Lycopene inhibits angiogenesis both in vitro and in vivo by inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways
Abstract
Scope
Limited in vitro data show that lycopene may be anti-angiogenic but with unclear mechanisms. Here, we employed ex vivo and in vivo assays to substantiate the anti-angiogenic action of lycopene and determined its molecular mechanisms in human umbilical vein endothelial cells (HUVECs).
Methods and results
The anti-angiogenic activity of lycopene was confirmed by ex vivo rat aortic ring and in vivo chorioallantoic membrane assays. Furthermore, the in vivo matrigel plug assay in mice demonstrated that lycopene implanted s.c. at the highest dose used (400 μg/plug) completely inhibited the formation of vascular endothelial cells induced by vascular endothelial growth factor (VEGF). As expected, lycopene inhibited tube formation, invasion, and migration in HUVECs, and such actions were accompanied by reduced activities of matrix metalloproteinase-2, urokinase-type plasminogen activator, and protein expression of Rac1, and by enhancing protein expression of tissue inhibitors of metalloproteinase-2 and plasminogen activator inhibitor-1. Moreover, lycopene attenuated VEGF receptor-2 (VEGFR2)-mediated phosphorylation of extracellular signal-regulated kinase (ERK), p38, and Akt as well as protein expression of PI3K.
Conclusion
Our data demonstrate the anti-angiogenic effect of lycopene both in vitro and in vivo. The anti-angiogenic activity of lycopene may involve inhibition of MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways.
Abbreviations
-
- BHT
-
- butylated hydroxytoluene
-
- CAM
-
- chorio-allantoic membrane
-
- ECGS
-
- endothelial cell growth supplement
-
- ERK1/2
-
- extracellular signal-regulated kinase 1/2
-
- FBS
-
- fetal bovine serum
-
- HUVECs
-
- human umbilical vein endothelial cells
-
- MMP-2
-
- matrix metalloproteinase-2
-
- PAI-1
-
- plasminogen activator inhibitor-1
-
- THF
-
- tetrahydrofuran
-
- TIMP-2
-
- tissue inhibitors of metalloproteinase-2
-
- uPA
-
- urokinase-type plasminogen activator
-
- VEGFR
-
- vascular endothelial growth factor receptor
1 Introduction
Angiogenesis, the process of vascular growth by sprouting of preexisting vessels [1], is a critical process in the adult organism such as pregnancy, the female reproductive cycle, woundhealing, and revascularization of ischemic tissues [2]. In addition, angiogenesis supports the increasing demands for metabolic supplies such as nutrients, various growth factors, and molecular oxygen at sites of tissue repair or regeneration [2]. In contrast, abnormal angiogenesis is involved in many pathological processes, including tumor growth, metastasis, diabetic retinopathy, and arthritis [3]. Angiogenesis is also an important event in the development of solid tumors because tumors cannot grow beyond 2–3 mm3 owing to a lack of oxygen and other essential nutrients [4]. Thus, inhibition of angiogenesis presents a promising strategy for cancer treatment [5].
During angiogenic process, MMPs/urokinase plasminogen activator (uPA) system plays a major role in extracellular matrix (ECM) degradation, which is deeply involved in regulation of endothelial cell survival, migration, and invasion [6]. uPA binding to urokinase plasminogen activator receptor (uPAR) can activate the conversion of plasminogen to plasmin [7-9]. This conversion is mediated by specific and fast-acting plasminogen activator inhibitors (PAIs) [10]. The activated plasmin can mediate the conversion of pro-MMPs to MMPs, which can be inhibited by the specific endogenous inhibitors called tissues inhibitor of matrix metalloproteinases (TIMPs) [11]. Therefore, the inhibition of MMPs/uPA system is regarded as a therapeutic strategy against angiogenesis.
Lycopene is a major carotenoid in tomatoes and other red fruits and vegetables including watermelon, papaya, pink grapefruit, and guava [12]. Lycopene contains 11 conjugated and two nonconjugated double bonds, which renders it highly reactive toward oxygen and free radicals [13-15]. Unlike β-carotene, lycopene lacks a β-ionone ring, and therefore exhibits no pro-vitamin A activity. Epidemiological studies suggest that elevated intakes of lycopene is associated with decreased risks of chronic diseases such as cancer and cardiovascular diseases [16]. Preclinical and experimental studies have demonstrated the multiple biological functions of lycopene, including antioxidant activity [17], antimetastasis [18-20], antiproliferation [16, 21-23], induction of gap junction intercellular communication (GJIC) [24], and induction of apoptosis [25, 26].
