Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death
Abstract
Scope
Resveratrol (RSV) has been proposed to prevent tumor growth; nevertheless, these preventive effects are controversial since RSV pharmacokinetics studies show a low bioavailability. Recent clinical trials show that patients with colorectal cancer and receiving oral RSV have high levels of RSV conjugates in the colorectum, mainly RSV-3-O-sulfate (R3S), RSV-3-O-glucuronide, and RSV-4′-O-glucuronide. However, their potential biological activity has not yet been established. This study thus investigated in human colorectal cancer cell lines whether RSV main metabolites retain anticarcinogenic properties as their parental molecule.
Methods and results
Proliferation, apoptosis assays and cell cycle analysis were performed to study the effect of RSV, R3S, RSV-3-O-glucuronide, or RSV-4′-O-glucuronide alone or of a mixture of the three metabolites. R3S inhibits colon cancer cells proliferation and an accumulation of cells in S phase. Interestingly, the mixture induced a synergistic effect. This process was associated with an induction of DNA damages and apoptotic process, which allowed sensitization of colon cancer cells to the anticancer drugs.
Conclusion
Altogether, our data provide significant new insight into the molecular mechanism of RSV and support the notion that despite low bioavailability in vivo, RSV biological effects could be mediated by its metabolites.
Abbreviations
-
- Ab
-
- antibody
-
- BrdU
-
- 5-bromo-2-deoxyuridine
-
- cdk
-
- cyclin-dependent kinase
-
- PI
-
- propidium iodide
-
- RSV
-
- resveratrol
-
- R3G
-
- RSV-3-O-glucuronide
-
- R3S
-
- RSV-3-O-sulfate
-
- R4G
-
- RSV-4′-O-glucuronide
-
- UV
-
- ultraviolet
1 Introduction
A wide variety of plant-derived compounds, including polyphenols, are present in the human diet and may protect against vascular diseases, cancers, viral infections, neurodegenerative, and associated inflammatory processes. The impetus sparking this scientific inquiry was the result of many epidemiologic studies that showed protective effects of plant-based diets on cardiovascular diseases and cancers. Among these bioactive compounds, several epidemiological studies 1 revealed that resveratrol (trans-3,4′,5-trihydroxystilbene, RSV) may be one of the main wine microcomponents responsible for health benefits. Indeed, RSV can prevent important pathologies, i.e. vascular diseases, cancers, or neurodegenerative processes (see for review 2, 3). These preventive actions of RSV have been extensively studied at the molecular and cellular level, such as cellular signaling, enzymatic pathways, apoptosis, and gene expression 4-6. Particularly, we have previously demonstrated that the ability of RSV to prevent the occurrence of carcinomas is related to the inhibition of tumor cell cycle 7, 8 and the induction of tumor cell death 9. Furthermore, there are also compelling evidences that RSV can sensitize tumor cells to various anticancer drugs 10, 11 and cytokines such as TRAIL 12, suggesting its potential use as an adjuvant for new chemotherapy strategies. These in vitro observations are correlated with numerous in vivo evidences showing that RSV can prevent or delay the onset of cancer, heart diseases, ischemic and chemically induced injuries, diabetes, pathological inflammation, and viral infection in various animal models 13. However, these preventive effects of RSV are very controversial since RSV pharmacokinetics studies show a low bioavailability and rapid clearance from the circulation 13-15. These low circulating levels could be explained by a rapid and extensive phase II metabolism which generates glucuronide and sulfate conjugates 16-19. A potential answer to controversies between the low RSV bioavailability and its in vivo evidence as a preventive agent is provided by recent phase I/II clinical trials from the University of Leicester, UK. Indeed, Patel et al., have shown that patients with confirmed colorectal cancer and who received daily for 8 days before surgery 0.5 and 1.0 g of RSV, had high levels of RSV conjugates in the colorectum, mainly RSV-3-O-sulfate (R3S), RSV-3-O-glucuronide (R3G), and RSV-4′-O-glucuronide (R4G) (Fig. 1) 20. This study also show that daily oral doses of RSV at 0.5 and 1.0 g can generate sufficiently high levels of metabolites in the human gastrointestinal tract for the polyphenol to exert antiproliferative properties 20. Moreover, other RSV trials in humans after single 19, 21 or multiple daily doses of up to 600 mg/day administered over 2 or 3 days 22, 23, showed that RSV is safe under the tested conditions and that the main metabolites found in the circulation are R3S, R4G, and R3G, with particularly high levels in the case of the sulfo-conjugate 24.
