kits. Platelet-activating factor acetylhydrolase (PAF-AH) activity was measured in serum (Cayman Chemical, Ann Arbor, MI, USA). Finally, paraoxonase/arylesterase (PON) activity was determined in serum through the measurement of the capacity for cleavage of phenyl acetate resulting in phenol formation (Zeptometrix Corporation, Buffalo, NY, USA). A fluorimetric methodology based on the oxidation of HDL particle in the presence of dihydrorhodamine 123 (DHR) by measuring increasing fluorescence due to the production of reactive oxygen species over time was performed according to previous work 28. The products of redox cycling are detected as time-dependent oxidation of the fluorogenic probe DHR to fluorescent rhodamine 123. The rate of DHR oxidation in the presence of HDL reflects the antioxidant activity of the particle.
2.5 HDL subclass distribution and composition analyses
HDL-subclass distribution was measured in plasma by the Lipoprint-HDL System (Quantimetrix, Manhattan Beach, CA, USA), in which following electrophoresis, lower density lipoproteins (VLDL, LDL) remain at the beginning of the band (Rf = 0.0) and the albumin moves to the front (Rf = 1.0). Between these two points, we were able to find up to nine HDL bands with intermediate Rf values. The first three bands corresponded to a large HDL subclass (HDL2), and fourth to ninth bands corresponded to a small HDL subclass (HDL3). Taking all this into account, we calculated both proportions of HDL2 and HDL3 subtypes, as previously described 29. HDLs were isolated by a density gradient ultracentrifugation method 30 using preparation solutions of 1.006 and 1.21 density. TC, free cholesterol (FC), and phospholipids (PL) in HDL were quantified by using automatic enzymatic methods (Spinreact, Barcelona, Spain). Esterified cholesterol (EC) was calculated subtracting FC from TC. TGs were determined in these samples by automatic enzymatic methods (ABX-Horiba Diagnostics). Apo-AI and Apo-AII were determined by automatic immunoturbidimetric methods (ABX-Horiba Diagnostics, and Spinreact, respectively). For assuring the purity of HDL fractions, apolipoprotein-B100 and albumin levels were also determined in these samples by automatic immunoturbidimetric methods (ABX-Horiba Diagnostics).
2.6 APOE genotyping
DNA was isolated from the buffy coat of blood collected into EDTA tubes using a standardized method (FlexiGene DNA Kit; Qiagen). Allelic discrimination of the APOE gene variants was performed with TaqMan PCR technology (QuantStudioTM 12K Instrument; Applied Biosystems) and Assay-on-Demand single-nucleotide polymorphism genotyping assays (Applied Biosystems). The APOE haplotypes (E2/E2, E2/E3, E2/E4, E3/E3, E3/E4, and E4/E4) were determined from the alleles for the APOE single-nucleotide polymorphisms rs7412 and rs429358.
2.7 Sample size and power analyses
The sample size of 30 individuals allows at least 80% power to detect a statistically significant difference among groups of 3 mg/dL of HDL-C and an SD of 1.9, assuming a dropout rate of 15% and a Type I error of 0.05 (two-sided).
2.8 Statistical analyses
Normality of continuous variables was assessed by normal probability plots. Non-normally distributed variables were log-transformed if it was necessary. Noncompliance volunteers were excluded from the analysis. To compare means (for normal distributed variables) or medians (for non-normal distributed variables) among groups, ANOVA or Kruskal–Wallis test were performed, respectively; whereas χ2 and exact F-test, as appropriate, were computed to compare proportions. Pearson and Spearman correlation analyses were used to evaluate relationships among variables. Linear regression models were used to adjust postintervention values for preintervention values. A general linear model for repeated measurements was used to assess the effect of intra- and inter-interventions. To check whether APOE4 carrier genotype modified the results, we tested the interaction of this variable (as between individual factor) with the treatment effect defined as the posttreatment value adjusted with its pretreatment value (as within individual factor) in a general lineal model (GLM) for repeated measurements. When interaction p-value was borderline or significant with a study variable, this variable was analyzed without APOE4 carrier participants in GLM for repeated measurements. A value of p < 0.05 was considered significant. Carry-over effect was discarded in all variables. Statistical analyses were performed by SPSS13.0 software (IBN Corp.) and R2.12.0 software (R Development Core Team).