Recently, lycopene has been shown to inhibit tube formation and migration in human umbilical vein endothelial cells (HUVECs) [27]. In addition, our previous studies have demonstrated that lycopene decreases the vascular endothelial growth factor (VEGF) plasma levels in nude mice transplanted with prostate tumor PC-3 cells and in nude mice injected via tail vein with hepatocarcinoma SK-Hep-1 cells [20, 28]. However, only limited in vitro and indirect data suggest that lycopene possesses anti-angiogenic activity, and the molecular mechanisms are unclear. In this study, we employed ex vivo and in vivo assays, including chorioallantoic membrane (CAM), rat aortic ring, and matrigel plug model, to substantiate the anti-angiogenic action of lycopene and determined the mechanism underlying such actions of lycopene in HUVECs.
2 Materials and methods
2.1 Lycopene solubilization
Lycopene was purchased from Wako (Kawagoe-shi, Saitama-ken, Japan) and the purity of commercial lycopene was approximately 98%, as claimed by the supplier (Wako). Lycopene was dissolved in tetrahydrofuran (THF)/butylated hydroxytoluene (BHT) to form a stock solution of 10-mM lycopene, which was diluted with THF at indicated ratios (1:1, 1:3, 1:7), and then diluted with fetal bovine serum (FBS) at the indicated ratio (1:9) [29]. THF/BHT-FBS-lycopene was added to the culture medium at a calculated final concentration of 2.5, 5, or 10 μM. THF at 0.2% (v/v) and FBS at 1.8% (v/v) served as the solvent control for lycopene, which did not significantly affect the assays described later.
2.2 Rat aortic ring assay
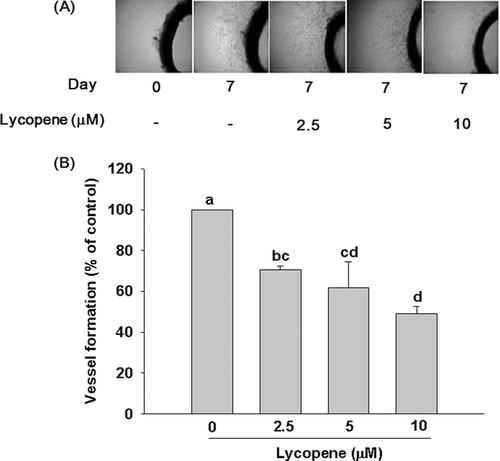
The ex vivo rat aortic ring assay was performed as described by Go and Owen [30] with minor modification. Twenty-four well tissue culture plates were precoated with 0.5 mL type I collagen gels mixed with Dulbecco's modified Eagle medium (DMEM) and allowed to gel for 1 h at 37°C. Thoracic aortas were isolated from 8-week-old male Sprague–Dawley rats. The thoracic aortas were quickly harvested and rinsed with phosphate-buffered saline (PBS) containing 4% gentamicin and sectioned into 1–1.5 mm aortic rings. The rings were embedded in a 24-well plate, and then 0.5 mL DMEM containing 2% FBS and various concentrations of lycopene (0–10 μM) was added and changed every day for 7 days. After incubation for 7 days, vessel sprouting around the rat aortic rings were photographed with an optical microscope at a 10× magnification and quantified by microvessel length using image software (Image-Pro Plus 5.0). Four fields were counted for each well and the mean number was expressed for each condition.
