Effect of flavonoids on circulating levels of TNF-α and IL-6 in humans: A systematic review and meta-analysis
Abstract
Epidemiological or in vitro evidence suggests a potential role for flavonoids as anti-inflammatory agents; we investigated the effect of flavonoids-rich foods or supplements on tumor necrosis factor- alpha (TNF-α) and interleukin-6 (IL-6) in long-term placebo-controlled human intervention trials. From 110 human intervention studies selected (MEDLINE, EMBASE, CHORANE, and FSTA databases), 32 long-term placebo-controlled trials were suitable for meta-analysis. After sensitivity analysis, seven studies imputed of bias were excluded and 25 studies were analyzed (TNF-α, n = 2404; IL-6, n = 2174). Levels of TNF-α decreased after flavonoid consumption in the fixed model only (mean difference (MD) (95% CI): −0.098 (−0.188, –0.009), p = 0.032), but metaregression results showed that neither higher dose, nor a longer duration of intervention were associated with a greater effect size. Subgroup analysis did not reveal any significant effect for quercetin and soy, but other sources (red wine, pomegranate, and tea extracts) showed a significant effect size both in fixed (MD (95% CI): TNF-α −0.449 (−0.619, –0.280), p < 0.001; IL-6 −0.346 (−0.612, –0.079), p = 0.011) and random (MD (95% CI): TNF-α −0.783 (−1.476, –0.090), p = 0.027; IL-6, −0.556 (−1.062, –0.050), p = 0.031) effect models. High-quality placebo-controlled trials are needed in order to identify flavonoids as the active ingredients.
Abbreviations
-
- ApoE
-
- apolipoprotein E
-
- CVD
-
- cardiovascular disease
-
- EGCG
-
- epigallocatechin 3-gallate
-
- IL-6
-
- interleukin-6
-
- MD
-
- mean difference
-
- TNF-α
-
- tumor necrosis factor-alpha
1 Introduction
Chronic low-grade inflammation is a common pathogenetic denominator in many diseases 1-3 and inflammatory markers are significant predictors of mortality in humans 1. Tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are important mediators of chronic low-grade systemic inflammation, which plays a central role in several pathologies, such as diabetes, metabolic syndrome, and cardiovascular disease (CVD) 4-6. Besides, these cytokines have been also implicated in complication related to obesity 6, as well as in geriatric 7 and cancer-induced cachexia 8. Therapeutic blockade of IL-6 9 and TNF-α 10 signaling are emerging in clinical management of immune-related inflammatory diseases. Following that, the effect of dietary habit on TNF-α and IL-6 represents an important subject with significant clinical implications.
A large body of evidence suggests a primary role for plant food-based diet in reducing risk of mortality from inflammatory stress-mediated diseases. Studies have suggested an inverse correlation between fruit and vegetable consumption and serum levels of inflammatory markers 11-13. The molecules, contained in foods of plant origin, responsible for the protective action are not known, but flavonoids have been recognized as potential candidates 14, 15. Flavonoids, divided in different subclasses: flavonols (quercetin and kaempferol), flavones (luteolin and apigenin), flavanols (catechins and proanthocyanidins), anthocyanidins, flavanones (naringenin and hespertin), and isoflavones (genistein and daidzein), are widely present in the majority of food of plant origin 16, 17. Flavonoid-rich food intake was significantly and inversely related to TNF-α and IL-6 1, 18, 19. Mounting evidence suggests that flavonoids in vitro inhibit TNF-α 20-22 and IL-6 21, 23 productions. However, evidence in humans are limited and the results are contrasting, mainly due to the fact that only few studies measure flavonoid plasma levels 24. In fact, the health effects of flavonoids depend on their bioavailability, which is low in humans and is affected by several variables, such as intestinal absorption, metabolism by the microflora, intestinal and hepatic metabolism, nature of circulating metabolites, binding to albumin, cellular uptake, accumulation in tissues, and biliary and urinary excretion 17. Thus, the effects observed in vitro may not reflect the in vivo situation and the current knowledge remains limited and inconclusive. Moreover, the large majority of evidence is obtained with nonphysiological concentrations of flavonoids and without considering the nature of the metabolites found in bloodstream 21, 23, 24 compared to the scarce results obtained with metabolites 20, 22. The objective of this meta-analysis was to investigate the effect of flavonoids-rich foods or supplements on the more frequently studied inflammatory cytokines (TNF-α and IL-6). To this aim, a systematic review and meta-analysis of chronic intervention studies with flavonoids or flavonoid-rich foods and circulating concentrations of TNF-α or IL-6 with outcome has been conducted in this work.
2 Methods
2.1 Study selection
We performed a systematic search in the MEDLINE, EMBASE, COCHRANE, and FSTA databases for literature examining the effect of flavonoids or flavonoid-rich foods on TNF-α or IL-6 circulating levels, up to July 2012. The search terms and the limits are depicted in Fig. 1. Given that the aim of present review was to focus on the consumption of food-derived flavonoids, interventions that involved herbal extracts or sylimarin supplements were excluded.