3 Results
3.1 Participant characteristics and dietary adherence
From 62 subjects who were assessed for eligibility, 29 were excluded. Finally, 33 eligible participants (19 men, 14 women) entered the study. A discontinued single intervention was occurred in three volunteers for the investigator decision. We could not identify any adverse effects related to OO intake. Participants’ baseline characteristics are shown in Table 1, no significant differences exist between orders. No changes in daily energy expenditure in leisure-time PA were observed from the beginning to the end of the study. No changes were observed in the main nutrients (Table 2) and medication intake throughout the study, and participants’ compliance was good as reflected in the urinary phenols after OO interventions (Table 3).
| Order 1a) (n = 11) | Order 2a) (n = 11) | Order 3a) (n = 11) | Totala) (n = 33) | p-Value | |
|---|---|---|---|---|---|
| General | |||||
| Sex: man | 5 (45.6%) | 7 (63.6%) | 7 (63.6%) | 19 (57.6%) | 0.742 |
| Age (years) | 54.91 ± 12.57 | 55.27 ± 11.88 | 55.45 ± 7.84 | 55.21 ± 10.62 | 0.856 |
| BMI (kg/m2) | 25.63 ± 3.68 | 26.31 ± 5.25 | 27.85 ± 4.71 | 26.64 ± 4.54 | 0.529 |
| Hypolipidemic medication: no | 7 (63.6%) | 9 (81.8%) | 3 (27.3%) | 19 (57.6%) | 0.047 |
| Physical activity (kcal/wk) | 3498.75 (1755.00;8092.50) | 1188.75 (742.50;1687.50) | 3322.50 (861.25;3663.75) | 2423.25 (897.38;4543.75) | 0.094 |
| Diastolic blood pressure (mmHg) | 68.09 ± 13.53 | 72.27 ± 9.31 | 71.91 ± 13.43 | 70.76 ± 12.01 | 0.678 |
| Systolic blood pressure (mmHg) | 125.09 ± 18.70 | 128.27 ± 16.69 | 130.45 ± 17.93 | 127.94 ± 17.37 | 0.778 |
| Systemic lipid profile and glycaemia | |||||
| Total-cholesterol (mg/dL) | 228 ± 43 | 232 ± 33 | 219 ± 31 | 226 ± 35 | 0.680 |
| Triglycerides (mg/dL) | 94 (75;149) | 119 (95;168) | 117 (81;126) | 114 (85;145) | 0.517 |
| Glucose (mg/dL) | 89 ± 12 | 93 ± 13 | 91 ± 11 | 91 ± 12 | 0.683 |
| HDL-cholesterol (mg/dL) | 53 ± 13 | 53 ± 13 | 53 ± 20 | 53 ± 11 | 0.992 |
| LDL-cholesterol (mg/dL) | 150 ± 32 | 152 ± 28 | 142 ± 26 | 148 ± 28 | 0.700 |
| ApoA-I (g/L) | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.2 | 0.458 |
| Apolipoprotein-B100 (g/L) | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.529 |
- a) Values expressed as mean ± SD or median (25th to 75th percentile).
