Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells—implications for diet-induced insulin resistance
Abstract
Scope
Inflammasome-mediated inflammation is a critical regulator of obesity-induced insulin resistance (IR). We hypothesized that saturated fatty acids (SFA) directly prime the NLRP3 inflammasome via TLR4 concurrent with IR. We focused on dendritic cells (DCs) (CD11c+CD11b+F4/80−), which are recruited into obese adipose tissue following high-fat diet (HFD) challenge and are a key cell in inflammasome biology.
Methods and results
C57BL/6 mice were fed HFD for 16 weeks (45% kcal palm oil), glucose homeostasis was monitored by glucose and insulin tolerance tests. Stromal vascular fraction (SVF) cells were isolated from adipose and analyzed for CD11c+CD11b+F480− DC. Following coculture with bone marrow derived DC (BMDC) insulin-stimulated 3H-glucose transport into adipocytes, IL-1β secretion and caspase-1 activation was monitored. BMDCs primed with LPS (100 ng/mL), linoleic acid (LA; 200 μM), or palmitic acid (PA; 200 μM) were used to monitor inflammasome activation. We demonstrated significant infiltration of DCs into adipose after HFD. HFD-derived DCs reduce adipocyte insulin sensitivity upon coculture co-incident with enhanced adipocyte caspase-1 activation/IL-1β secretion. HFD-derived DCs are skewed toward a pro-inflammatory phenotype with increased IL-1β secretion, IL-1R1, TLR4, and caspase-1 expression. Complementary in vitro experiments demonstrate that TLR4 is critical in propagating SFA-mediated inflammasome activation.
Conclusion
SFA represent metabolic triggers priming the inflammasome, promoting adipocyte inflammation/IR, suggesting direct effects of SFA on inflammasome activation via TLR4.
Abbreviations
-
- BMDC
-
- bone marrow derived DC
-
- BMM
-
- bone marrow-derived macrophages
-
- DCs
-
- dendritic cells
-
- EAT
-
- epididymal adipose tissue
-
- FMO
-
- fluorescence minus one
-
- HFD
-
- high-fat diet
-
- IR
-
- insulin resistance
-
- LA
-
- linoleic acid
-
- PA
-
- palmitic acid
-
- SFA
-
- saturated fatty acids
-
- SVF
-
- stromal vascular fraction
-
- TLR
-
- toll-like receptor
-
- T2DM
-
- type 2 diabetes mellitus
1 Introduction
Adipose tissue inflammation has been linked with a plethora of pro-inflammatory cytokines, which play a key role in the attenuation of insulin sensitivity 1. Several recent studies suggest a functional role for the NLRP3 inflammasome, a lipid responsive protein complex involved in processing IL-1β and IL-18, in obesity, with specific roles in adipogenesis and development of adipocyte insulin resistance (IR) 2, 3.
Impeding caspase-1 activation and IL-1RI signaling in adipose attenuates the impact of obesity-induced IR 3, 4. Inflammasome activation by islet amyloid polypeptide (IAPP) initiates pancreatic β-cell destruction 5, an event which promotes progression of IR to overt type 2 diabetes mellitus (T2DM).
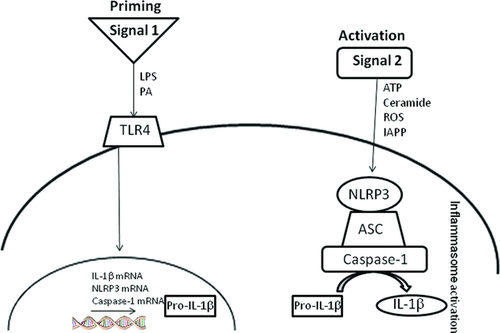
A key feature of inflammasome-mediated inflammation is the priming of pro-IL-1β to mature IL-1β. This process requires two signals—signal 1 induces pro-IL-1β production while signal 2 activates caspase-1, which cleaves pro- to active IL-1β. While the NLRP3 inflammasome is instrumental in the cleavage of IL-1β, the priming of pro-IL-1β is reliant on activation of surface receptors such as toll-like receptor (TLR)4 6. There is little doubt that enhanced production of IL-1β is key to driving obesity-induced IR (2, 3), however, the identity of signals 1 and 2 within adipose tissue remains elusive. Recently, Wen et al. 7 demonstrated the potential of saturated fatty acids (SFA) to promote inflammasome activation in vitro in macrophages. However, mechanisms responsible for this priming were not evaluated and concentrations of SFA used were unphysiologically high.