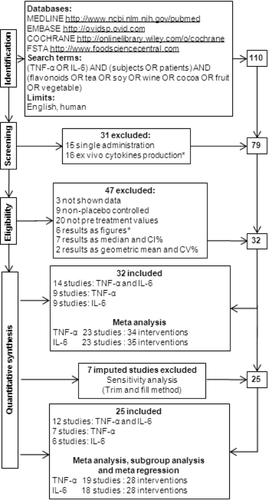
Any study that met the following criteria was included for meta-analysis: studies that appear in an edited journal (peer-review criterion) and are written in English (language criterion), randomized placebo-controlled trials (research criterion), of a duration ≥7 d (duration criterion), focused on the effect of flavonoid-rich food or supplements on TNF-α or IL-6 levels (topic criterion), the plasma levels of cytokine were measured by ELISA before and after placebo and treatment (method criterion). All human chronic studies were included if they met the above criteria regardless of the flavonoids’ source and dose or the healthy status of subjects. First trials were identified through title or abstract. When a title and abstract could not be rejected with certainty, the full text of the article was obtained. Then, based on inclusion and exclusion criteria, eligible studies were included through full text and reviewed by two independent authors (I.P. and A.R.). Discrepancies were resolved by discussion with the third reviewer (M.S.).
Using the search criteria, after exclusion of irrelevant references, a total of 110 intervention studies after flavonoids-rich food and beverages consumption or pure molecules and food extracts administration were identified as suitable and were retrieved for complete review 25-134. Figure 1 depicts the flow of studies in this review and the four-phase diagram of meta-analysis, according to the PRISMA Statement 135. First, of the 110 reviewed, we excluded studies referring to single administration or addressing ex vivo cytokines production. Then, we excluded studies referring to not shown data, nonplacebo-controlled trials, not randomized studies and papers reporting posttreatment but not pretreatment values. Usually, normally distributed data are given as mean ± SD and not normally distributed variables as medians (25–75 percentiles). Thus we excluded studies with data presented as medians, as well as by figures, but retained them for discussion. The remaining 32 studies met our inclusion criteria and could provide data for the analyses. After this first meta-analysis, studies imputed of bias by Trim-and-fill sensitivity analysis (Fig. 2) were excluded and a second meta-analysis was performed on 25 studies, 12 of which reported data on both cytokines, seven only data on TNF-α and eight only data on IL-6. Meta regression and subgroup analysis were also performed on these 25 studies reporting 28 data sets from 19 studies for TNF-α and 28 data sets from 18 studies for IL-6.

2.2 Data extraction and analysis
A data extraction form including quality characteristics was designed for the review (Table 1) and piloted by all of those involved in the meta-analysis (I.P., A.R., and M.S.). All data extraction and quality assessment were performed independently by two reviewers (I.P. and A.R.) to ensure uniformity, and all data were entered by these reviewers, who checked the extracted data and validity criteria against the original published report. Discrepancies were resolved by discussion between the two reviewers and unresolved disagreement was referred to a third reviewer (M.S.).
| General checklist | Data extracted | Subgroup and metaregression | Quality score (values between 0–1) | ||||
|---|---|---|---|---|---|---|---|
| Reference | Year, authors, journal | Controls | Number | Type of intervention | Pure molecule, extract, food, or beveragea | Proper placebo | 0.30 |
| Male | Number | Pg/mL before treatment | Mean ± SD/SEM | Dose | Mg/die of flavonoid | Intra and interstudy baseline comparability | 0.30 |
| Female | Number | Pg/mL after treatment | Mean ± SD/SEM | Duration | Days | Compliance assessment | 0.10 |
| Dropout | % | Treated | Number | Status | Healthy, risk: menopausal, overweight, dislipidemia disease | Dietary record (Food records or food-frequency questionnaires throughout the study) | 0.06 |
| Body mass index (BMI) | Mean | Pg/mL before treatment | Mean ± SD/SEM | Food antioxidant intake in subjects selection criteria (Flavonoid-rich food or antioxidant supplement consumption) | 0.05 | ||
| Age | Mean | Pg/mL after treatment | Mean ± SD/SEM | Wash out/run-in period | 0.05 | ||
| Study design | Crossover, parallel | Delta pg/mL | Mean ± SD/SEM | Marker of bioavailability | 0.05 | ||
| Double blinding | 0.05 | ||||||
| No funding support | 0.03 | ||||||
| No food donation | 0.01 | ||||||
- a Due to the low number of intervention, data are subgrouped in: quercetin, soy, and other sources.
We were interested in the following outcomes or endpoints, pre- and post-IL-6 and TNF-α concentration for both treatment and control. Also other data were extracted to assess each trial's risk of bias and perform a validity assessment as described in Table 1. Considering that restriction to high-quality studies may exclude much information, while inclusion of low-quality studies may bias the summary effect estimate, we assigned a quality score as described in Table 1. Withdrawal was not considered as a bias because in dietary or flavonoid-based intervention, the compliance of subjects is generally due to factors not imputable to treatments or researchers.