These findings are very important, as it has been speculated that RSV metabolites may contribute to the pharmacological efficacy of its parent molecule 13, 25 notably in its anticancer action. Nevertheless, this hypothesis remains actually unsolved. At the concentrations estimated in plasma of patients 24, we studied for the first time the biological activities of RSV metabolites alone or in combination on colon cancer cells, as well as their potential to sensitize metastatic colon cancer cells to anticancer agents such as SN38 (7-ethyl-10-hydroxycamptothecin) or oxaliplatin used in chemotherapy.
2 Materials and methods
2.1 Cell lines
SW480 human colon carcinoma cell line and its derived metastatic cell line SW620 obtained from the American Tissue Culture Collection (ATCC, Rockville, MD, USA) were maintained in RPMI 1640 medium, (Biowhittaker Co, Fontenay sous Bois, France) supplemented with 10% fetal calf serum and 2 mM l-glutamine (Biowhittaker).
2.2 Drugs, chemical reagents, and antibodies
Trans-RSV, SN38, and oxaliplatin were obtained from Sigma-Aldrich (St Quentin Fallavier, France). R3S, R3G, and R4G were obtained from Bertin Pharma (Montigny-le-Bretonneux, France). For all experiments except those of dose–response, the three metabolites were used at 30 μM. For chemosensitization assays, RSV and RSV metabolites were used at 10 and 20 μM alone and the mixture (M) of metabolites was performed by using 10 or 20 μM of each metabolite. Polyclonal antibodies (Abs) against cyclin A, cyclin B1, cyclin E, Cdk1, Cdk2, γH2AX, ATM, ATR, p21, and p53 were purchased from Santa-Cruz Biotechnology (Santa-Cruz, CA, USA), human caspase-3 active fragments and PARP from Cell Signalling (Beverly, MA, USA), and mouse monoclonal Ab against β-actin from Sigma-Aldrich.
2.3 Stability studies
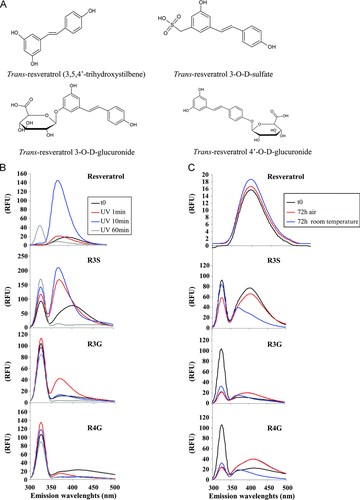
Emission spectra of trans-RSV (30 μM in water with 0.1% ethanol) and RSV metabolites alone (10 μM in water in 0.1% ethanol) were carried out in a 1-cm quartz cell with a spectrofluorimeter. Ultraviolet (UV) irradiations were obtained by exposure of solutions in quartz cells on a 6 × 15 W/312 nm UV transilluminator table. Metabolites (10 μM in water in 0.1% ethanol) were also exposed to air or to room temperature for 72 h before analysis by spectrofluorimetry. Data are expressed as relative fluorescence units.
2.4 Cell viability measurements
SW480 and SW620 cells were seeded 24 h before treatment into 24-well plates in complete growth medium. The next day, cells were challenged for 24, 48, or 72 h with RSV or with various concentrations of its metabolites alone, i.e. R3S, R3G, and R4G or with a mixture of these three metabolites (M). All control and treated cells received the same amount of ethanol (0.1%). After the indicated times, cells were washed with PBS and then stained with crystal violet (0.5% (w/v) for 5 min and then rinsed thrice with water. Absorbance was read at 540 nm after extraction of the dye by 0.1 M sodium citrate in 50% ethanol.