2.3 CAM assay
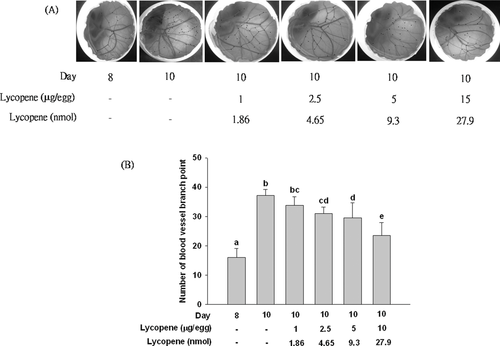
Eight-day-old fertilized chick eggs were used to examine the in vivo anti-angiogenic activity of lycopene as described by Ribatti et al. [31] with minor modification. Fertilized eggs (five eggs per group) were incubated at 37°C with 65% relatively humidity. On day 8, a 1-cm2 window was carefully created on the air-cell side of the egg to assure the existence of embryonic blood vessels. Corn oils (20 μL) containing 1–15 μg lycopene were applied on a 0.5-cm2 filter paper disk to the CAM, and the permeable sticky tape was immediately appended to the window. After incubation for 2 days, the eggshell was pushed aside around the window, and the blood vessels were photographed. The anti-angiogenic effect of lycopene on CAMs was quantified by counting the number of blood vessel branch points, which were marked using artistic software on the photos.
2.4 Matrigel plug assay
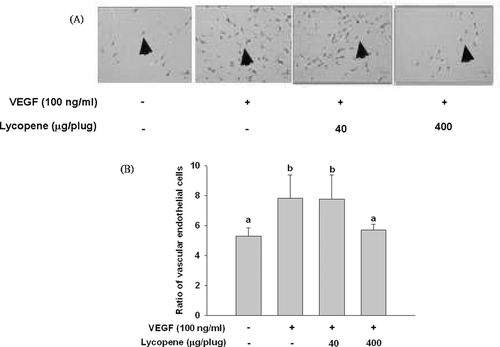
The in vivo matrigel plug assay was conducted according to the procedures described by Komi et al. [32] in which the lipid-soluble test compound, acyclic retinoid, was dissolved in dimethyl sulfoxide (DMSO) and mixed with matrigel before implantation subcutaneously (s.c.) to mice. In this study, lycopene (40 or 400 μg per plug or per mouse) was first solubilized in DMSO, of which 30 μL was added to the 0.5 mL matrigel. The final concentration of DMSO was 6% (v/v) in matrigel, which did not affect the plug formation. These doses of lycopene were based on those of our previous studies [20, 28], in which lycopene (1–20 mg/kg) was given orally and repeatedly to the nude mice twice a week for 12 weeks. C57BL/6 male mice (6-wk-old) were obtained from the Animal Center of the National Science Council. The mice were housed individually in cages with controlled temperature (25 ± 2°C), humidity (65 ± 5%) with 12-h light/dark cycles. After acclimatization for 1 week, the mice were randomly divided into four groups (n = 5 for each group) as follows. Group 1: control (implanted s.c. with 0.5 mL of matrigel containing 100 unit/mL of heparin and 6% DMSO); group 2: VEGF (implanted s.c. with 0.5 mL of matrigel containing 100 unit/mL of heparin, 100 ng/mL of VEGF, and 6% DMSO); group 3: low dose of lycopene (implanted s.c. with 0.5 mL of matrigel containing 100 unit/mL of heparin, 100 ng/mL of VEGF, and 40 μg lycopene); and group 4: high dose of lycopene (implanted s.c. with 0.5 mL of matrigel containing 100 unit/mL of heparin, 100 ng/mL of VEGF, and 400 μg lycopene). After implantation for 1 week, the matrigel formed a plug, and the mice were killed by CO2 anesthetization; after which the plugs were removed carefully, fixed in 10% formalin, embedded in paraffin, and then sectioned for staining with hematoxylin and eosin. The vascular endothelial cells were quantified using image software (Image-Pro Plus 5.0), and the formula for calculation the anti-angiogenic effect of lycopene is (total vascular endothelial cells area ÷ total matrigel plug area) × 1000. The study protocol was approved by the Animal Research Committee of National Chung Hsing University (IAUCC Approval No: 99-71).
2.5 Cell culture and cell proliferation assay
HUVECs (BCRC H-UV001) with a passage of five were used in this study.