For the meta-analysis, we calculated the mean change of plasma concentrations of selected cytokines from baseline to follow-up for each intervention and control group, if not reported, and the SD of delta concentration was estimated as previously described 136. All values were converted to pg/mL, if necessary. If more than one time point for follow-up was reported, we included the value corresponding to the longer treatment. For each study with more than one intervention group, we divided the control group evenly according to the number of intervention groups 137. When the data were presented as SEMs, SD was obtained by multiplying SEM by the square root of the sample size.
Statistical analysis has been performed with MIX software 138 and meta-analyst software 139. Mean difference (MD) and confidence intervals 95% (CI 95%) were calculated by fixed (inverse variance, unequal, small sample corrected) random (DerSimonian & Laird, unequal, small sample corrected) and quality effect (inverse variance, unequal, small sample corrected) models for continuous data. We used the quality score as probability modifier to perform quality effect model analysis, with modifier multiplication as additional weighting scheme with inverse variance as weighting method.
Visual inspection of funnel plots and Egger's weighted regression statistics were used to determine the presence of publication bias. Symmetry or asymmetry of funnel plots was defined through visual examination. In addition, trim-and-fill sensitivity analysis was conducted. Statistical heterogeneity was assessed by using the I2, τ2, and Q statistics, for fixed, random, and quality models, respectively.
Investigation of sources of heterogeneity will increase both the scientific and the clinical relevance of the results of meta-analyses. Subgroup analysis and metaregression are usual methods to explore heterogeneity of effect. To explore the effects of modifier factors on the primary outcomes, metaregression was performed when at least ten studies were available, while subgrouping was used when data from ≥ three studies and ≥ five interventions were included. These factors were the type of intervention, the participants’ characteristics, the dose and the duration of intervention.
3 Results
3.1 Description of included studies
Studies characteristics and the results of the assessment of risk of bias of the included trials (quality assessment) are shown in Table 2. Average length is 7–730 days and the number of participants in individual trials ranged from 11 to 672. Six studies selected only females and four only males. The remaining trials included percentage of male participants from five to 85. Mean age ranged between 23 and 66 years and mean body mass index between 22 and 40 kg/m2. Participant withdrawal rate from the trial was between 0 and 37%.
| Reference | Subjects | Study design | Treatment | Days | Bioavailability | Bias | Score | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Asghari 2012 | Dyslipidemic 45 D12% BMI ≤ 35 Age 53 | Randomized, double-blind, parallel | Pomegranate seed oil capsule (800 mg) | 28 | 1. No antioxidant or food consumption in selection criteria 2. No wash out or run in 3. Capsule supplied by Vitane Pharma | 0.84 | TNF ↓ Baseline levels: TNF-α 12–15 pg/mL | |
| Bae 2009 | Rheumatoid arthritis 1M, 19W D37% BMI 22 Age 52 | Randomized, double-blind, cross-over | Quercetin (498 mg) + vitamin C 399 mg | 28 | 1. No compliance 2. Vitamin C dosage differs between treatment | 0.55 | TNF ↔ IL-6 ↔ Baseline levels: TNF-α 3–4 pg/mL IL-6 3–4 pg/mL | |
| Basu 2011a | Obese with metabolic syndrome 27M, 8W D17% BMI 36 Age 43 | Randomized, blind, parallel | 1. Green tea (928 mg) 2. Green tea extract (870 mg) | 56 | 1. Funding by WhiteWave Foods | 0.49 | IL-6 ↔ Baseline levels 19–26 μg/L | |
| Basu 2011b | Obese with metabolic syndrome 31W D11% BMI 40 Age 52 | Randomized, double-blind, parallel | Cranberry juice (458 mg) | 56 | 1. No wash out or run in 2. No dietary record | 0.6 | IL-6 ↔ Baseline levels 19–26 μg/L | |