| VOO | FVOO | FVOOT | Inter-intervention p-Value | ||||
|---|---|---|---|---|---|---|---|
| Preinterventiona) | Postinterventiona) | Preinterventiona) | Postinterventiona) | Preinterventiona) | Postinterventiona) | ||
| Energy (kcal) | 1975.07 ± 424.42 | 1882.66 ± 482.78 | 1916.48 ± 555.76 | 1913.81 ± 394.38 | 2019.96 ± 463.32 | 1899.11 ± 547.85 | 0.865 (VOO–FVOOT) |
| 0.688 (FVOO–FVOOT) | |||||||
| 0.559 (VOO–FVOO) | |||||||
| Carbohydrate (%) | 41.13 ± 5.01 | 42.38 ± 7.61 | 43.93 ± 9.20 | 42.61 ± 6.51 | 40.89 ± 6,19 | 42.55 ± 7.41 | 0.935 (VOO–FVOOT) |
| 0.252 (FVOO–FVOOT) | |||||||
| 0.293 (VOO–FVOO) | |||||||
| Protein (%) | 18.45 ± 3.68 | 19.80 ± 4.53 | 19.81 ± 4.49 | 19.76 ± 3.93 | 19.58 ± 4.60 | 19.72 ± 4.24 | 0.417 (VOO–FVOOT) |
| 0.846 (FVOO–FVOOT) | |||||||
| 0.380 (VOO–FVOO) | |||||||
| Total fat (%) | 41.12 ± 7.33 | 38.68 ± 8.49 | 38.29 ± 12.47 | 37.55 ± 7.70 | 41.55 ± 7.35 | 38.35 ± 7.52 | 0.823 (VOO–FVOOT) |
| 0.542 (FVOO–FVOOT) | |||||||
| 0.435 (VOO–FVOO) | |||||||
| Saturated fat (%) | 12.41 ± 3.88 | 11.33 ± 4.00 | 10.78 ± 2.98 | 11.60 ± 4.98 | 12.21 ± 3.06 | 10.71 ± 3.40 | 0.750 (VOO–FVOOT) |
| 0.124 (FVOO–FVOOT) | |||||||
| 0.087 (VOO–FVOO) | |||||||
| Monounsaturated fat (%) | 17.74 ± 3.19 | 16.23 ± 3.91 | 16.13 ± 5.31 | 16.22 ± 4.47 | 16.56 ± 3.73 | 16.09 ± 3.42 | 0.303 (VOO–FVOOT) |
| 0.765 (FVOO–FVOOT) | |||||||
| 0.135 (VOO–FVOO) | |||||||
| Polyunsaturated fat (%) | 4.84 ±1.14 | 4.54 ±0.88 | 4.88 ±1.45 | 4.78 ±1.08 | 4.69 ±1.61 | 4.90 ±1.32 | 0.231 (VOO–FVOOT) |
| 0.388 (FVOO–FVOOT) | |||||||
| 0.654 (VOO–FVOO) | |||||||
- a Values expressed as mean ± SD.
- Intra- and inter-intervention p-values were not significant.
- Abbreviations: VOO, virgin olive oil; FVOO, functional VOO enriched with its own phenolic compounds; FVOOT, functional VOO enriched with its own phenolic compounds plus additional complementary ones from thyme.
| VOO | FVOO | FVOOT | Inter-intervention p-value | ||||
|---|---|---|---|---|---|---|---|
| Preinterventiona) | Postinterventiona) | Preinterventiona) | Postinterventiona) | Preinterventiona) | Postinterventiona) | ||
| Hydroxytyrosol | 6.13 | 5.50 | 6.11 | 11.09 | 6.35 | 9.08 | 0.402 (VOO–FVOOT) |
| sulfate | (3.66; 14.67) | (2.57;11.71) | (2.97;9.95) | (7.63;20.86)b) | (3.57; 13.43) | (4.43;12.61) | 0.328 (FVOO–FVOOT) |
| (μmoles/24 h-urine) | 0.013 (VOO–FVOO)d) | ||||||
| Thymol sulfate | 38.89 | 30.93 | 59.16 | 36.81 | 83.53 | 455 | 0.000 (VOO–FVOOT)d) |
| (μmoles/24 h- | (11.94;81.21) | (7.28;90.33) | (11.64;92.00) | (9.37;72.06) | (10.43; 108) | (120;880)c) | 0.000 (FVOO–FVOOT)d) |
| urine) | 0.339 (VOO–FVOO) | ||||||
- a Values expressed as median (25th to 75th percentile).
- b p-Value for intra-intervention: p < 0.05.
- c) p-Value for intra-intervention: p < 0.001.