Saturated fats have long been suspected as the culprits that drive adipose tissue inflammation during obesity. Key studies showed lack of TLR4 markedly protected mice from high fat diet (HFD)-induced IR 8-10. Furthermore, saturated fats, but not unsaturated fats, have been shown to increase cytokine mRNA expression in vitro 11. However, the major flaw in these findings was the extremely high, and arguably physiologically irrelevant, doses of SFA that were required to drive a minimal inflammatory response in vitro. The emergence of an adverse role for the NLRP3 inflammasome in obesity and the potential of saturates to promote pro-IL-1β production have shed more light into the mechanisms by which SFA induce inflammation. It is likely that previous in vitro studies were merely missing the second signal, which is inherently present within obese adipose tissue in vivo, to process pro- to active IL-1β and thus the effects of SFA on inflammation were underappreciated in vitro. We hypothesized that exposure to dietary SFA represents the key metabolic stressor relevant to both priming and processing of IL-1β and that this priming is dependent on TLR4. This study focused on TLR4/inflammasome signaling, given their potential deleterious role in obesity and IR.
HFD-induced obesity and IR are associated with a pro-inflammatory state in adipocytes, which is heightened by immune cell infiltration into adipose tissue 12-14. Adipose tissue macrophage infiltration, preceded by CD8+ effector T-cells, in obesity has important functional effects with respect to IR 15-17. We chose to focus on dendritic cells (DCs), potent antigen presenting cells that direct the adaptive immune response 18. Several studies have conclusively demonstrated the presence of DC in adipose tissue depots 19-21. Furthermore omental DC have the capability to present antigen to T cells 22, 23. However, the maturation status of DC in adipose tissue during obesity and the functional consequences of DC and DC-derived cytokines on adipocyte biology remain relatively unexplored.
This study addressed the hypothesis that SFA directly prime TLR4 and inflammasome activation in adipocytes and immune cells, with adverse effects on adipocyte insulin sensitivity. We demonstrate that CD11c+CD11b+F4/80− DC, with enhanced maturation and activation markers, were increased in insulin resistant HFD-fed animals. Bone marrow derived DC (BMDC) derived from insulin resistant HFD-fed animals have heightened IL-1β and IL-12p70 responses which coincide with significantly enhanced IL-1R1, TLR4, and caspase-1 expression. Complementary in vitro studies demonstrated that the SFA palmitic acid (PA) primes BMDC with pro-IL-1β, which is then processed by caspase-1 and released as mature IL-1β upon stimulation, but the polyunsaturated fatty acid (PUFA) linoleic acid (LA) did not. We also demonstrate that TLR4 is critical in propagating SFA-mediated inflammasome activation. These studies provide evidence that SFA-enriched diets prime the NLRP3 inflammasome via TLR4 in BMDC skewing toward a pro-inflammatory, insulin resistant phenotype in HFD-induced obesity.
2 Materials and methods
2.1 Materials
LPS from Escherichia coli (serotype 127:B8) was obtained from Alexis Chemicals (Cayman Chemicals, Cambridge, UK). Insulin ELISA was purchased from Mercodia (Uppsala, Sweden); triglyceride and free fatty acid kits were purchased from Randox (Antrim, NI). 3T3L1 cells were obtained from American Type Culture Collection (ATCC, Manassas, CA). Deoxy-D-glucose 2-[1,2-3H(N)]- was purchased from Perkin-Elmer Analytical Sciences (Boston, MA). BSA (Fraction V, low heavy metals) was purchased from Calbiochem (EMD, Darmstadt, Germany). Primers, probes, and TaqMan Universal Mastermix were purchased from Applied Biosystems (ABI, Carlsbad, CA). High-fat diet (HFD, 45% kcal from palm oil) and nutrient-matched standard chow diet (10% kcal from palm oil) was purchased from Research Diets (New Brunswick, NJ). All other reagents were purchased from Sigma-Aldrich (Dorset, UK) unless otherwise stated.
2.2 Animal experiment and nutritional intervention
All experiments were performed in accordance with current EU and Irish Department of Health guidelines on the use of experimental animals. Male C57BL/6 and C3H/HeJ mice were purchased from Charles River, UK. Mice were placed on a HFD (45% palm oil) or chow diet (10% palm oil) starting at 6 weeks of age. Food and water were offered ad libitum. At termination of study, mice were anesthetized using isofluorane and plasma collected by cardiac puncture followed by cervical dislocation.