| Beavers 2009 | Healthy 31W D6% BMI 26 Age 54 | Randomized, blind, parallel | Soy milk (98 mg) | 28 | 1. No wash out or run in | 0.86 | TNF ↔ IL-6 ↔ Baseline levels: TNF-α 2–4 pg/mL IL-6 2–3 pg/mL | |
| Bogdanski 2012 | Obese hypertensive 28M, 28W D0% BMI 33 Age 50 | Randomized, double-blind, parallel | Green tea extract (379 mg; 208 mg EGCG) | 93 | 1. No wash out or run in | 0.9 | TNF ↓ Baseline levels: TNF-α 5.2–5.5 ng/L | |
| Buscemi 2012 | High risk of CVD 10M, 9F D0% BMI 32 Age 48 | Randomized, single-blind, cross-over | Red orange juice (500 mL/209.5 mg) | 7 | 1. No wash out or run in | 0.55 | TNF ↓ IL-6 ↓ Baseline levels: TNF-α 100–200 pg/mL IL-6 30–37 pg/mL | |
| Chiva-Blanch 2012 | High risk of CVD 67M D8% BMI 29 Age 60 | Randomized, cross-over | Red wine (2933 mg) Dealcoholized red wine (2695 mg) | 28 | 1. A unique basal value for both groups | 0.6 | TNF ↔ IL-6 ↓ Baseline levels: TNF-α 3–7 pg/mL IL-6 2–3 pg/mL | |
| Chu 2011 | Metabolic syndrome 41M, 49W D1% BMI 29 Age 51 | Randomized, double-blind, parallel | Tea extract (1000 mg) | 93 | 1. No compliance 2. No wash out or run in 3. No dietary record 4. No antioxidant or food consumption in selection criteria | 0.39 | TNF ↓ IL-6 ↓ Baseline levels: TNF-α 19–25 pg/mL IL-6 25–35 pg/mL | |
| Deibert 2011 | Middle aged 35M D12% BMI 27 Age 57 | Randomized, parallel | Soy | 84 | 1. No wash out or run in 2. No dietary record 3. No antioxidant or food consumption in selection criteria 4. Funding by Almased Wellness Corp | 0.4 | IL-6 ↔ Baseline levels: 1–4 pg/mL | |
| Egert 2009 | Obese with metabolic syndrome 42M, 51W D2% BMI 30 Age 45 | Randomized, double-blind, cross-over | Quercetin 150 mg | 42 | Plasma quercetin 250 nM Flavonols 350 nM | No bias | 1.00 | TNF ↓ Baseline levels: 2–3 pg/mL |
| Egert 2010 | Overweight/obese Apo E 3 (24M, 36F) Apo E 4 (15M, 11F) D2% BMI 31 Age 46 | Randomized, double-blind, cross-over | Quercetin 150 mg | 42 | Plasma n = 60 Q 262.7 nM Kaempherol 30.6 Isorhamnetin 30.6 Total flavonols 347.8 | No bias | 1.00 | TNF-α ↓ Apo E 3 TNF-α ↓ Apo E 4 Baseline levels: 2–4 pg/mL |
| Ellis 2011 | Overweight/obese 10M, 14W D8% BMI 29 Age 60 | Randomized, blind, parallel | Strawberry beverage 94.7 mg | 42 | 1. No compliance 2. No dietary record | 0.74 | TNF ↔ IL-6 ↔ Baseline levels: TNF-α 1–2 pg/mL IL-6 1–2 pg/mL | |
| Estruch 2004 | Healthy 40M D5% BMI nr Age 38 | Randomized, blind, cross-over | Red wine 201mg (Control gin) | 28 | Plasma epicatechin 42 μg/L | No bias | 0.65 | TNF ↔ Baseline levels of 70–80 pg/mL |
| Heinz 2010 | Healthy 120W D0% BMI 26 Age 47 | Randomized, double-blind, parallel | 1 Quercetin (500 mg) 2 Quercetin (1000 mg) | 84 | Plasma quercetin 350 μg/L 500 μg/L | 1. No dietary record 2. No antioxidant or food consumption in selection criteria 3. Funding by Coca-Cola and Quercegen Pharma. | 0.85 | TNF ↔ IL-6 ↔ Baseline levels: TNF-α 1–2 pg/mL IL-6 1–3 pg/mL |
| Hermansen 2005 | Hypercholesterolemic 58 M, 42W D11% BMI 25 Age 59 | Randomized, double-blind, parallel | Soy (100 mg isoflavones) | 168 | 1. Funding by Nutripharma | 0.91 | TNF ↔ Baseline levels: TNF-α 4–5 pg/mL | |
| Jenkins 2002 | Hypercholesterolemic + menopausal 23M, 18W D2 0% BMI 25 Age 62 | Randomized, blind, cross-over | Soy 1 M (70 mg) 2 W (70 mg) 3 M (10 mg) 3 W (10 mg) | 28 | 1. No antioxidant or food consumption in selection criteria | 0.82 | TNF ↔ IL-6 ↑ W Baseline levels: TNF-α 1–2 pg/mL IL-6 0–2 pg/mL | |
| Knab 2011 | Healthy overweight 260M, 412W D3% BMI 27 Age 45 | Randomized, double-blind, parallel | 1 Quercetin (500 mg) 2 Quercetin (1000 mg) | 84 | Plasma quercetin 400 μg/L 600 μg/L | 1. Vitamin C dosage differs between treatment 2. No dietary record 3. No antioxidant or food consumption in selection criteria 4. Funding by Coca-Cola and Quercegen Pharma. | 0.55 | TNF ↔ IL-6 ↓ Q1000 Baseline levels: TNF-α 1–2 pg/mL IL-6 1–2 pg/mL |
| Lenn 2002 | Healthy 6M, 5W D27% BMI 24 Age 23 | Randomized, parallel | Soy isoflavones (120 mg) | 30 | 1. No compliance 2. No dietary record 3. No antioxidant or food consumption in selection criteria | 0.39 | TNF ↔ IL-6 ↔ Baseline levels: TNF-α 0–2 pg/mL IL-6 1–11 pg/mL | |