- d) p-Value for inter-intervention: p < 0.001.
- Abbreviations: VOO, virgin olive oil; FVOO, functional VOO enriched with its own phenolic compounds; FVOOT, functional VOO enriched with its own phenolic compounds plus additional complementary ones from thyme.
3.2 Systemic biomarkers
No changes were observed in blood pressure and BMI throughout the study. Although no changes were observed in lipid profile in the entire sample, when the analyses were performed in the participants without hypolipidemic medication, HDL-C increased 5.74% (p < 0.05) after FVOOT consumption versus its baseline.
Changes in enzymes related with HDL metabolism and antioxidant status are shown in Table 4. An improvement of LCAT activity was observed after FVOOT intervention compared with VOO (p = 0.020), while a borderline decrement was observed after VOO intake versus its baseline (p < 0.09). A rise in PON was observed after FVOOT consumption versus its baseline (p < 0.05), while a decrement reaching a borderline significance (p < 0.09) was observed after VOO intake versus its baseline. A borderline improvement of CETP activity was observed after FVOO intervention compared with VOO (p = 0.089). No changes were observed in PAF-AH activity. With regard to the DHR test, there were no significant changes in the intra- and intertreatment analysis. In addition, an inverse correlation between the induced oxidation rate of DHR and the activity of the glutathione peroxidase was observed after VOO and FVOOT treatments (rVOO = –0.49, rFVOOT = –0.57; p < 0.05).
| Baselinea) | Post-VOO interventiona),b) | Post-FVOO interventiona),b) | Post-FVOOT interventiona),b) | Inter-intervention p-value | |
|---|---|---|---|---|---|
| Paraoxonase-arylesterase (103 U/L) | 119 ± 30 | 116 ± 26 | 115 ± 25 | 118 ± 24c) | 0.287 (VOO–FVOOT) 0.174 (FVOO–FVOOT) 0.587 (VOO–FVOO) |
| Platelet-activating factor acetylhydrolase (10−3 U/L) | 15 264 ± 3498 | 15 321 ± 2874 | 15 345 ± 3030 | 15 239 ± 2374 | 0.861 (VOO–FVOOT) 0.821 (FVOO–FVOOT) 0.932 (VOO–FVOO) |
| Lecithin-cholesterol acyltransferase (μg/mL) | 1161 ± 210 | 1160 ± 144 | 1202 ± 195 | 1228 ± 130 | 0.020 (VOO–FVOOT)d) 0.341 (FVOO–FVOOT) 0.098 (VOO–FVOO) |
| Cholesteryl-ester transfer protein (103 U/L) | 26 117 ± 5983 | 25 617 ± 3333 | 26 717 ± 5350 | 26 667 ± 4767 | 0.159 (VOO–FVOOT) 0.955 (FVOO–FVOOT) 0.089 (VOO–FVOO) |
| Glutathione peroxidase (U/L) | 723 ± 105 | 703 ± 77 | 709 ± 79 | 717 ± 94 | 0.151 (VOO–FVOOT) 0.513 (FVOO–FVOOT) 0.584 (VOO–FVOO) |
| DHR oxidation rate (Fluorescence units/s) | 4.07 ± 1.47 | 4.07 ± 1.34 | 3.95 ± 1.37 | 4.21 ± 1.26 | 0.228 (VOO–FVOOT) 0.104 (FVOO–FVOOT) 0.494 (VOO–FVOO) |
- a Values expressed as postintervention mean ± SD.
- b Postintervention was adjusted by its preintervention.
- c p-Value for intra-intervention: p < 0.05.
- d) p-Value for inter-intervention: p < 0.05.
- Abbreviations: VOO, virgin olive oil; FVOO, functional VOO enriched with its own phenolic compounds; FVOOT, functional VOO enriched with its own phenolic compounds plus additional complementary ones from thyme; DHR, dihydrorhodamine 123.