HUVECs were cultured in M199 medium (Gibco, Carlsbad, CA, USA), supplemented with 20% FBS, 30 μg/mL endothelial cell growth supplement (ECGS), 25 U/mL heparin, 2 mM l-glutamine, 1.5 g/L sodium bicarbonate, and 1% antibiotic–antimycotic (Gibco). The supplementation of ECGS to M199 medium [33] provides several essential growth factors such as acidic fibroblast growth factor (FGF) and endothelial cell growth factor (ECGF) for the growth of endothelial cells, as claimed by the supplier (Millipore, Billerica, MA). For cell proliferation assay, cells (5 × 104 cells/mL) were seeded onto 24-well precoated 1% gelatin. HUVECs at 80% confluence were incubated with lycopene at the indicated concentration for 6, 12, and 24 h. The cell proliferation was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as described previously [34].
2.6 Determination of tube formation in HUVECs
The effect of lycopene on angiogenesis in vitro was estimated by the tube formation assay, as described previously [35] with minor modification. Briefly, μ-slides were precoated with the 10 μL/well ECMatrixTM (Chemicon, Darmstadt, Germany) and incubated for 2 h at 37°C. ECMatrixTM, a soluble basement membrane isolated from Engelbreth-Holm-Swarm mouse sarcoma, has been used for in vitro assays of invasion and angiogenesis as well as for in vivo assays of angiogenesis [36, 37]. After pretreatment with lycopene for 6, 12, and 24 h, the number of HUVECs was adjusted to 8 × 104 cells/mL, and 50 μL M199 containing 2% (v/v) FBS and 8 × 104 cells was placed in the μ-slides followed by incubation for 6 h. The tube formation was examined microscopically. For each replicate, the neovascularization in five randomly selected fields was determined.
2.7 Cell invasion and migration assays
Cell invasion and migration were assayed using transwell chambers (Millipore, Darmstadt, Germany) according to the method reported by Repesh [38] with minor modification. The main difference between cell invasion and cell migration assays is that each filter was coated with 100 μL of a 1:20 diluted matrigel in cold DMEM to form a thin continuous film on the top of the filter for cell invasion assay. In both assays, transwell chambers with 6.5-mm polycarbonate filters of 8-μm pore size were used. After HUVECs were pretreated with lycopene for 6 h, the number of cells was adjusted to 1.25 × 105/mL, and 400 μL of M199 serum-free medium containing 5 × 104 cells was placed in the upper chamber. The lower chamber was loaded with 600 μL of M199 containing 20% FBS. After an incubation of 6 h (for the migration assay) or 24 h (for the invasion assay), the cells on the upper surface of the chamber were completely wiped away with a cotton swab. The cells on the lower surface of the filter were immobilized in methanol, stained with Giemsa, and counted under a microscope. For each replicate, the cells in five randomly selected fields were selected, and the counts were averaged.
2.8 Gelatin and plasminogen zymography
The activities of MMP-2 and uPA were assayed using the gelatin and plasminogen zymography, respectively, according to the methods reported by Yang et al. [39] with minor modifications. The cells (5 × 104 cells/mL) were pretreated with lycopene for 6 h in M199 medium containing 20% (v/v) FBS and incubated for an additional 24 h in M199 serum-free medium, after which the culture medium was harvested and stored at –80°C until use. For the assay of gelatin and plasminogen zymography, the culture medium was electrophoresed in a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel containing 0.1% (w/v) gelatin (for assay of MMP-2 activity) or 0.25% (v/v) plasminogen (for assay of uPA activity). After electrophoresis, gels were washed with 2.5% Triton X-100 and then incubated in reaction buffer (40 mM Tris–HCl, pH 8.0; 10 mM CaCl2 and 0.01% NaN3) for 12–15 h at 37°C. Then, gel was stained with Coomassie brilliant blue R-250 for 30 min and destained in 10% acetic acid (v/v) and 50% methanol (v/v). The relative MMP-2 anduPA activities were quantified using Matrox Inspector 2.1 software.
2.9 Western blotting
Protein expression of Rac1, TIMP-2, PAI-1, PI3K, Akt, p-Akt, ERK, p-ERK, p38, p-p38, c-Jun N-terminal kinase (JNK), p-JNK, and VEGFR2 was measured by Western blotting. Cells were lysed with cold radio-immuno-precipitation assay (RIPA) buffer containing protease inhibitor cocktail. The cell lysates were then centrifuged at 12 000 × g for 30 min at 4°C. An amount of protein (40 μg) from the supernatant was resolved by SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane. After blocking with Tris Buffered Saline buffer (20 mmol/L Tris–HCl, 150 mmol/L NaCl, pH 7.4) containing 5% nonfat milk, the membrane was incubated with monoclonal antibody followed by horseradish peroxidase-conjugated anti-mouse IgG, and then visualized using an Electrogenerated chemiluminescence (ECL) chemiluminescent detection kit (Amersham, Uppsala, Sweden).