| Llanezaa 2011 | Obese postmenopausal women 70 W D19% BMI 35 Age 57 | Randomized, double-blind, parallel | Soy isoflavones extract (Fisiogen® 80 mg) | 180 | 1. No antioxidant or food consumption in selection criteria | 0.9 | TNF ↔ Baseline levels: 6–8 pg/mL | |
| Maskarinec 2009a | Healthy overweight 183W D8% BMI 26 Age 43 | Randomized, double-blind, parallel | Soy (100 mg) | 730 | Urinary isoflavone 59.8 nmol/mg creatinine | 1. Support through food donations from The Solae Company, | 0.99 | IL-6 ↔ Baseline levels: 1–2 pg/mL |
| Maskarinec 2009b | Healthy overweight 24M D17% BMI 28 Age 59 | Randomized, blinded, cross-over | Soy (70 mg) | 93 | 1. Support through food donations from Aloha Tofu Factory, DrSoy Nutrition, and The Solae Company | 0.89 | IL-6 ↔ Baseline levels: 0 pg/mL | |
| Miraghajani 2012 | Type II diabetic 25 (60%W) D13% BMI 28 Age 51 | Randomized, cross-over | Soy milk (240 mL) | 28 | 1. No antioxidant or food consumption in selection criteria | 0.85 | TNF ↔ IL-6 ↔ Baseline levels: TNF-α 1.7–1.9 pg/mL IL-6 1.3–1.6 pg/mL | |
| Mukamal 2009 | Diabetics 10M, 18W D10% BMI 29 Age 55 | Randomized, blind, parallel | Black tea (318 mg) | 180 | Urinary 4-O-methylgallic acid 13 μM | 1. No antioxidant or food consumption in selection criteria 2. Support through food donations from Templar Foods | 0.89 | TNF ↔ IL-6 ↔ Baseline levels: TNF-α 1–3 pg/mL IL-6 1–2 pg/mL |
| Naruszewicz 2007 | After myocardial infarction 33M, 11W D0% BMI 27 Age 66 | Randomized, double-blind, parallel | Chokeberry extract (255 mg) | 42 | 1. No compliance 2. No dietary record 3. No antioxidant or food consumption in selection criteria 4. Funding by Agropharm | 0.35 | IL-6 ↔ Baseline levels: IL-6 4–6 ng/mL | |
| Nieman 2009 | Healthy 32M, 7W D0% BMI nr Body composition 15% fat Age 26 | Randomized, double-blind, parallel | 1. Quercetin (1000 mg) 2. (Quercetin 1000 mg + EGCG 120 mg + isoquercetin 400 mg) | 14 | Plasma quercetin 550 μg/L 700 μg/L | 1. Vitamin C dosage differs between treatment 2. No dietary record 3. No antioxidant or food consumption in selection criteria 4. Funding by Quercegen Pharma | 0.56 | TNF ↔ Baseline levels: 3–5 pg/mL IL-6 (fig) ↔ |
| Rajaram 2010 | Healthy 17M, 14W D7% BMI<30 Age 41 | Randomized, blinded, crossover | 1. Low-almond diet 2. High-almond diet | 28 | 1. A unique basal value for both groups | 0.60 | IL-6 ↔ Baseline levels 1, 18 ng/L | |
| Ryan-Borchers 2006 | Healthy overweight postmenopausal 52W D9% BMI 27 Age 56 | Randomized, double-blind, parallel | 1. Soy (71.6 mg) 2. Isoflavone tablets (70 mg) | 112 | Soy: Plasma: daidzein ↔; genistein ↑ (0.15 μM); equol ↑ (0.1 μM); Urine: daidzein ↑ (13 μM); genistein ↑ (5 μM); equol ↑ (18 μM); Supplement: Plasma: daidzein ↔; genistein ↑ (0.35 μM); equol ↑ (0.03 μM); Urine: daidzein ↑ (24 μM); genistein ↑ (18 μM); equol ↑ (10 μM) | 1. No compliance | 0.64 | TNF-α ↔ Baseline levels 2–2.5 ng/mL |
| Rytter 2010 | Diabetes 18M, 22W D15% BMI 28 Age 62 | Randomized, double-blind, parallel | Fruit, berries, and vegetable supplement 1 (736 mg) 2 (1472 mg) | 56 | 1. Support through food donations from White Wave, Inc. (Boulder, CO) and Archer Daniels Midland Co. (Decatur, IL, USA). | 0.85 | IL-6 ↔ Baseline levels: 2–3 ng/L | |
| Tome-Carneiro 2012 | CVD 34M, 41W D0% BMI 31 Age 60 | Randomized, triple-blind, parallel | 1. Grape extract (66.8mg/6 months + 133.6 mg/6 months) 2. Grape extract + resveratrol (66.8 mg + 8 mg/6 months + 133.6 mg + 16 mg/6 months) | 365 | 1. No wash out or run in 2. Product provided by Actapharma | 0.89 | TNF ↔ IL-6 ↔ Baseline levels: TNF-α 1.7–1.9 pg/mL IL-6 1.3–1.6 pg/mL | |
| Urpi-Sarda 2012 | High risk of CVD 106 Registration number: NCT01449110 | PREDIMED study | 1. Virgin oil (1L/week) 2. Nuts (30 g/d) | 93 | No bias | 1 | IL-6 ↓ Baseline levels: IL-6 5–7 pg/mL | |
| Zemel 2010 | Overweight and obese 14M, 6W D2% BMI 28 and 32 Age 31 | Randomized blinded, crossover | 1. Soy (overweight) 2. Soy (obese) | 28 | 1. No compliance 2. No dietary record 3. No antioxidant or food consumption in inclusion criteria | 0.39 | TNF ↑ IL-6 ↑ obese Baseline levels: TNF-α 440–460 pg/mL IL-6 83–90 pg/mL |
- D: dropout (when not reported it has been calculated confronting the numbers in methods and in results).