3.3 HDL subclass distribution and composition
After the FVOOT intervention, a rise in the HDL2-subclass was observed (p < 0.05). This increase was significant versus changes in VOO (p = 0.047). HDL3-subclass decreased after FVOOT intervention (p < 0.05; Fig. 2). Moreover, after FVOOT intervention an increment in HDL2/HDL3 was observed (p < 0.05).

HDL subclass distribution after interventions. Values represent postintervention mean ± SD. Postintervention values were adjusted with its preintervention ones. #Intra-intervention p-value < 0.05; *Inter-intervention p-value < 0.05 compared with VOO intervention.
Abbreviations: VOO, virgin olive oil; FVOO, functional VOO enriched with its own phenolic compounds; FVOOT, functional VOO enriched with its own phenolic compounds plus additional complementary ones from thyme.
An increment in HDL EC/FC was observed after FVOOT consumption compared with VOO (p = 0.029) and FVOO (p = 0.007). Also, an increment in HDL PL/FC was observed after the FVOOT consumption compared with VOO (p = 0.028) and FVOO (p = 0.027). No changes were observed in TC, TG, Apo-AI, Apo-AII, FC, EC, and PL in HDL after interventions. Nevertheless, a borderline decrease in HDL FC/TC after FVOOT consumption versus VOO (p = 0.056) and FVOO (p = 0.067) was observed. Moreover, a borderline increase in HDL EC/TC after FVOOT consumption versus VOO (p = 0.056) and FVOO (p = 0.067) was observed (Table 5).
| Baselinea) | Post-VOO interventiona),b) | Post-FVOO interventiona),b) | Post-FVOOT interventiona),b) | Inter-intervention p-value | |
|---|---|---|---|---|---|
| HDL total-cholesterol (mg/dL) | 31 ± 11 | 31 ± 11 | 32 ± 11 | 31 ± 11 | 0.893 (VOO–FVOOT) 0.478 (FVOO–FVOOT) 0.445 (VOO–FVOO) |
| HDL triglycerides (mg/dL) | 7.48 ± 2.44 | 7.29 ± 1.19 | 7.25 ± 1.36 | 7.17 ± 1.46 | 0.628 (VOO–FVOOT) 0.749 (FVOO–FVOOT) 0.882 (VOO–FVOO) |
| HDL apolipoprotein A-I (mg/dL) | 66 ± 15 | 65 ± 11 | 66 ± 12 | 64 ± 14 | 0.271 (VOO–FVOOT) 0.150 (FVOO–FVOOT) 0.510 (VOO–FVOO) |
| HDL apolipoprotein A-II (mg/dL) | 16.85 ± 3.64 | 16.91 ± 2.20 | 17.61 ± 3.10 | 16.80 ± 3.38 | 0.818 (VOO–FVOOT) 0.152 (FVOO–FVOOT) 0.158 (VOO–FVOO) |
| HDL free-cholesterol (mg/dL) | 11.81 ± 5.38 | 12.98 ± 4.47 | 13.61 ± 5.90 | 12.53 ± 5.42 | 0.381 (VOO-FVOOT) 0.107 (FVOO–FVOOT) 0.303 (VOO–FVOO) |
| HDL esterified-cholesterol (mg/dL) | 19.49 ± 8.05 | 18.18 ± 6.30 | 18.25 ± 5.78 | 18.56 ± 7.36 | 0.624 (VOO-FVOOT) 0.625 (FVOO–FVOOT) 0.909 (VOO–FVOO) |
| HDL phospholipids (mg/dL) | 60.28 ± 17.04 | 59.73 ± 16.26 | 61.45 ± 14.89 | 59.49 ± 15.88 | 0.883 (VOO–FVOOT) 0.357 (FVOO–FVOOT) 0.395 (VOO–FVOO) |
| HDL free-cholesterol/total-cholesterol | 0.38 ± 0.12 | 0.43 ± 0.06 | 0.42 ± 0.08 | 0.40 ± 0.08 | 0.056 (VOO–FVOOT) 0.063 (FVOO–FVOOT) 0.898 (VOO–FVOO) |
| HDL esterified-cholesterol/total-cholesterol | 0.62 ± 0.12 | 0.57 ± 0.06 | 0.58 ± 0.08 | 0.60 ± 0.08 | 0.056 (VOO–FVOOT) 0.063 (FVOO–FVOOT) 0.898 (VOO–FVOO) |
| HDL phospholipids/free-cholesterol | 5.39 ± 1.68 | 4.97 ± 0.81 | 4.99 ± 0.89 | 5.44 ± 1.39 | 0.028 (VOO–FVOOT)c) 0.027 (FVOO–FVOOT)c) 0.900 (VOO–FVOO) |
| HDL esterified-cholesterol/free-cholesterol | 1.74 ± 0.77 | 1.53 ± 0.35 | 1.50 ± 0.33 | 1.73 ± 0.56 | 0.029 (VOO–FVOOT)c) 0.007 (FVOO–FVOOT)c) 0.604 (VOO–FVOO) |
- a Values expressed as postintervention mean ± SD.