2.5 Cell cycle analysis by flow cytometry
Cell cycle analysis was performed as described previously 7. In brief, SW620 cells were grown in 6-well plates, treated or not with RSV or metabolites alone or with the mixture of the three metabolites (30 μM each). After 48 h of treatment, cells were labeled with 30 μg/mL 5-bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich) for 1 h 30 min at 37°C. Then, detached and adherent cells were collected and pooled, washed with PBS, fixed in cold 80% ethanol and then incubated with a mouse anti-BrdU monoclonal antibody (Dako, Denmark) for 1 h at room temperature. After washing, pellets were subsequently incubated with a FITC-conjugated anti-mouse antibody (Dako) for 40 min and were then counterstained with a 50 μg/mL solution of propidium iodide (PI) containing 200 μg/mL of RNAse A. Stained cells were analyzed with a Galaxy flow cytometer (Partec, Münster, Germany) equipped with a 488 nm blue laser providing acquisition of 20 000 events. Red fluorescence from PI was detected through a 625 nm long pass filter (FL3, DNA content), and green fluorescence from FITC was detected through a 520/10 nm band pass filter (FL1, BrdUrd incorporation). The acquired data were analyzed with FlowMax software (Partec) and displayed as dual-parameter PI versus log-FITC histograms.
2.6 Western blotting
Cells washed in PBS were lysed in a RIPA buffer (10 mM Tris-HCl, pH 7.2, 150 mM NaCl, 0.5% Nonidet NP40, 0.5% Na deoxycholate, 0.1% SDS, 2 mM EDTA, and 50 mM NaF) in the presence of 1/25 complete protease inhibitor cocktail tablets (Roche Diagnostics Corporation) for 15 min on ice. Cell lysates were cleared by a 15 min centrifugation at 20 000 × g. Protein concentration was measured in the supernatant using the BCA protein assay kit (Sigma-Aldrich). Thirty micrograms of proteins were diluted in loading buffer (125 mM Tris-HCl, pH 6.8, 10% β-mercaptoethanol, 4.6% SDS, 20% glycerol and 0.003% bromophenol blue), boiled for 5 min, separated on a polyacrylamide SDS containing gel and transferred onto a polyvinylidene difluoride membrane (Bio-Rad). After blocking nonspecific binding sites for 2 h with 3% nonfat milk and 1% BSA in TBST (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20, pH8), the membrane was incubated overnight with the primary antibody diluted in TBST, washed twice with TBST and incubated for 45 min at room temperature with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit antibody (Santa-Cruz Biotechnology). The membrane was washed twice with TBST and revealed using an enhanced chemiluminescence detection kit (Amersham, Les Ulis, France) and autoradiography.
2.7 Apoptosis identification
Apoptosis was identified by staining the nuclear chromatin of trypsinized cells (untreated and RSV metabolites-treated cells) with 1 μg/mL Hoechst 33342 (Sigma-Aldrich) for 15 min at 37°C. The percentage of apoptotic cells was determined by analyzing 300 cells from randomly selected fields for each condition of treatment.
2.8 Chemosensitization assays
SW620 cells were seeded 24 h before treatment into 24-well plates. The next day, cells were left untreated or pretreated in triplicate wells with RSV, RSV metabolites or mixture of metabolites for 24 h and then challenged for additional 24 h with either SN38 (50 nM) or oxaliplatin (500 nM). After treatments, cells were washed with PBS and then stained with crystal violet (0.5% (w/v) as described previously in Section 2.3.
2.9 Statistical analysis
Data are expressed as means ± SD (n = 6) of at least three independent experiments. The significance of differences was established with the Student's paired t-test. Values of p < 0.05 were considered significant.