The characteristics and comorbidity status of the participants varied. Eleven studies recruited only healthy participants. The remaining studies evaluated the effect of the intervention among patients with rheumatoid arthritis, diabetes, and metabolic syndrome or subjects with risk factors of CVD.
All the 32 studies were randomized placebo-controlled trials (10 blinded and 18 double blinded), 11 of which had a crossover design.
The more frequent limitation was incomplete food or antioxidant monitoring (19/32) and funding source or support by profit-making companies (12/32). In three studies, vitamin C differs between treatment and control, however this factor has been taken into account in score assignment (no proper placebo) (Table 1). All but five studies described flavonoid concentration of ingested food (soy, almond, nuts, virgin oil), beverage (teas, red wine, and fruit juice) or supplement (quercetin, pomegranate seed oil capsules, isoflavones supplements, tea, grape, and chokeberry extracts), with a flavonoids intake ranging from 10 to 2933 mg/d, but only nine assessed polyphenols bioavailability with both plasma and urinary levels in the range of micromolar concentrations. However, in these studies, bioavailability was unrelated to anti-inflammatory effect. In diabetic subjects, IL-6 and TNF-α were unaffected by a daily ingestion of three cup of black tea, despite urinary 4-O-methylgallic acid was excreted in urine 98. Estruch 65 et al. found unchanged levels of TNF-α after 4 weeks of red wine consumption but increased epicatechin gallate plasma concentrations.
The sources of flavonoids used in the selected studies are shown in Table 2 and quercetin is the only pure molecule used in these interventions. The inflammatory cytokines IL-6 30, 104 and TNF-α 30 were unaffected by quercetin supplementation, also when taken in combination with vitamin C 30 or epigallocatechin 3-gallate 104. To the contrary, quercetin alone decreased TNF-α in subjects with metabolic syndrome 58 and with apolipoprotein E (ApoE) genotype ApoE3 and ApoE4 57. All but one of these studies reported also data of bioavailability but the absorption of quercetin in the mainstream was not related to any reduction in circulating cytokines. However, Knab 83 reported no effects on TNF-α, but reduced levels of IL-6 concomitantly with increased concentrations of plasma quercetin.
The majority of chronic intervention studies using flavonoid-rich food or extract as source of bioactive molecules showed no changes in TNF-α and IL-6 levels. Besides, Zemel 133, in an intervention study providing 10 g of soy proteins, reported increased levels of TNF-α in both obese and overweight subjects and increased levels of IL-6 in obese only. Similarly, Jenkins 78 observed, after soy consumption, increased levels of IL-6 in hypercholesterolemic postmenopausal women. Contrarily, recent studies, conducted in subjects at high risk of CVD 28, 37, 44, 48, 122 or with metabolic syndrome 49, reported decreased levels of TNF-α 28, 37, 44 and IL-6 44, 48, 122 after consumption of red wine 48, tea extract 37, 49, pomegranate capsules 28, red orange juice 44, olive oil 122, and nuts 122.
3.2 Data synthesis
Since there were 13 studies with different treatment groups or with different sources of subjects, 34 datasets from 23 studies for TNF-α and 35 datasets from 23 studies for IL-6 were analyzed in this meta-analysis. The number of subjects was: for TNF-α 2163 of which 448 in crossover for a total of 2611: 1575 received flavonoids and 1036 placebo; for IL-6 2078 of which 275 in crossover for a total of 2353 (1467 treated and 886 controls).
No significant difference was detected with meta-analysis for the change of plasma concentration between flavonoid ingestion and control for both TNF-α and IL-6 in random (MD (95%CI): TNF-α –0.160 (−0.374, 0.055); IL-6 −0.064 (−0.225, 0.096)) and quality (MD (95%CI): TNF-α −0.108 (−45.155, 44.940); IL-6 0.015 (−184.585, 184.614)) effect models. On the other hand, decreased levels of TNF-α (MD (95%CI): −0.097 (−0.187, −0.008), p = 0.033) but not of IL-6 (MD (95% CI): 0.014 (−0.004, 0.033)) were found after flavonoid consumption in fixed effect model. From low to medium, statistical heterogeneity was found for TNF-α (I2 65%, τ2 0.15, Q 20%, Egger intercept −0.882, p = 0.461) and IL-6 (I2 60%, τ2 0.06, Q 12%, Egger intercept 0.869, p = 0.003), respectively. Funnel plots showed asymmetric distribution of results and Trim-and-fill analysis suggesting potential publication bias TNF-α (4 bias) and IL-6 (3 bias) (Fig. 2). After this sensitivity analysis, seven studies 32, 33, 44, 65, 100, 113, 133 have been excluded for higher baseline levels compared with other studies.