- b Postintervention was adjusted by its preintervention.
- c) Inter-intervention p-value: p < 0.05.
- Intra-intervention p-values were not significant.
- Abbreviations: VOO, virgin olive oil; FVOO, functional VOO enriched with its own phenolic compounds; FVOOT, functional VOO enriched with its own phenolic compounds plus additional complementary ones from thyme.
Analyses after OOs intake of HDL-subclass distribution showed cross-linked correlations with HDL composition and with HDL metabolism enzymes. The increase in HDL2/HDL3 directly correlated with ApoA-I after OOs intake (rVOO = 0.41, rFVOO = 0.48, rFVOOT = 0.44; p < 0.05). The increase in HDL2/HDL3 directly correlated with the decrease in CETP after VOO intake (r = –0.39; p < 0.05) and borderline correlated with the decrease in CETP after FVOO intake (r = –0.30; p < 0.09).
3.4 APOE genotype
A total of 66.67% of the participants have APOE3/E3 genotype, 15.15% have APOE2/E3 genotype, 15.15% of volunteers have APOE3/E4 genotype, and 3.03% have APOE4/E4 genotype. In the analyses of possible interaction among the studied variables and APOE4 carrier variable, only a borderline interaction with HDL EC/FC, HDL PL/FC, and PAF-AH activity was observed (p < 0.09). When these variables were analyzed without APOE4 carrier volunteers, similar results as entire sample were obtained, except for HDL PL/FC increment after FVOOT consumption versus FVOO, which did not reach significance (p = 0.053).
4 Discussion
We performed a randomized, double-blind, crossover, controlled trial with a VOO, an OO enriched with its own PCs, and another with them plus complementary PCs from thyme. From our results, VOO enriched with its PC plus those of thyme improved parameters related with HDL functional profile, such as HDL-subclass distribution, HDL composition, and enzymes related with HDL metabolism and antioxidant status. For the first time, the additional benefits achieved with complementary phenol-enriched OO consumption on the HDL functional profile in hypercholesterolemic volunteers have been demonstrated with the highest degree of evidence. Moreover, such benefits can be obtained without increasing the individual's fat intake.