3 Results
3.1 RSV metabolites inhibit colon cancer cell proliferation
To determine whether RSV metabolites exert a potential anticancer action, we first compared in human colorectal cancer cells, the antiproliferative capacities of the three main RSV metabolites identified in both healthy volunteers 19 and colon cancer patients 20 who received oral doses of RSV, R3S, R3G, and R4G, to 30 μM of RSV aglycone (R30). At this concentration, we have previously shown that RSV inhibits cancer cells proliferation 26, 27 without cytotoxicity in normal human monocyte and in normal rat intestinal IEC18 cells 8, 29. As various studies have shown that RSV is not stable after UV exposure or to air 28, we have tested the stability of RSV metabolites under UV exposure or air or room temperature (Fig. 1B and C). It appeared that emission spectra of RSV metabolites are modified by UV exposure with a shift to the left (Fig. 2B). We also observed instability of the glucuronide metabolites when they were exposed to sunlight or air for 72 h with a lowering of the spectrum (Fig. 2C).
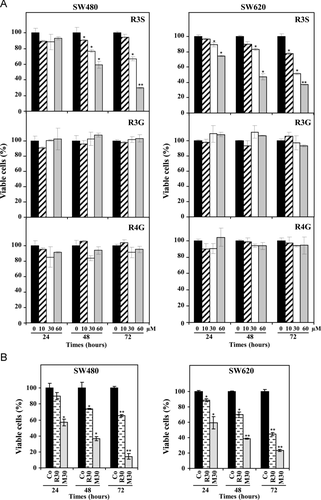
As shown in Fig. 2A, only R3S was able to inhibit SW480 and its metastatic-derived SW620 cell line in a time and dose-dependent manner. Moreover, we observed that after 72 h of treatment inhibition rate induced by R3S was similar to that induced by R30, with 50% and 35% of inhibition in SW480 and SW620, respectively (Fig. 2A and B). Contrary to R3S, glucuronide metabolites did not significantly affect colorectal cancer cell lines growth (Fig. 2A). R3S, R3G, and R4G are found together in gastrointestinal tract 20, 24, we next tested the efficiency of an equimolar mixture of the three metabolites (M30) to determine a potential synergy. Interestingly, although RSV glucuronides used alone had no effect on cell proliferation, the mixture showed a potent synergic, time, and dose-dependent antiproliferative effect which was even more potent than RSV or R3S alone (Fig. 2B). Indeed, the combination of 30 μM of each metabolite inhibited by 65% and 80% cell proliferation after 48 and 72 h, respectively (Fig. 2B). This is an important point because it has been recently shown that after an oral administration of 1.0 g of RSV for 29 days, the plasma mean concentrations of the three RSV metabolites were around 30 μM 24. At this concentration, which is described in plasma levels of patients 24, the proliferation inhibition induced by the mixture was specific since it did not increased cell permeability after PI labeling (data not shown). Thus, in the subsequent experiments we used the combination of 30 μM of each metabolite.
3.2 RSV metabolites accumulate colon cancer cells in S phase of the cell cycle
We have previously shown that RSV affects cell proliferation by disturbing cell cycle progression 7. In order to determine whether metabolites, in particular R3S and M30, have the same effect we analyzed by flow cytometry the distribution of cells in cell cycle 7. Colon cancer cells were exposed for 48 h to the different compounds alone or with the mixture before addition of BrdUrd in the last hour of treatment. Cells with high fluorescence were S phase cells that incorporated BrdUrd. Those with background fluorescence (Fig. 3A) represented G1 or G2/M phase cells, depending on DNA content (2C or 4C, respectively).