Thus, 28 datasets from 19 studies for TNF-α (n = 2404: 1464 treated and 940 controls) and 28 datasets from 18 studies for IL-6 (n = 2174: 1374 treated and 800 controls) were analyzed. Trim-and-fill analysis showed no publication bias for both TNF-α and IL-6 (not shown). Also this analysis revealed, in fixed effect model, decreased levels of TNF-α after flavonoid consumption but not for IL-6 (Table 3). No significant difference was detected with meta-analysis for the change of plasma concentration between flavonoid ingestion and control for both TNF-α and IL-6 in random and quality effect models (Table 3).
| Studies/interventions | Fixed effect | Random effect | Quality effect | |
|---|---|---|---|---|
| (subjects) | MD (95% CI) | MD (95% CI) | MD (95% CI) | |
| p (I2%) | p (τ2) | p (Q%) | ||
| TNF-α | 19/28 (2404) | −0.098 (−0.188 to –0.009) | −0.151 (−0.350 to 0.048) | −0.108 (−0.287 to 0.070) |
| 0.032 (64%) | 0.138 (0.12) | 0.191 (20%) | ||
| Quercetin | 6/10 (1494) | −0.032 (−0.196 to 0.133) | −0.032 (−0.196 to 0.133) | −0.052 (−0.353 to 0.249) |
| 0.707 (0%) | 0.707 (0) | 0.609 (32%) | ||
| Soy | 6/9 (391) | 0.087 (−0.051 to 0.224) | 0.087 (−0.051 to 0.224) | 0.087 (−0.157 to 0.332) |
| 0.216 (0%) | 0.216 (0) | 0.253 (15%) | ||
| Other sources | 7/9 (519) | −0.449 (−0.619 to −0.280) | −0.783 (−1.476 to –0.090) | −0.456 (−0.779 to −0.113) |
| <0.001 (82%) | 0.027 (0.51) | 0.109 (16%) | ||
| Disease | 7/8 (525) | −0.186 (−0.316 to −0.055) | −0.505 (−1.118 to 0.108) | −0. 184 (−0.472 to 0.104) |
| 0.005 (89%) | 0.107 (0.44) | 0.308 (13%) | ||
| Risk | 8/14 (1678) | 0.021 (−0.115 to 0.157) | 0.021 (−0.115 to 0.157) | 0.018 (−0.228 to 0.264) |
| 0.763 (0%) | 0.763 (0) | 0.828 (29%) | ||
| Healthy | 4/6 (201) | −0.209 (−0.497 to 0.080) | −0.209 (−0.497 to 0.080) | −0.209 (−0.664 to 0.245) |
| 0.157 (0%) | 0.157 (0) | 0.194 (16%) | ||
| IL-6 | 18/28 (2174) | 0.015 (−0.004 to 0.034) | −0.033 (−0.180 to 0.114) | 0.015 (−0.059 to 0.089) |
| 0.122 (57%) | 0.659 (0.04) | 0.869 (12%) | ||
| Quercetin | 3/5 (1097) | −0.064 (−0.236 to 0.107) | −0.064 (−0.236 to 0.107) | –0.056 (−0.560 to 0.448) |
| 0.463 (0%) | 0.463 (0%) | 0.629 (43%) | ||
| Soy | 7/10 (438) | 0.017 (−0.001 to 0.036) | 0.132 (−0.018 to 0.281) | 0.017 (−0.171 to 0.205) |
| 0.069 (48%) | 0.085 (0.02) | 0.091 (11%) | ||
| Other sources | 9/13 (639) | −0.346 (−0.612 to −0.079) | −0.556 (−1.062 to −0.050) | −0.383 (−0.775 to 0.009) |
| 0.011 (65%) | 0.031 (0.48) | 0.046 (26%) | ||
| Disease | 6/8 (323) | 0.122 (−0.014 to 0.257) | −0.135 (−0.567 to 0.297) | 0.129 (−0.070 to 0.327) |
| 0.078 (45%) | 0.541 (0.14) | 0.087 (15%) | ||
| Risk | 8/14 (1614) | 0.013 (−0.006 to 0.032) | −0.051 (−0.269 to 0.167) | 0.013 (−0.031 to 0.057) |
| 0.180 (68%) | 0.646 (0.07) | 0.592 (11%) | ||
| Healthy | 4/6 (237) | −0.060 (−0.385 to 0.265) | −0.058 (−0.470 to 0.355) | −0.060 (−1.306 to 1.185) |
| 0.719 (27%) | 0.784 (0.07) | 0.761 (30%) |
- MD, mean difference.
We evaluated the effect of dose and duration of supplementation of flavonoids with metaregression on TNF-α concentration, however neither a higher dose (range 10–2933 mg) nor a longer duration (range 14–365 days) of treatment were associated with a greater effect (Fig. 3).
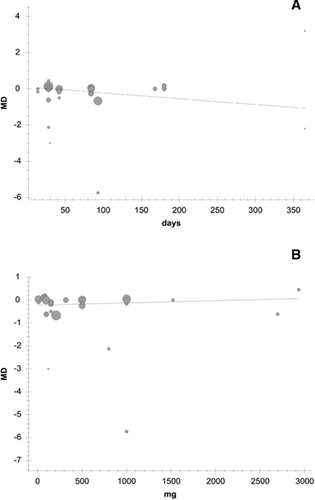
To differentiate between the effect of whole foods with respect to flavonoids, we did subgroups analyses. However, due to the low number of studies, we could subgroup only soy for foods and quercetin for supplements, from the other sources of flavonoids (foods, beverages, or extracts) as described in Table 3. Not statistically significant differences between treated and controls have been observed with quercetin or soy for both TNF-α and IL-6 (Table 3). On the contrary, other sources subgroup revealed a significant effect size on TNF-α in fixed and random effect models, and IL-6, in fixed, random and quality effect models (Table 3). When subjects were stratified on the basis of their “health status”, a significant effect on TNF-α was revealed in disease subjects as described in Table 3.