2.10 Statistical analysis
Values are expressed as means ± SD and analyzed using one-way analysis of variance (ANOVA) followed by least significant difference (LSD) for comparisons of group means. Student's t-test was used for comparisons of two groups. All statistical analyses were performed using SPSS for Windows, version 10. Unless specified otherwise, a p < 0.05 is consideredsignificant.
3 Results
3.1 Lycopene inhibits angiogenesis both ex vivo and in vivo
We used both ex vivo and in vivo models to confirm the anti-angiogenic effect of lycopene. In the rat aortic ring assay (an ex vivo model), treatment of lycopene (2.5–10 μM) induced a significant and concentration-dependent inhibition of the sprouting of vessels from rat aortic rings, with an inhibition of 51% (p < 0.01) at 10 μM lycopene, as compared with control group (Fig. 1). In the in vivo CAM experiment, neovascularization was markedly increased during 8–10 days of incubation (Fig. 2). Treatment of fertilized eggs with lycopene (1–15 μg or 1.86–27.9 nmol/egg) significantly inhibited the number of blood vessel points, when the dose reached 2.5 μg or 4.65 nmol/egg. The inhibitory effect of lycopene continued to increase in a dose-dependent manner, reaching an inhibition of 38% (p < 0.05) at 15 μg/egg, as compared with the control group (Fig. 2).
In the in vivo matrigel plug assay, subcutaneous injection of mice with matrigel containing VEGF (100 ng/mL) induced a significant angiogenic response, as evidenced by increased vascular endothelial cells, as compared with the control group (Fig. 3). Lycopene treatment at the low dose (40 μg/plug or 74.5 nmol/plus for each mouse) did not significantly affect the number of vascular endothelial cells induced by VEGF, whereas lycopene at the high dose (400 μg/plug or 745 nmol/plug for each mouse) completely inhibited the formation of vascular endothelial cells (Fig. 3).
3.2 Lycopene inhibits tube formation in HUVECs
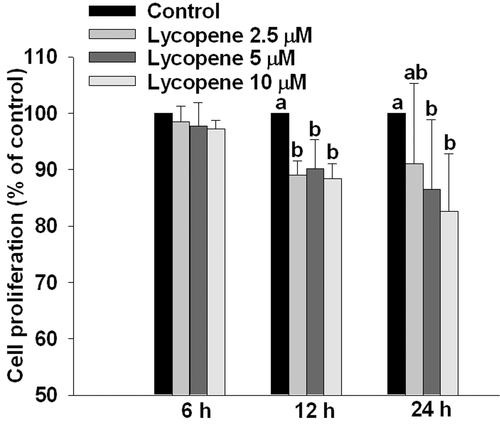
Pre-incubation of HUVECs with lycopene (2.5–10 μM) for 6, 12, and 24 h significantly inhibited tube formation in a concentration-dependent manner (Fig. 4), and the strongest inhibitory effect occurred at 6 h of incubation with an inhibition of 39% (p < 0.05) at 10 μM lycopene (Fig. 4). In addition, lycopene did not affect cell proliferation at either 6 or 12 h of incubation, but slightly inhibited cell proliferation at 24 h of incubation with an inhibition of 18% (p < 0.05) at 10 μM lycopene in HUVECs (Fig. 5). Based on these results, we excluded the possibility that the inhibitory effect of lycopene on tube formation is attributed to inhibition of cell proliferation. Therefore, we chose a pre-incubation time of 6 h or less than 6 h for the following studies.