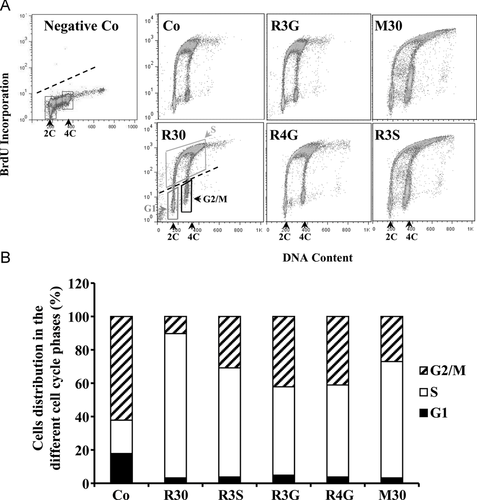
The results reveal that at a time of treatment inducing an arrest of cell proliferation such as 48 h, all RSV metabolites induce marked modifications of the cell cycle at 30 μM (Fig. 3A). We particularly noticed that R3S increased by +65% the number of cells in the S phase which was comparable to the accumulation in this cell cycle phase induced by RSV that was used as a positive control. Interestingly, contrary to the action on cell proliferation, RSV glucuronides, R3G and R4G, induced an accumulation almost equivalent to that observed with R3S. In a same manner, the metabolites mixture induced a strong accumulation of cancer cells in this DNA replication phase with 75% of BrdUrd positive cells (Fig. 3B). These effects induced by RSV metabolites were associated with a decrease in the percentage of tumoral cells in G1 and in G2/M phases, respectively (Fig. 3B).
Furthermore, bivariate BrdUrd/DNA histograms shows that R3S and M30 treatments shifted BrdUrd positive cells to higher DNA content which suggest the induction of polyploidy processes (Fig. 3A).
3.3 RSV metabolites modulate key regulators of cell cycle
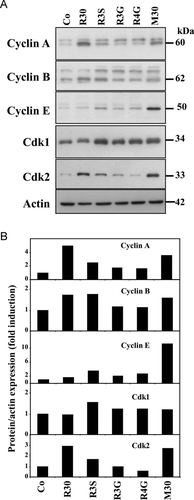
Cancer cells accumulation in S phase prompted us to investigate whether the key regulators of the cell cycle progression, cyclins and their partners, the cyclin-dependent kinases (Cdks), could be affected upon exposure to the studied compounds. As we have previously published 7, RSV (R30) increases cyclins A and B expression as well as Cdk 2 protein levels which have been shown to be required for DNA synthesis 30 (Fig. 4A). In this study, immunoblotting demonstrate that R3S and more strongly the mixture (M30) dramatically increased cyclin A, cyclin E, Cdk1, and Cdk2 expressions (Fig. 4). A concomitant cyclin B expression was also observed. Indeed, quantitative analysis show that after 48 h of treatment, R3S increases by 2.43-fold cyclin A, by 3.51-fold cyclin E, by 1.56-fold Cdk1, and by 1.67-fold Cdk2 protein expression levels as compared to control (Co) (Fig. 4B). In agreement with the previous results obtained on cell proliferation, the mixture (Mix 30) more potently enhanced the expression levels of key cell cycle (+3.49-fold for cyclin A, +11.26-fold for cyclin E, 2.72-fold for Cdk2) (Fig. 4B). In these experiments, RSV glucuronides slightly increased cyclins and Cdks expression after 48h of treatment (Fig. 4). These results underline the importance of cylin E and Cdk1 in the disruption of cell cycle progression induced by RSV metabolites, which we have previously demonstrated in colon cancer cells after RSV treatment 3, 7, 27.
3.4 RSV metabolites induce p53 and H2AX phosphorylation
Recent reports have correlated the delay through S→G2/M progression in the cell cycle to the increase in p53 phosphorylation and to the induction of DNA damages. Both processes are reported to depend on the DNA damage checkpoint kinases ataxia telangiectasia mutated (ATM)/ataxia telangiectasia-Rad3-related (ATR) 31 and Chk (checkpoint kinase) 32. ATR is a protein that is well known as a central mediator of responses to DNA double strand breaks and subsequent replication stress. Some studies have reported that RSV induces S phase arrest via an activation of DNA checkpoint pathways including ATM/ATR kinases 33.