2.3 Intraperitoneal glucose tolerance test (GTT)
Mice were fasted for 6 h prior to commencement of metabolic tests. Mice were injected intraperitoneally with 25% (w/v) glucose (1.5g/kg; B. Braun Medical Ltd., Dublin, Ireland). Glucose levels were monitored at base line and at indicated time points postmetabolic challenge via tail-vein blood sampling using a glucometer from Accu-Chek (Roche Ltd., Dublin, Ireland).
2.4 Isolation of stromal vascular fraction (SVF)
Epididymal adipose tissue (EAT) depots were removed following chow or HFD diet. Adipose tissue was collagenase (2mg/mL) digested for 60 min at 37°C and cell suspensions were filtered and washed prior to centrifugation and resuspended in PBS/2%BSA. Cells were stained with fluorescently labeled antibodies, F4/80-FITC, CD11B-AF647/PE, and CD11C-RPE (AbD Serotec). Unstained, single stains, and fluorescence minus one (FMOs) were used for setting compensations and gates. Flow cytometry was performed on a Dako CyAn ADP platform (Beckman-Coulter Ltd., UK) and analyzed using Summit v4.3 software.
2.5 Isolation and culture of BMDC/BMM
BMDC were prepared by culturing bone marrow cells obtained from the femurs and tibia of mice in RPMI 1640 medium in the presence of granulocyte macrophage-colony stimulating factor (GM-CSF) (50 ng/mL). Cells were isolated from animals fed HFD or chow diet to compare effects of diet on DC activation state. Cells were cultured in GM-CSF alone, no exogenous fatty acids were added during culture. Following 7 days culture, cells were stimulated for 24 h with LPS (100ng/mL). Bone-marrow monocytes were isolated from mouse femurs and tibias of chow- and HFD-fed mice and were cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 30% L929 conditioned medium for 7 days to differentiate to a mature macrophage (BMM) phenotype.
In a separate study to determine the chronic effect of PA on BMDC, PA was dissolved in sterile DMSO. Cells were isolated from chow-fed animals and were incubated with PA (0–100 μM) or DMSO from day 1 of culture. DCs were seeded at a density of 200 000 cells/mL in 12-well plates and were stimulated ± LPS (100 ng/mL) for 3 h.
Acute experiments involved stimulating chow BMDC for 3 h with LPS (100 ng/mL), PA (200 μM), or LA (200 μM) for 3 h to prime pro-IL-1β, ATP (5 mM; 1 h) was used to stimulate inflammasome-mediated IL-1β secretion. Supernatants were stored for cytokine analysis by ELISA and cells were harvested for RNA analysis or flow cytometry analysis as indicated.
2.6 Culture of adipocytes
3T3L1 cells were differentiated to adipocytes as described previously 24. Briefly, confluent cells were incubated in differentiation media (DMEM, 10% FBS, 1% penicillin/streptomycin, insulin [1.7 μM], dexamethasone [1 μM], isobutylmethylxanthine [IBMX; 500 μM]) for 72 h. Adipogenic media was then replaced with complete media (DMEM, 10% FBS and 1% penicillin/streptomycin) and cells differentiated for 7 days.
2.7 Glucose uptake assays
BMDC were isolated and seeded on Corning transwell filters (0.4μm, Sigma-Aldrich Ltd.) for 7 days, Transwells were transferred to differentiated 3T3L1 adipocytes (day 7 post differentiation) and were left to coculture for a further 48 h; mock control cells were incubated in media alone. On the day of glucose-uptake assay, cells were serum starved for 4 h prior to a glucose-free step-down in PBS + 0.2% BSA for 30 min. Cells were then stimulated ± insulin (100 nM) for 15 min prior to addition of 3H-deoxyglucose (5 μCi per mL cold glucose (1 mM)) for an additional 15 min. Cells were washed in PBS prior to lysing in RIPA buffer and 3H-glucose uptake measured by liquid scintillation counting. Fold increase in glucose uptake over basal (non-insulin stimulated) is presented.
2.8 Cytokine analysis
Supernatants from the DC activation experiments were analyzed for IL-12p70, IL-10, TGFβ, IL-18, IL-1β, and IFNγ by commercial DuoSet or Quantikine ELISA kits (R&D Systems, Abdingdon, UK) according to the manufacturer's instructions.