4 Discussion
Results of meta-analysis, including all the 32 selected studies, show a decrease of TNF-α, but not of IL-6 circulating levels, following flavonoid chronic consumption. With Trim-and-fill sensitivity analysis, we were able to remove publication bias excluding seven studies from the meta-analysis. The second analysis confirmed the effect only for TNF-α, but neither the administration of higher doses, nor longer duration of intervention were related to this effect. However, subsequent subgroup analysis showed that sources of flavonoids different from soy and quercetin reduce plasma levels of TNF-α and IL-6 in humans.
The major limitation of this meta-analysis is that it excludes studies that presented data as figures, medians, or geometric means 29, 36, 42, 47, 51, 53, 55, 72, 74, 80, 89, 90, 97, 110, 125. However, the exclusion of these studies did not affect the results considering that also these studies suggest that chronic ingestion of fruit 36, 52, 80, 90, wine 55, 89, 125, 90, 72 and tea 53, 72 reduces TNF-α 72, 80, 89 and IL-6 36, 80, 89, 125, while results on soy intervention are more contrasting 29, 47, 74 and none of these studies used quercetin as pure molecule.
To understand the impact of flavonoids on human health, it is essential to know the nature of the main compounds ingested and their bioavailability, which is known to be low in humans, because they are substrates of phase I (cytochrome P450), phase II (conjugation) and phase III (transporters) drug metabolism/transport systems 140. First, only about 28% of the selected studies reported data of bioavailability. Second, investigators rarely (only in 41% of cases) consider other dietary origin and the amounts consumed during the study. Third, potential limitations of included trials included short duration of follow-up and a small sample size. Again, all the included trials varied in terms of study population, source, and amount of flavonoids ingestion. In addition, the number of studies contributing substantial data to the meta-analysis were limited, thus we did not perform a subgroup analysis for different food sources other than soy.
The only pure molecule used in the selected studies was quercetin and almost all of these studies (5/6) reported data of bioavailability, however this flavonol did not affect both TNF-α and IL-6 levels. Also, treatment with soy, containing primarily isoflavones 16, unaffected both TNF-α and IL-6 levels.
On the other hand, results suggest that sources different from quercetin and soy, such as tea extract 37, 49 and red wine 48, both rich in flavanols 16, or fruit extract 28, containing a wide variety of flavonoids including flavanones 16, may decrease both TNF-α and IL-6 circulating levels. In the group “other sources”, only one of the studies included in this subgroup analysis was conducted with healthy subjects. Inflammation states may interfere with flavonoid metabolism, due to modulating effects of inflammatory cytokines on cytochrome P450 141 and transporter gene expression 142, thus healthy and disease-affected subjects may metabolize flavonoids differently. On the other hand, the interaction of flavonoid-rich foods or beverages with some drugs commonly used in clinical management of chronic patients is well known 143-150. Thus, in order to understand if there was an anti-inflammatory effect of flavonoids supplementation linked to the presence of diseases in the subjects, we performed subgroup meta-analysis in healthy or pathological subjects. Statistically significant effects were found for TNF-α in subjects affected by different diseases, but only in fixed effect model and with high heterogeneity, making difficult to draw any conclusions. The high variability in terms of different pathologies 30, 37, 49, 58, 96, 98, 119, treatments, duration, and dosage, make unfeasible a further stratification of the data to clarify if flavonoids might be more effective in certain disease state with respect to others.
We could not assess the effects of important clinical factors that might influence outcomes of flavonoid consumption. For example, we could not adequately investigate sex, age, and body mass index differences, due to the low number of subjects. We could not analyze also the effects of different study designs (crossover/parallel). For the parallel design studies, the small sample sizes could have led to ineffective randomization and potential confounding, while without complete information about bioaccumulation and pharmacokinetic of flavonoids, the correct wash out period for crossover design is difficult to establish. Last, due to the lack of a proper placebo with same aspect and same taste of the treatment, blinding of subjects in dietary interventions trials is also difficult to obtain.
In conclusion, results of our subgroup meta-analysis suggest an effect of grouped dietary sources of flavonoids (wine, pomegranate, and tea extracts), but not for soy and quercetin subgroups, on TNF-α and IL-6 plasma levels in humans. Due to the high heterogeneity of intervention, the low number of evidences and the lack of data on bioavailability, high-quality double-blind randomized trials with proper placebo are needed in order to identify flavonoids as the active ingredients involved in the anti-inflammatory effect of plant foods.
Acknowledgment
We thank Dr. Claudio Andrew Gobbi for English revision of the manuscript.
The authors have declared no conflict of interest.