RSV metabolites, which are able to induce a polyploidization (Fig. 3A), especially R3S and M30, induced a strong increase in phosphorylated-histone H2AX (γH2AX) which reflects DNA damage levels. Indeed, as well as RSV, R3S, and M30 strongly enhanced H2AX serine-139 phosphorylation after 48 h of treatment (Fig. 5) which represent +1.66- and +2.45-fold increases, respectively. In various models, RSV has been shown to induce DNA damages through the activation phosphoinositide kinase homologs such as ATR 31, 33, 34. Our results show that RSV metabolites alone or together increased ATR expression as compared to nontreated cells (Fig. 5). These activations lead to p53 activation, which has been shown to respond to DNA damage by an increase in its phosphorylation at serine-20 (Fig. 5). In agreement with the activation of p53, we observed that R3S and M30 induced p21 expression, which has been described as one of the transcriptional targets of tumor suppressor p53 protein (Fig. 5).
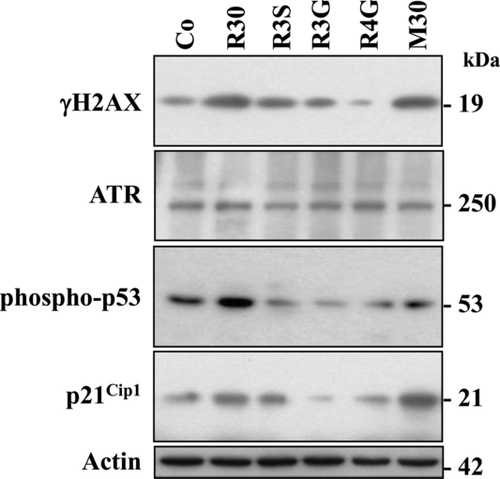
3.5 RSV metabolites induce apoptosis
Following DNA damages, cells either repair the damages or undergo apoptotic death mostly in a p53-dependent manner. To precise the relationships between tumoral cells accumulation in the S phase, the increase in polyploidy cells and the induction of ATR/p53 pathway signaling, we investigated the cell death induced by RSV metabolites. As we have previously shown 9, when cultured in the presence of RSV (R30), colon cancer cells underwent apoptosis (Fig. 6). This process was also observed with R3S and M30, as evidenced by tumor cells staining with Hoechst 33342, which showed characteristic apoptotic changes, i.e. condensation and fragmentation of nuclear chromatin (Fig. 6A). This apoptotic induction was not observed with glucuronide metabolites (Fig. 6A and B). R3S and M30-mediated apoptosis and DNA damages are confirmed with the cleavage of the nuclear DNA repair enzyme poly(ADP-ribose)-polymerase (PARP) into an N-terminal 89 kDa fragment and by the cleavage of the Mr 32 000 proform of caspase-3 into its active fragment (Fig. 6C).

3.6 Synergic effects of RSV metabolites with SN38 or oxaliplatin on colon cancer cells
We and others have shown that RSV could be used as a chemosensitizing agent or a chemotherapeutic adjuvant particularly due to, in some cancer models, cell cycle arrest, cyclins and Cdks modulations, and apoptosis induction 7, 10, 11.
The ability of RSV metabolites to induce (i) cell cycle arrest in DNA replication phase, (ii) apoptotic cell death, (iii) important DNA damages shown by γH2AX induction, seems to suggest that RSV metabolites could act as chemosensitizing agents. In order to evaluate this potential, we looked for a synergistic effect of RSV metabolites with SN38 (irinotecan's active metabolite), a classical anticancer agent widely used in the treatment of metastatic colon cancer 35. Recent studies have shown that SN38 resistant colon cancer cells present a huge reduction of DNA double strand breaks formation compared to sensitive cancer cells 36. In our previous experiment (Fig. 4), we observed a γH2AX increase in SW620 metastatic colon cells induced by R3S and the mixture, reflecting a DNA double strand break formation. Pretreatment with RSV, R3S or the mixture at 10 or 20 μM for 24 h dramatically enhanced SN38-induced growth inhibition of SW620 cells (Fig. 7A and B). Moreover, it is noted that R3G and R4G, that also induced ATR pathway (Fig. 5), cooperated with SN38 to kill tumor cells (Fig. 7). Similar results were obtained with another anticancer agent acting on DNA repair processes, oxaliplatin, which is a third generation platinum derivative (Fig. 7). These results are very important since at the concentrations used (10 and 20 μM) in these chemosensitizing experiments, RSV metabolites concentrations are compatible with plasmatic concentrations detected in patients receiving an oral dose of RSV 24.