2.9 Analysis of BMDC mRNA
Cells were harvested in TRI reagent. Total RNA was extracted from the cells according to the manufacturer's instructions. Single stranded cDNA was prepared using the High Capacity cDNA archive kit (Applied Biosystems, Warrington, UK). mRNA expression was quantified by real-time PCR on an ABI 7700 Sequence Detection System (Perkin-Elmer Applied Biosystems, Warrington, UK). TaqMan real-time PCR was performed for IL-1R1, Caspase-1, TLR4, GLUT4 using Pre-Developed Assay Reagent Kits.
2.10 Flow cytometry
The expression of cell surface markers on DC was assessed using an anti-mouse CD14 (FITC anti-mouse, eBioscience; Hatfield, UK), TLR4-MD2 (PE anti-mouse, eBioscience), CD40 (FITC anti-mouse, eBioscience), CD80 (PE anti-rat, eBioscience), MHCII (FITC anti-rat, eBioscience), CD86 (PE anti-rat). Unstained, single stains and FMOs were used for setting compensations and gates. After incubation for 30 min at 4°C, immunofluorescence analysis was performed on a Dako CyAn ADP platform (Beckman-Coulter Ltd., UK) and analyzed using Summit v4.3 software.
2.11 Caspase-1 assay
BMDCs were primed for 3 h with either LPS (100 ng/mL), PA (200 μM), or LA (200 μM) then stimulated for 1 h with ATP (5mM) before the addition of FAM-YVAD-fmk (5-carboxyfluorescein–Tyr-Val-Ala-Asp–fluoromethylketone) according to the manufacturer's instructions (Immunochemistry Technologies). Caspase-1 activation was determined by degree of fluorescence.
2.12 Analysis of secreted IL-1β in BMDC by Western blotting
BMDCs were primed for 3 h with either LPS (100 ng/mL) or PA (200μM) and cells were lysed in RIPA buffer. Protein was separated by electrophoresis on a 4–20% SDS-PAGE gel. Samples were transferred to nitrocellulose and blocked in 10% BSA prior to detection with anti-IL-1β (MAB4011; 39 kDa; R&D Systems).
2.13 Statistics
Two-way ANOVA was used to determine significant differences between dietary conditions. Data are presented as means ± SEM. When this indicated significance (p < 0.05), post hoc Bonferroni test analysis was used to determine which conditions were significantly different from each other. There was no significant difference between cells alone and DMSO (vehicle control) treated cells, therefore DMSO was used as the reference treatment. All statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA).
3 Results
3.1 HFD promotes recruitment of DC into adipose tissue in a time-dependent manner
Immune cell recruitment into adipose tissue was monitored over a timecourse of high-fat feeding and for the first time we demonstrate significant infiltration of DCs (F4/80−/CD11b+/CD11C+) into the expanding adipose mass (Fig. 1A) coincident with the development of systemic IR (Fig. 1B and Table 1).
| Chow | HFD | |
|---|---|---|
| Body weight (grams) | 22.72 ± 0.395 | 43.01 ± 1.57*** |
| EAT weight (grams) | 0.28 ± 0.02 | 2.1 ± 0.19** |
| Plasma NEFA (mmol/L) | 1.44 ± 0.19 | 1.79 ± 0.4 |
| Plasma triglycerides (mmol/L) | 0.75 ± 0.07 | 1.84 ± 0.41** |
| Plasma insulin (ng/mL) | 0.62 ± 0.07 | 1.66 ± 0.19*** |
| Plasma glucose (mmol/L) | 8.03 ± 0.28 | 7.9 ± 0.42 |
| HOMA-IR | 1.3 ± 0.4 | 3.3 ± 0.43* |
| Plasma resistin (pg/mL) | 1700.83 ± 135.11 | 2754.17 ± 333.04* |
| Plasma leptin (pg/mL) | 1631.67 ± 240.08 | 13009.06 ± 6504.53* |
- Plasma was isolated from chow and HFD mice by cardiac puncture and levels of metabolic markers were analyzed enzymatically.
- *p < 0.05, **p < 0.01, and ***p < 0.001 with respect to chow-fed animals n ≥ 10.
- HOMA-IR (Homeostatis Model Assessment-IR [(glucose × insulin)/22.5]).
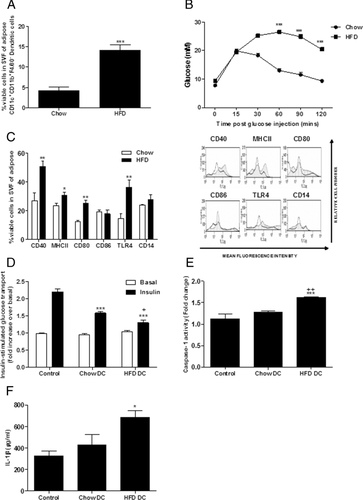
An array of cell surface maturation markers representative of antigen presenting cells, such as DCs were further analyzed in the SVF of lean and obese adipose tissue. CD40, MHCII, CD80, and TLR4 surface expression on SVF cells was significantly increased after HFD compared to age-matched chow-fed controls indicative of heightened activation state of infiltrated immune cells (Fig. 1C).