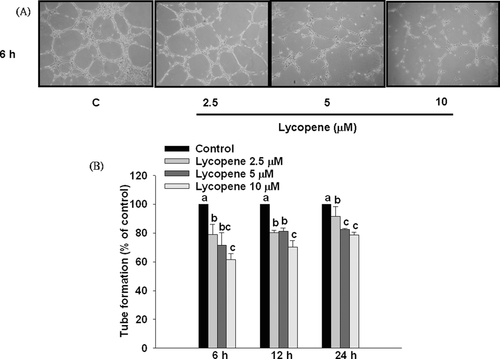
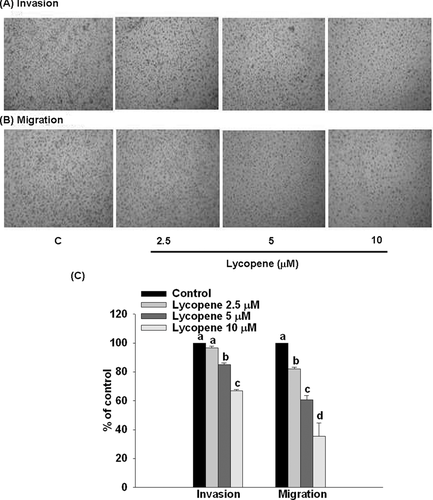
3.3 Lycopene inhibits invasion and migration in HUVECs
Pretreatment of HUVECs with lycopene (2.5–10 μM) for 6 h markedly inhibited cell invasion and migration in a concentration-dependent manner, with an inhibition of 33% (p < 0.05) and 65% (p < 0.01), respectively, when added at 10 μM lycopene (Fig. 6).
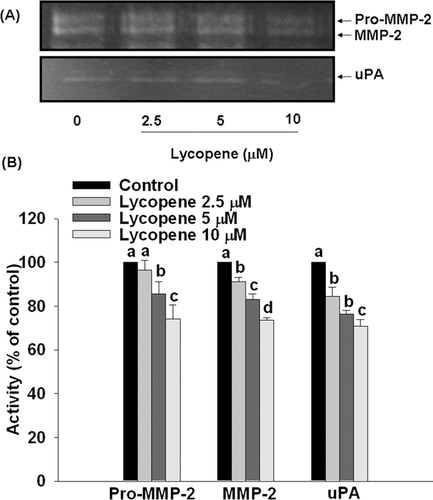
3.4 Lycopene inhibits activities of proMMP-2, MMP-2, and uPA in HUVECs
Pre-incubation of HUVECs with lycopene (2.5–10 μM) for 6 h significantly inhibited activities of proMMP-2, MMP-2, and uPA in a concentration-dependent manner, with an inhibition of 26% (p < 0.05), 27% (p < 0.05), and 30% (p < 0.05) at 10 μM lycopene, respectively (Fig. 7).
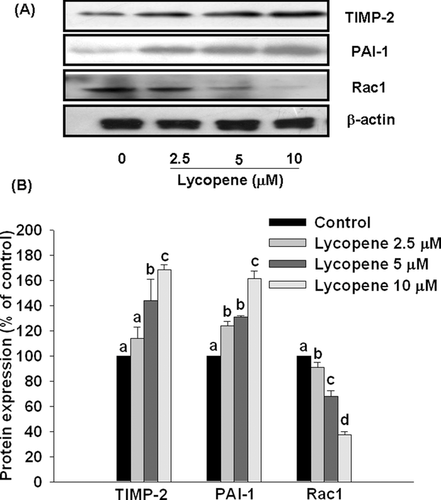
3.5 Effect of lycopene on TIMP-2, PAI-1, and Rac1 protein expression in HUVECs
Pre-incubation of HUVECs with lycopene (2.5–10 μM) for 6 h significantly increased protein expression of TIMP-2 and PAI-1 in a concentration-dependent manner, with a promotion of 68% (p < 0.01) and 60% (p < 0.01), respectively, at 10 μM lycopene (Fig. 8). In contrast, lycopene significantly inhibited protein expression of Rac1 in a concentration-dependent, with an inhibition of 63% (p < 0.01) at 10 μM lycopene (Fig. 8).