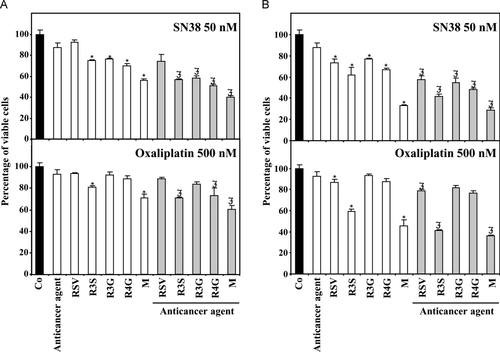
4 Discussion
There are compelling evidences that RSV can act as a chemopreventive agent or a chemotherapeutic drug against various cancers through the regulation of multiple therapeutic targets 3. Nevertheless, these chemopreventive effects remained controversial due to its low bioavailability level 21, 37. Recently, Brown's team has shown in a phase I/II clinical trial that the levels of RSV and RSV metabolites (R3S, R3G, and R4G) measured in human colorectal tissue after repeated oral ingestion of 1 g 20 were correlated to a 5% (p = 0.05) reduction in tumoral cell proliferation thus suggesting potential anticarcinogenic properties of RSV metabolites. Consequently, the potential activity of RSV metabolites is an important question to solve in order to better understand RSV mechanisms of action. The present study shows that RSV metabolites, especially the sulfate conjugate, R3S, inhibited proliferation of colon adenocarcinoma cells, SW480, and their metastatic-derived cells SW620 due to an arrest of cell cycle in S phase. Interestingly, we showed that the mixture of three RSV metabolites, (R3S, R3G, and R4G) induced an antiproliferative activity greater than that of RSV or its metabolites alone. These antiproliferative effects were accompanied by a blockage of cells in S cell cycle phase, by the induction of DNA damages and by the induction of apoptotic cell death. All these processes favored the antitumoral activity of SN38 and oxaliplatin in SW620 metastatic cancer cells. All these events suggest that in vivo RSV metabolism could produce chemoprotective metabolites, which in combination may efficiently act on cancer cells.
RSV has been proposed to function as a cancer chemopreventive agent through the inhibition of different steps of carcinogenesis 3. RSV also has direct cytostatic 27, 38 or cytotoxic effects 9, 39-41 through a cell cycle disruption. The later effect can be mediated by apoptosis through activation of both extrinsic and the intrinsic pathways leading to caspase activation, and to oncoproteins and death kinases pathways 9, 39-42. Pharmacokinetics studies of RSV in human and animal models show that RSV is efficiently absorbed upon oral administration with levels being recovered in plasma and urine 37. These low levels in circulation could be explained by a rapid and extensive phase II metabolism which generates conjugates and five distinct metabolites that are present in the urine: RSV monosulfate, two isomeric forms of RSV monoglucuronide, dihydroRSV monosulfate, and dihydroRSV monoglucuronide 16-19. The nature and quantity of metabolites may differ among subjects due to interindividual variability 19, 21, 37. Once in the bloodstream, metabolites can be subjected to phase II metabolism with further conversions occurring in the liver, where enterohepatic transport in the bile may result in some recycling back to the small intestine 43. We have already shown in hepatic cells, that RSV is highly conjugated after 4 h of incubation into mono-(3-sulfate-RSV and 4′-sulfate-RSV) and disulfate (3,4′-disulfate- RSV and 3,5-disulfate- RSV) derivatives but no glucuronide conjugates could be found 16. Several phase I/II clinical trials in humans are currently underway for oral RSV administration, including National Cancer Institute-sponsored studies at the University of Michigan, USA, and at the University of Leicester, UK. This last study revealed that after daily RSV administration (0.5, 1.0, 2.5, or 5.0 g) for 29 days in 40 healthy volunteers, no significant adverse effects were observed, only mild to moderate gastrointestinal symptoms with 2.5 and 5.0 g of RSV 44. Other reports from RSV trials in humans after single 19, 21 or multiple daily doses of up to 600 mg/day administered over 2 or 3 days 22, 23, show that RSV is safe under the tested conditions. In these studies, circulating levels of RSV sulfo-conjugate is the most important. With the highest RSV oral dose, authors also found peak circulating levels of the aglycone molecule approaching the concentrations reported to have pharmacological activity in vitro 14. These finding are very important since it has been speculated that RSV metabolites may contribute to the pharmacological efficacy of the parent molecule 13. Nevertheless, little information is available concerning the biological effects of RSV metabolites. Recent in vitro studies hint at the possibility that R3S and R4S may contribute to some of the pharmacological properties elicited by their parent molecule 45, 46. Indeed these studies showed that R3S and R4S were able to inhibit NO production and cyclo-oxygenase-1/2 activities 45, 46.