3.2 Coculture of HFD-derived DCs with 3T3L1 adipocytes results in greater loss of insulin sensitivity compared to chow-derived DCs
Given the marked effects of macrophages and T cells on adipocyte functionality 16, 25, we speculated that DCs could also cross talk with resident adipocytes within adipose and contribute to the development of IR. To this end we set up a transwell coculture system of high-fat and chow-derived DCs with 3T3L1 adipocytes and monitored insulin sensitivity after 48 h in culture. Both chow and HFD-derived DC significantly attenuated adipocyte insulin-stimulated glucose uptake in 3T3-L1 adipocytes, however, this effect was more potent in adipocytes cocultured with HFD-derived DC (Fig. 1D). Adipocyte caspase-1 activation and IL-1β secretion was significantly enhanced when cocultured with HFD DC from obese mice (Fig. 1E and F).
3.3 HFD primes inflammasome-mediated IL-1β signaling altering the cytokine profile of DC
Given the enhanced immunogenic phenotype of SVF from HFD-fed mice, we speculated that high-fat feeding may be priming cells directly in the bone marrow prior to recruitment into adipose. To address this hypothesis, we isolated BMDC from mice after chow or HFD and monitored their cytokine secretion profiles ex vivo after stimulation with LPS. HFD skewed BMDC to a pro-inflammatory phenotype with greater IL-1β secretion in response to an LPS stimulus compared to age-matched chow-derived BMDC (Fig. 2A). The classical DC pro- and anti-inflammatory cytokines IL-12 and IL-10 were also induced in response to HFD. TNFα concentrations were also examined and increases were observed in response to HFD (data not shown).
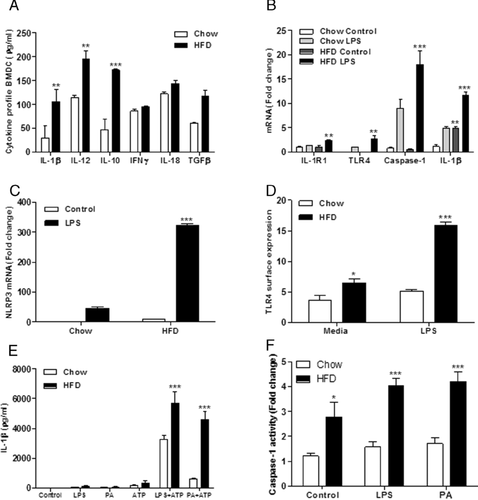
IL-1RI, caspase-1, TLR4, and NLRP3 mRNA expression was markedly increased in HFD-derived BMDC compared to chow fed controls (Fig. 2B and C). Furthermore, cell surface TLR4, which is indicative of functionality, was significantly increased in HFD-derived BMDC (Fig. 2D). Ex vivo HFD-derived BMDC were more reactive to LPS and PA challenges, with increased IL-1β secretion and caspase-1 activation following stimulation with ATP, a potent inflammasome activator (Fig. 2E and F). Maturation markers of BMDC, CD40, CD80, and CD86 were markedly enhanced post-LPS stimulation in ex vivo BMDC derived from HFD-fed versus chow-fed mice (Supporting Information Fig. 1A).
3.4 SFA exposure specifically primes the BMDC cytokine profile
PA was the predominant SFA in the HFD and is typical of westernized diets. We sought to determine the direct effect of PA on BMDC in vitro to ascertain whether the observed ex vivo effects were attributable to the SFA rather than components of the obese phenotype. BMDC chronically treated with PA (0–100 μM) showed a dose-dependent increase in LPS-induced IL-1β and IL-12p70 secretion (Fig. 3A and B). Furthermore, in vitro PA exposure significantly increased BMDC CD40, CD80, and MHCII expression post-LPS (Supporting Information Fig. 1B). BMDC CD80 and MHCII expressions were significantly increased by PA pretreatment alone prior to LPS stimulation (Supporting Information Fig. 1B).