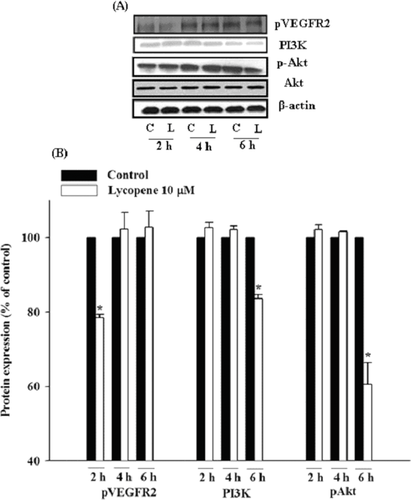
3.6 Lycopene attenuates VEGFR2-mediated PI3K-Akt axis and ERK/p38 pathway in HUVECs
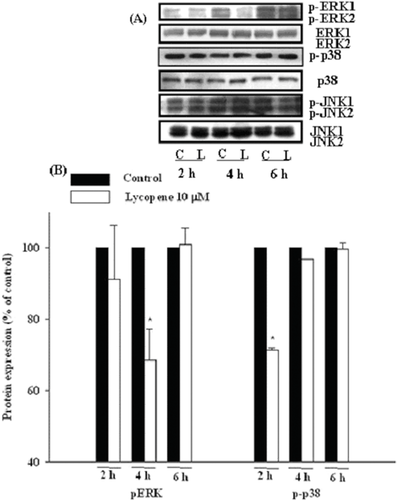
HUVECs were incubated with lycopene (10 μM) for 2, 4, and 6 h, and the protein expression of VEGFR2, PI3K-Akt, and MAPK family members (ERK1/2, JNK1/2, and p38) was determined by Western blotting. Lycopene (10 μM) significantly decreased the protein expression of VEGFR2 at 2 h of incubation, with an inhibition of 22% (p < 0.05; Fig. 9). In addition, lycopene (10 μM) significantly reduced the protein expression of PI3K and the phosphorylation of Akt at 6 h of incubation with inhibition of 17% (p < 0.05) and 40% (p < 0.05), respectively, without affecting total protein expression of Akt (Fig. 9). Moreover, lycopene (10 μM) significantly inhibited the phosphorylation of p38 and ERK1/2 at 2 h and 4 h of incubation, with an inhibition of 32% (P < 0.05) and 29% (P < 0.05), respectively, without changing their protein expression levels (Fig. 10). In contrast, lycopene did not affect the phosphorylation and total protein expression of JNK1/2 during 6 h of incubation (Fig. 10).
4 Discussion
Our present data substantiate that lycopene is a potent anti-angiogenic agent both in vitro and in vivo. We used both ex vivo and in vivo models for screening the anti-angiogenic activity of lycopene, including rat aortic ring, CAM, and matrigel plug assay. The rat aortic ring is an ex vivo organ culture assay commonly used in angiogenesis research [40]. We found that lycopene significantly and concentration-dependently inhibited tube formation in rat aortic ring assay. The CAM model, one of the commonly used in vivo models for determining angiogenesis, is relatively simple and inexpensive for rapidly screening the anti-angiogenic compound [31, 41]. However, CAM itself possesses a well-developed vascular network and it is difficult to distinguish new blood vessel from existing ones [41]. To solve this difficulty, we used the 8-day-old fertilized eggs as initial control and found that the blood vessel branch point significantly increased after 2 days of incubation. In addition, lycopene (15 μg/egg or 27.9 nmol/egg) significantly decreased new blood vessel formation, as compared with untreated group. In the in vivo matrigel plug assay, we quantified the angiogenic response by counting vascular endothelial cells derived from matrigel and demonstrated that administration (s.c.) of lycopene at the highest dose used (400 μg/plug or 745 nmol/plug for each mouse) completely inhibited the formation of vascular endothelial cells induced by VEGF. These results strongly suggest that lycopene is an anti-angiogenic agent, although it remains to be seen whether lycopene is indeed anti-angiogenic in humans.
Using HUVECs as a cell model, we found that lycopene inhibited the invasion, migration, and tube formation, and these results are consistent with those of a previous study [27]. Blood vessels comprise three main components, including vascular smooth muscle cells, endothelial cells, and ECM [42]. The ECM degradation is primarily mediated by proteases such as uPA and MMPs, which are inhibited by endogenous inhibitors (PAIs and TIMPs), and such degradation is the result of an imbalance between these activators and inhibitors [11]. Among the MMPs, MMP-2 is the predominately expressed MMP in endothelial cells capable of degrading type IV collagen and is directly involved in endothelial cell migration during angiogenesis [43]. In this study, we demonstrated that the inhibitory effects of lycopene on invasion, migration, and tube formation were accompanied by reduced activities of MMP-2 and uPA and by enhanced protein expression of TIMP-2 and PAI-1. In consistency with the finding of Wagner et al. [44], we did not detect MMP-9 activity in HUVECs.