LCAT is an enzyme that mediates the esterification of the FC of nascent HDL monolayers to EC. As a result, this hydrophobic EC move into the center of the particles 31. The cholesterol esterification by LCAT mediates the gradual conversion of nascent discoidal HDL into small spherical HDL (HDL3) with two ApoA-I molecules/particle and, finally, into large spherical HDL particles (HDL2) that contain three ApoaA-I molecules 32, 33. Another enzyme intimately related with the HDL metabolism is CETP that extracts EC from the HDL core to TG-rich lipoproteins, returning TCs from TG-rich lipoproteins to HDL 34. These EC-poor-TG-rich particles are substrates for hepatic lipase that hydrolyses the TGs. Hepatic lipase depletes the particles of core lipids, generating a redundancy of surface constituents. A TG-poor HDL core may imply better functional properties of the particle. When the HDL core is TG-rich, ApoA-I is more loosely bound to the HDL surface 35. Accordingly, after the intervention with FVOOT, which increases LCAT activity, we observed an increase in HDL2-particle subclass percentage and a decrease in the HDL3 one, while after VOO intervention we detected a decrease in LCAT activity and in HDL2-particle subclass percentage. Moreover, the increase in HDL2/HDL3 directly correlated with the increase in ApoA-I after the three interventions. In addition, an increase in CETP activity inversely correlated HDL2/HDL3 after VOO and FVOO intake. Numerous population studies have suggested that HDL2-particles may be more cardioprotective than HDL3 36, 37. Low levels of HDL2 and/or high levels of HDL3 are present in CHD 38, ischemic stroke 39, type II diabetes mellitus 40, and peripheral arterial disease patients 41. Although there are also a number of in vitro studies that reveal that HDL3 has some similar effects to HDL2 42, increased small HDL in serum may indicate an aberration in the maturation of HDL and further impaired reverse cholesterol transport 43, 44. HDL2-particles moreover bind better to SR-B1 45, thus they are more effective in promoting cholesterol efflux via this receptor 46. A similar antioxidant status between HDL2 and HDL3 has been described 47-49, nevertheless in our study, an increase in HDL2-subclass and in antioxidant enzyme activities was observed after FVOOT intake.
An HDL with an increase in EC/FC, and an HDL monolayer with more PL/FC were observed after FVOOT consumption, which also augmented LCAT activity. Hernáez et al. reported that VOO improves the fluidity of the HDL monolayer and the cholesterol efflux in a randomized, crossover, double-blind, controlled trial with healthy individuals 16. Cholesterol efflux from lipid-loaded cells is a key atheroprotective event that counteracts cholesterol uptake. The imbalance between cholesterol efflux and uptake determines the prevention or development of atherosclerosis. Four pathways for cholesterol efflux from cells to plasma are described: (i) aqueous diffusion mediated, (ii) SR-BI mediated, (iii) ABCA1 mediated, and (iv) ABCG1 mediated 50-53. The aqueous diffusion-mediated pathway mediates the bidirectional flux between the cell plasma membrane and HDL in the extracellular medium; the direction of net cholesterol mass transport is determined by the cholesterol concentration gradient as reflected by the proportion of FC and PL content in the monolayer of donor and acceptor particles 50. According to this, particles with more PL/FC, such as that which we obtained after FVOOT consumption, could be more efficient in enhancing aqueous diffusion cholesterol efflux. Furthermore, other mechanisms can increase the cholesterol efflux mediated by other transporters. Viksdet R et al. described that the active form of PLTP can increase the cholesterol efflux from macrophage foam cells and that underlying mechanism involves PLTP-mediated HDL conversion into preβ-HDL and large fused HDL particles, both of which are efficient acceptors for cellular cholesterol. These results suggested that an active form of PLTP could enhance ABCA1- and ABCG1-mediated efflux 54.