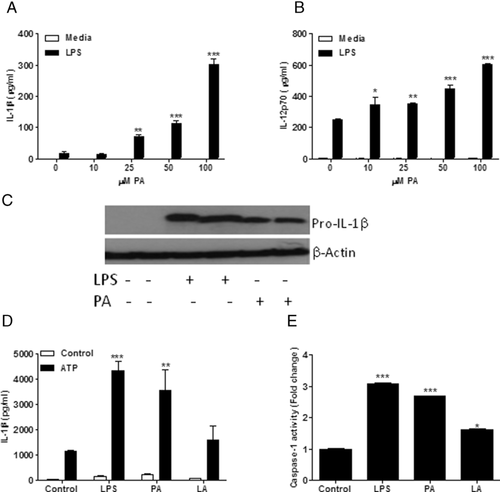
Given the enhanced IL-1β secretion upon HFD feeding and in vitro PA challenge, we aimed to determine the role of acute PA treatment in priming pro-IL-1β production. BMDC were primed with an acute 3 h LPS (100 ng/mL) or PA (200 μM) challenge to induce pro-IL-1β production. It is clear given the significant increase in pro-IL-1β production in the presence of both LPS and PA (Fig. 3C) that PA acts as a priming agent of the inflammasome. In contrast the PUFA LA failed to induce IL-1β secretion or activate caspase-1 activation compared to PA or LPS (Fig. 3D and E).
Bone marrow macrophages from chow- and HFD-fed animals were also isolated and stimulated with LPS (100 ng/mL) and PA (200 μM). It is interesting to note that while both PA and LPS induce IL-1β secretion, the concentration produced by these cells are significantly lower than that of BMDC (Supporting Information Fig. 2A).
3.5 Inhibition of IL-1β and TLR4 signaling alters the cytokine profile of BMDC
Given the potential role of TLR4 in relaying lipid-mediated pro-inflammatory signals we determined the responsiveness of TLR4 deficient BMDC to the SFA/PA challenge. TLR4 deficient and WT BMDC were stimulated with ATP (5 mM) following 3-h treatment with LPS and PA. TLR4 deficient BMDC remained unresponsive to both LPS and PA and failed to efficiently secrete IL-1β (Fig. 4A). We also showed that the lipid challenge failed to prime IL-1β with lack of pro-IL-1β expression in TLR4−/− BMDC compared to WT (Fig. 4B). IL-12p70 is consistently induced in BMDC treated with LPS and PA in line with enhanced IL-1β concentrations. Indeed there was a significant reduction of IL-12p70 concentrations in BMDC derived from TLR4-deficient mice following treatment with LPS and PA (Fig. 4C). Furthermore, treatment of IL-1R1−/− BMDC with LPS and PA yielded significantly reduced quantities of IL-12p70 (Fig. 4C) indicating an intrinsic link between IL-12p70 and IL-1β signaling pathways.
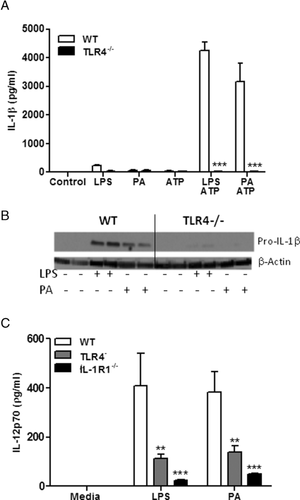
4 Discussion
IL-1β represents a critical pathological mediator of obesity-induced IR 26. Indeed the NLRP3 inflammasome, responsible for IL-1 and IL-18 processing and activation, has recently come to the forefront as a sensor of metabolic stress which triggers an inflammatory cascade contributing to the insulin resistant phenotype 4. Unlike other inflammasome complexes, NLRP3 is unique at recognizing DAMPs, which are directly involved in cellular metabolism 27. Furthermore, NLRP3 expression is NF-κB dependent and therefore has the potential to be regulated by dietary components 28. While a wide range of metabolic compounds (glucose, IAPP, and ceramide) have been assessed in relation to inflammasome-mediated IR and diabetes 27, the potential direct effects SFA have only recently come to light. Wen et al. [7] highlighted the role of the SFA PA in priming the inflammasome with pro-IL-1β. We add to this new knowledge by further demonstrating the pivotal role of TLR4 in terms of PA-induced inflammasome priming. It is important to note that stimulation of DCs with either PA or LPS alone in the absence of a “second signal” results in negligible amounts of secreted IL-1β, but induces both IL-1 mRNA and pro-IL-1β protein levels. In this study, we used the classical inflammasome activator ATP as our second signal to activate caspase-1 and cleave pro- to active IL-1β, but the identity of this second signal in obesity is still relatively unexplored. However, it is interesting to note that experiments carried out by Vandanmagsar et al. demonstrate that ceramide may represent a physiological activator of the NLRP3 inflammasome complex rather than their free fatty acid counterparts 4. Indeed we also demonstrated that a commercially available ceramide analogue exhibited a moderate, albeit not equivalent to ATP, inflammasome activation response (data not shown).