Mechanistically, we found that lycopene attenuated VEGFR2-mediated signaling pathways, including ERK1/2-p38 and PI3K-Akt axis. VEGFRs comprise three subtypes-VEGFR1, R2, and R3, and VEGFRs-mediated signaling pathways are considered the rate-limited steps in physiological angiogenesis [45]. Among the VEGFRs, VEGFR2 is the major receptor, which mediates angiogenic activity of VEGF via diverse signaling pathways, including MAPK family and PI3K-Akt axis that regulate proliferation, migration, and tube formation of endothelial cells [46]. Cell migration plays an important role in many physiological and pathological circumstances, including angiogenesis, cancer metastasis, embryonic development, and wound healing [47, 48]. A large amount of evidence reveals that MAPK family members, such as ERK1/2, JNK1/2, and p38, are the major signaling molecules that mediate endothelial cell migration [49, 50]. Activation of MAPK family members in response to extracellular stimuli could activate intracellular signaling molecules and induce the alterations in cytoskeleton-related proteins, such as Rho-small GTPases that are critical in cell migration process [51]. Carotenoids like acyclic retinoid, a synthetic retinoid, have been shown to inhibit endothelial cell growth, migration, and tube formation through the inhibition of VEGFR2-MAPK pathway [32]. In addition, the activation of PI3K-Akt axis has been shown to promote cell survival, migration, and cytoskeletal rearrangement [52]. Herein, we demonstrated that lycopene inhibited VEGFR2-ERK/p38 pathway followed by inhibition of Rac1 protein expression leading to reduced migration and tube formation of HUVECs. In addition to VEGFR2-ERK/p38 pathway, PI3K-Akt axis is also involved in anti-angiogenic action of lycopene (Fig. 11). However, because these factors were determined at different time points, the chronology of events leading to anti-angiogenic actions of lycopene is somewhat speculative.
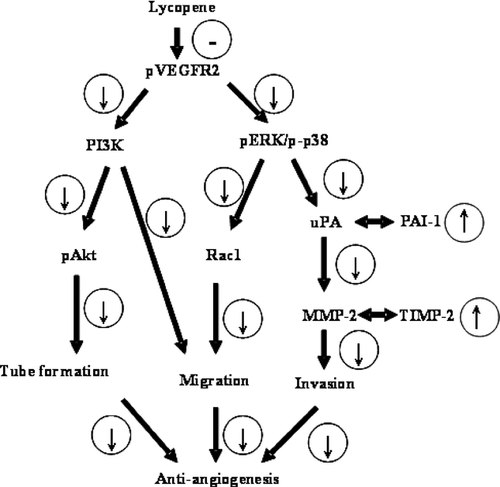
 Inhibited by lycopene;
Inhibited by lycopene;  Promotion; or
Promotion; or  inhibition as a result of upregulation or downregulation of upstream signaling molecules. MMP-2: matrix metalloproteinase-2; PAI-1: plasminogen activator inhibitor-1; TIMP-2: tissue inhibitors of metalloproteinase-2; uPA: urokinase-type plasminogen activator; VEGFR-2: vascular endothelial growth factor receptor-2.
inhibition as a result of upregulation or downregulation of upstream signaling molecules. MMP-2: matrix metalloproteinase-2; PAI-1: plasminogen activator inhibitor-1; TIMP-2: tissue inhibitors of metalloproteinase-2; uPA: urokinase-type plasminogen activator; VEGFR-2: vascular endothelial growth factor receptor-2.In conclusion, this study demonstrates that lycopene inhibits angiogenesis both in vitro and in vivo. Mechanistically, we demonstrate that the anti-angiogenic activity of lycopene may involve inhibition of MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways leading to reduced invasion, migration, and tube formation of HUVECs (Fig. 11).
ACKNOWLEDGMENTS
The authors have declared no conflict of interest.