The antioxidant properties of OOPC in vivo are well known. The EUROLIVE study showed a decrease in in vivo lipid oxidative damage in a dose-dependent manner with the phenolic content of the OO administered 2. Since an increment in HDL-C after a high polyphenol-OO intake was reported in healthy volunteers, a rise in HDL-C was expected. Accordingly an increment in the HDL-C was observed in the subsample of volunteers without hypolipidemic medication, although no changes in lipid profile were appreciated when all volunteers were included in the analysis. In 2011, the European Food Safety Authority recognized the PC-rich OO effects on protecting LDL from oxidation 55. The PC acquired through diet can also directly or indirectly protect the HDL antioxidative status. An improvement of antioxidant status, reflected in an increase in PON and LCAT activities after the FVOOT intervention, was observed in the present study. In contrast, PON decreased after the FVOO intervention. PON1, which is associated with HDL, exerts a protective effect against the oxidative damage of cells and lipoproteins. It also modulates the susceptibility of HDL and LDL to atherogenic modifications such as homocysteinylation 56. A less proinflamatory and oxidized HDL could be more efficient in its pleotropic function. In addition, LCAT is an enzyme related to HDL antioxidant activity and prevents the oxidation of LDL 57. Hydroxytyrosol and the main phenols of the VOO, the secoiridoid group, have been described as acting in a similar manner to phenolic acids, inhibiting the lipid oxidation by trapping free and peroxy radicals. Moreover flavonoids, the main antioxidants of thyme, also help to control the extent of lipid peroxidation by chelating metal ions 58. Furthermore, the enrichment of VOOs with hydroxytyrosol derivatives combined with complementary phenols from aromatic herbs, such as thyme used for oil flavoring, might be a good strategy to provide the optimum balance among the different kinds of OOPC such as flavonoids, monoterpenes, and phenolic acids 23 (Fig. 1). In this sense, some non-human studies with flavonoid-enriched OO have been performed. Rosenblat et al. published that an extra VOO enriched with green tea polyphenols (mostly epicatechin gallate) attenuates atherosclerosis development enhancing macrophage cholesterol efflux in Apo-E-deficient mice more than an extra VOO. These OOs also showed a decrease in the serum oxidative stress in Apo-E-deficient mice compared with placebo treatment 59. Recently, a study has reported that an OO-pomegranate sauce prolongs lipid oxidation and can retard undesirable quality changes in anchovy 60.
One strength of our study is its crossover and randomized design, which permitted the participants to consume all OO types and thus eliminated the interindividual variability. Moreover, the laboratory analyses were centralized and all the time-series samples from the same volunteer were measured in the same run. It lacks a related-HDL oxidative status parameter to stand up the association between the PC-enriched OO and the functionality HDL-related characteristics. A limitation of the study was its sample size, which could be responsible for reduced statistical power in some biomarkers with high interindividual variability. A synergistic effect on HDL parameters between PC and other OO components is as yet unknown. Another limitation is the inability to assess potential interactions among the OOs and other diet components and medication, although the controlled diet and medication followed during all clinical trial should have limited the scope of these interactions.
In conclusion, long-term consumption of complementary phenol-enriched OO induced changes in the HDL profile associated with low cardiovascular risk, such as higher levels of large HDLs, lower levels of small ones, increased HDL EC/FC content, increased HDL monolayer PL/FC, and increased HDL antioxidant enzymes. OO, a recognized healthy food, cannot be consumed in large quantities. Thus, the enrichment of OO with its PCs is a way of increasing its healthy properties while the same amount of fat is consumed. Moreover, these results show that an enrichment of OO with complementary antioxidants, according to their structure/activity relationship, promotes more benefits than an enrichment of OO with only its own phenolics. Our data suggest that a complementary phenol-enriched OO could be a good nutraceutical to enhance the functionality of HDL particles, and thus a complementary tool for the management of high cardiovascular risk individuals.
In conclusion, for the first time, the additional benefits achieved with complementary phenol-enriched OO consumption on the HDL functional profile in hypercholesterolemic volunteers have been demonstrated with the highest degree of evidence. Moreover, such benefits can be obtained without increasing the individual's fat intake. These findings suggest that FVOOT could be a useful dietary tool in the management of high cardiovascular risk patients.
Acknowledgments
This work has been done in the context of Universitat Autònoma de Barcelona (UAB) PhD Program in Biochemistry, Molecular Biology and Biomedicine, Department of Biochemistry and Molecular Biology. We thank Borges Mediterranean Group for providing the common OO used in the study. It was supported by the Spanish Ministry of Economy and Competitiveness (MINECO) financing the project AGL2009-13517-C03-01, AGL2009-13517-C03-02, AGL2009-13517-C03-03, and the FPI-fellowship (BES-2010-040766), by a contract from the Catalan Government and the ISCIII-FEDER (FIS-CP06/00100), by a Sara Borrell contract (CD10/00224), and by grants from ISCIII FEDER (CB06/03/0028), and AGAUR (2014SGR240). CIBEROBN, CIBERESP, and CIBERDEM are initiatives of ISCIII.
The authors have declared no conflict of interest.