Here, we show for the first time that R3S and the mixture of RSV metabolites (R4S, R3G, and R4G) inhibit human colon carcinoma and metastatic cell proliferation through an accumulation of cells in DNA replication phase. As demonstrated for RSV, R3S and the mixture induced a significant increase in cyclins A and E, as well as their kinases, Cdk1 and Cdk2. Cyclin A and Cdk2 may be responsible for the entry in S phase by activating components of the DNA replication initiation complex 47. These results are in accordance with previous reports showing that RSV alone increases these cell cycle key protein regulators involved in the formation of cyclinA/complex and which allows S phase progression 3, 7. This subsequent accumulation of tumor cells in S phase preceded apoptotic cell death. Interestingly, RSV glucuronides alone had no effect on cell proliferation and apoptosis induction but induced cell cycle disruption. The delay through S→G2/M progression in the cell cycle induced by RSV metabolites was associated with an increase in p53 phosphorylation resulting from the activation of the ATR pathway. ATR is a protein that is well known as a central mediator of DNA double strand breaks responses and subsequent replication stress. In fact, some studies have shown that RSV can activate ATM/ATR kinases signaling in response to DNA damage 33, 34. In our study, RSV metabolites promoted the phosphorylation of histone H2AX which is a marker of DNA damages. Once p53 is activated, one of the transcriptional targets, p21, is increased and plays a key role in cell cycle arrest. Moreover, the overexpression of this essential gene target was often found when DNA damage occurred. So, RSV metabolites activated p53 and subsequently enhanced p21 protein expression, which may be correlated to DNA damage in colon cancer cells 48. Consequently, by inducing DNA damages, RSV, R3S, and the mixture could counteract carcinogenesis and be used as chemopreventive agents since initiation of DNA damages is a basic element of cancer prevention 34, 49.
Furthermore, a recent report has shown that DNA double strand break formation is reduced in colon cancer cells resistant to a classical anticancer drug widely used in chemotherapy, SN38, an active metabolite of irinotecan 36. Thus, pretreatment of cells with RSV, R3S, and the mixture, all of them inducing DNA damages synergized with SN38 and also with oxaliplatin to induce cancer cells death. Hence, the use of RSV in pretreatment may not solely improve the anticancer drugs efficiency but may also help in reducing their dose and thus in reducing their cytotoxicity.
Altogether, our data obtained in colon cancer cells support the notion that RSV in spite of a low bioavailability could act through its metabolites mainly the sulfo-conjugate but also through the combination of sulfate/glucuronides derivatives. These findings provide significant new insight into the molecular basis of RSV biological activity. Moreover, the combination of RSV with agents that induce DNA damages such as SN38 could constitute a promising approach to counteract the development of resistance, which often limits the efficacy of anticancer drugs in metastatic colon cells.
Acknowledgments
This work was supported by the Ligue Inter-régionale Grand-Est Contre le Cancer.
The authors have declared no conflict of interest.