To further ascertain whether the effects observed for PA were specific for saturated fat, we monitored the effects of the n-6 PUFA linoleic acid on IL-1β priming and demonstrated that n-6 PUFA do not possess the same pro-inflammatory, IL-1β inducing properties as PA, as observed previously in a THP-1 monocytes 29.
Infiltration of innate and adaptive immune cells into obese adipose tissue is a major contributor to the pathogenesis of IR and T2DM. The role of macrophages and T cells has been well characterized over the last decade 15, 16. This study focused on the role of DC in adipose tissue biology and IR. DCs are classically thought of as antigen presenting cells. Indeed, immature DC are highly active and have the ability to acquire and process antigen, migrate to lymph nodes, and produce a milieu of cytokines involved in the differentiation of naïve T cells. It is possible that DC present in adipose tissue during the initial stages of adipose tissue inflammation may influence the early contribution of T cells in adipose tissue biology. Evidence from humans indicates that DC present to T cells in the omentum, further strengthening this hypothesis 22. While antigen presentation is a key feature of DC function, it is also important to acknowledge that during DC differentiation and maturation a series of cytokines are produced and secreted which have the ability to influence adipose tissue biology and indeed IR even after the initial onset of obesity. This study presents data demonstrating significant infiltration of DCs into adipose over a timecourse of high-fat feeding. Furthermore, expression of DC maturation markers including CD40, CD80, MHCII, and TLR4 were increased in HFD-derived adipose SVF cells, which may be indicative of enhanced migratory potential of DCs after HFD. DCs are known to be present in adipose tissue 20. A recent study has demonstrated that ablation of CD11c restores glucose homeostasis and reduces adipose tissue inflammation following HFD 30. The main aim of this study was to ablate M1 macrophages that express CD11C, but such an ablation system will also deplete adipose tissue DCs. Indeed in this study a significant proportion of the CD11C cells were not coexpressing the macrophage marker F4/80 and thus it is difficult to decipher whether the beneficial effects on glucose homeostasis were due to loss of M1 macrophages or depletion of adipose tissue DCs and infers an adverse pathogenic role for DCs during obesity. To further address the hypothesis that DCs may have an adverse role to play in adipose tissue biology, we created a coculture system of bone marrow DCs with 3T3L1 adipocytes to monitor crosstalk between the two cell types. Interestingly coculture of high-fat derived DCs resulted in greater induction of adipocyte IR compared to chow-derived DCs. Furthermore, high-fat DCs induced greater activation of caspase-1 within adipocytes with increased secretion of IL-1β within the coculture system. This presents a case whereby fatty acid-primed DCs interplay with resident adipocytes in obese adipose tissue resulting in inflammatory pathway activation and progressive loss of insulin sensitivity. However, whole body and specific depletion of DCs in vivo would be required to fully appreciate the adverse role for DCs in the development of IR.
The abundance of DC cell surface TLR4 and constant exposure to metabolic stressors create the perfect environment for both the priming and activation of the NLRP3 inflammasome and secretion of mature IL-1β. In agreement with this we have demonstrated in vitro that both bone marrow DCs and bone marrow-derived macrophages (BMM) are sensitive to priming by LPS and PA and secrete much higher concentrations of IL-1β in response to ATP stimulus. Indeed Masters et al. [5] demonstrate that DCs exposed to IAPP facilitate IL-1β-mediated destruction of pancreatic β cells, an event which may define the progression of IR to overt type 2 diabetes. As DC present a novel potential accessory in the pathology of adipose tissue inflammation, BMDC was employed for functional characterization and complementary in vitro experiments.
One of the most pronounced and arguably most important observations in our study was the heightened responsiveness of bone marrow DC derived from HFD mice to an LPS stimulus compared to chow-derived cells. This remarkable priming, sustained even after 7 days in culture, was coincident with increased cell surface expression of TLR4. HFD-derived DCs in turn exhibited enhanced IL-1RI, caspase-1, NLRP3, and TLR4 mRNA expression in response to LPS coincident with increased secretion of pro-inflammatory cytokines (IL-1β, IL-12). Furthermore, HFD-derived DCs secreted much greater levels of IL-1β after priming with PA compared to chow-fed DCs. This study demonstrated that exposure to HFD in vivo augmented the inflammatory potential of bone marrow DCs ex vivo. Diets enriched with saturated fats therefore appear to directly prime the cells within the bone marrow. Indeed the majority of immune cells recruited into adipose are of bone-marrow origin 15 and findings from this study suggest that these cells may already be primed after exposure to saturated-fat diets prior to movement into adipose where a second signal subsequently stimulates IL-1β release.
A major question in the area of HFD-induced obesity is whether dietary fatty acids or the underlying mechanical stress associated with obesity leads to the inflammation, which contributes to IR and diabetes. This study clearly demonstrated the role of saturated fatty acids as a metabolic stressor, which can directly prime inflammation in both adipocytes and innate immune cells independent of the obese phenotype. Indeed in vitro studies using PA show that enhanced BMDC reactivity in vivo was triggered by exposure to dietary SFA rather than the obese phenotype. Nguyen et al. [31] demonstrated that a mixture of free fatty acids increased BMDC pro-inflammatory cytokine and TLR4 expression. Importantly, our study clarified that chronic exposure of BMDC to a range of PA concentrations in vitro primed NLRP3 inflammasome-mediated IL-1β secretion augmenting the inflammatory response of LPS thus mimicking the ex vivo response observed in the PA-enriched diet that induced IR. Furthermore, we have established that these effects appear to be specific to SFA. We demonstrate that the PUFA LA does not induce IL-1β secretion in BMDC even following ATP activation. A recent study by Csak et al. 31 demonstrated a significant enhancement of IL-1β mRNA in response to PA, however, LA failed to increase IL-1β mRNA expression in this study indicating that LA does not act as a priming agent of the NLRP3 inflammasome.
TLR4 is a pattern recognition receptor present on the surface of immune cells including DC and is activated by LPS and SFA 32.C3H/Hej TLR4 deficient mice are partly protected from HFD-induced IR 10. In the present study, BMDC derived from HFD-fed mice have heightened TLR4 mRNA. More importantly surface expression of TLR4, indicating functionality, is heightened in both HFD-derived adipose tissue SVF and in BMDC, particularly post-LPS stimulation. In contrast C3H/HeJ TLR4 deficient BMDC did not respond to acute exposure to PA with a failure to prime and produce significant quantities of IL-1β even in the presence of ATP, a potent inflammasome activator. In addition to this, we have also demonstrated that BMDC derived from both C3H/HeJ TLR4 deficient mice and indeed IL-1R1−/− mice have a reduced ability to induce production of IL-12, a key BMDC cytokine, which we have shown to exacerbate IR in 3T3-L1 adipocytes (data not shown). This indicates that HFD-derived DC are primed to be more responsive to external stimuli such as LPS and metabolic stressors including SFA resulting in enhanced production of pro-IL-1β and other pro-inflammatory cytokines relevant to IR. This implicates PA-mediated activation of TLR4 and resulting cytokine secretion in the progression to IR in adipocytes and indeed adipose tissue. These findings are of key importance and demonstrate the potential of SFA as the link between diet-induced obesity and inflammasome-related IR.
In conclusion, this study establishes that ex vivo and in vitro exposure of BMDC to the SFA PA leads to TLR4-mediated priming of the NLRP3 inflammasome. A second signal in the form of ATP resulted in enhanced IL-1β secretion from high-fat derived DCs compared to chow-derived cells. This enhanced responsiveness to inflammatory stimuli was attributable to enhanced signaling via TLR4 and inflammasome priming. Also, HFD-derived BMDC block insulin-mediated glucose uptake in adipocytes. Complementary in vitro work shows that acute exposure to PA, enhanced activation, and functional potential of DC acted as a primer of the NLRP3 inflammasome. We thus speculate that SFA-mediated inflammasome priming plays a key role in the instigation of adipose tissue inflammation and IR.
ACKNOWLEDGMENTS
This work was supported by Science Foundation Ireland PI Programme. C.R. researched data and wrote manuscript. F.M. researched data and proofread manuscript. K.H. researched data and proofread manuscript. O.F. researched data and proofread manuscript. K.M. reviewed/edited manuscript. H.R. designed study, reviewed data and edited manuscript.
Potential conflict of interest statement: Kingston Mills is a cofounder and shareholder in Opsona Therapeutics Ltd., a startup company involved in the development of anti-inflammatory therapeutics. All other authors have no financial conflict of interest.




