High dose of dietary resveratrol enhances insulin sensitivity in healthy rats but does not lead to metabolite concentrations effective for SIRT1 expression
Abstract
Scope: trans-Resveratrol has been shown to improve insulin sensitivity and to enhance cellular glucose uptake. Evidence from recent studies indicates that these effects depend on SIRT1-pathways.
Methods and results: Since ingestion of resveratrol leads to the presence of resveratrol and resveratrol metabolites in the body, we aimed at investigating (i) whether a daily dose of 300 mg resveratrol/kg body weight in healthy male Wistar rats for a period of 8 wk affects the selected parameters of glucose and lipid metabolism and (ii) whether the resulting plasma concentrations of resveratrol metabolites were effective in modulating SIRT1 expression. The dietary dose was based on the results from preceding toxicity studies. The results from the feeding experiment revealed plasma concentrations of resveratrol and its metabolites below 1 μmol/L and showed that fasting glucose and insulin levels were decreased by 35 and 41%, respectively, in the resveratrol group compared with controls. Insulin sensitivity was enhanced by 70%, whereas liver SIRT1 protein expression was not affected. Treatment of HepG2 cells with 10 μM resveratrol (1.49-fold) or its diglucuronides (1.21-fold) increased SIRT1 expression.
Conclusion: These results suggest that the improved insulin sensitivity after dietary administration of 300 mg resveratrol/kg body weight does not involve increased protein expression of SIRT1.
1 Introduction
Resveratrol, 3,5,4′-trihydroxy-trans-stilbene, is a plant phytoalexin present in red grapes, peanuts, dried roots and various berries 1 and the average diet consumes about 10 mg/day 2. The biological impacts of resveratrol include its role in controlling oxidative stress and inflammation as well as evidence of anti-cancer and chemopreventative activities 1, 3, 4. Additionally, resveratrol has been shown to have beneficial properties in the maintenance of obesity and diabetes 5, 6.
Dietary resveratrol is rapidly absorbed 7 and high doses show few occurrences of adverse effects 8, 9. Resveratrol is primarily metabolised to resveratol glucuronides and resveratrol sulphates in enterocytes where it is transported into the plasma for distribution throughout the body 10-12. In a resveratrol pharmacokinetic study in humans, a high bolus dose of 5 g resveratrol resulted in a peak plasma resveratrol concentration of 2.4 μmol/L, a peak resveratrol-3-sulphate concentration of 14 μmol/L, and a peak resveratrol-monoglucuronide concentration of 4.3 μmol/L 9. A human repeated dose study with the administration of 150 mg resveratrol every 4 h for 13 doses revealed a peak plasma concentration of 0.27 μmol/L resveratrol 8. Administration of up to 5.0 g of resveratrol for 29 days in 40 human volunteers resulted in a peak plasma concentration of 4.2 μmol/L resveratrol 1 h after dosing 13, 14. The administration of a moderate dose of 85.5 mg/70 kg body weight (bw) of the naturally occurring resveratrol form, resveratrol-3-O-β-D-glycoside, resulted in a peak resveratrol-3-sulphate concentration of 0.95 μM after 1 h, a peak resveratrol-3-5-disulphate concentration of 0.94 μM after 8 h, and a peak concentration of 0.35 μM for resveratrol-diglucuronides 15.
Although the molecular mechanisms by which resveratrol exerts its biological effects are not completely understood, recent evidence has revealed a connection between resveratrol and the sirtuin, SIRT1 5, 6, 16. Sirtuins represent a family of nicotinamide adenine dinucleotide enzymes that couple lysine deacetylation on target proteins to NAD+ hydrolysis 17. The mammalian sirtuins are involved in a number of cellular and physiological functions, including chromatin remodelling, energy homeostasis, mitochondrial biogenesis, apoptosis and longevity 18-20. Several reports have shown contradictory results, indicating that resveratrol improves insulin sensitivity through SIRT1-independent and SIRT1-dependent pathways 5, 6, 21, 22. Evidence for the latter has been provided by Sun et al. 5 who demonstrated that resveratrol promotes increased glucose uptake in the presence of insulin in normal and insulin-resistant muscle myocytes through a SIRT1-dependent pathway. Resveratrol also attenuates adipogenesis in 3T3-L1 adipocytes through a SIRT1-dependent pathway and upregulates SIRT1 expression in differentiated adipocytes leading to lipolysis and mobilisation of free fatty acids (FFA) 23. However, the mechanism by which resveratrol or the resveratrol metabolites attenuate SIRT1 expression or function has not yet been determined 24. The aim of the current study was to determine the effect of resveratrol on the selected parameters of glucose and lipid metabolism, and liver SIRT1 protein expression in rats fed a high daily dose of 300 mg resveratrol/kilogram body weight (bw) for 8 wk. The dietary dose was based on the results from preceding acute oral and three-month toxicity experiments following Organization for Economic Co-operation and Development (OECD) guidelines. In addition, the gene expression of SIRT1 in human HepG2 cells after incubation with the most abundant resveratrol metabolites was determined to answer the question whether the plasma concentrations of resveratrol and its metabolites reached in the feeding trial are effective in modulating SIRT1 expression.
2 Materials and methods
2.1 Acute oral toxicity of trans-resveratrol in rats
An acute oral toxicity study was conducted according to the OECD Test Guideline 423 and following GLP Principles, German Chemicals Law, Appendix 1. SPF-Wistar rats (Crl:WI) at 7-wk of age were purchased from Charles River Deutschland (Sulzfeld, Germany). A specially prepared diet (1324N, Altromin International, Lage, Germany) and water was offered ad libitum, except during the exposure period. The animals were kept in an artificial 12-h light/dark cycle. trans-Resveratrol (≥98% purity, Bio De Tek, Bensheim, Germany) was dissolved in corn oil to a final concentration of 200 mg/mL. After a 16-h fast without food, the animals (3/sex/group) were given an oral gavage by stomach tube with a volume of 10 mL/kg bw followed by another 3 h fast. The animals were clinically observed daily for the following parameters: general condition, fur, grooming activity, visible mucous membranes, behavior and locomotor activity (lethargy, coma, convulsions, diarrhoea and salivation), central nervous symptoms, breathing patterns. Additionally, the individual bw was recorded (Datatox rC.10) to the nearest 0.1 g on days 0, 3, 7, and 14 At the end of the study, the animals were anesthetized with CO2, exsanguinated and necropsied.
2.2 Three-month repeated dose study in rats
A 90-day oral toxicity study was conducted on SPF-Wistar rats (Crl:WI) at 6-wk of age, purchased from Charles River Deutschland, according to OECD Test Guideline 408 following GLP Principles, German Chemicals Law, Appendix 1. A specially prepared diet (1324N, Altromin International) and water was offered ad libitum, except during the exposure period. The animals were kept in an artificial 12-h light/dark cycle. The animals (11/sex/group) were randomly assigned to one control group, fed 1% methocel (hydroxypropylmethylcellulose, Dow Chemical, USA) by oral gavage (5 mL/kg bw/day) or three dose groups of 50, 150 and 450 mg/kg bw/day trans-resveratrol (≥98% purity, Bio De Tek) dissolved in 1% methocel and fed by oral gavage (5 mL/kg bw/day). The animals were observed daily for clinical symptoms. The individual bw, food and water consumption by difference were recorded weekly (Datatox rC.10) to the nearest 0.1 g. In the last week of treatment, spontaneous locomotor activity was measured with a “Motitest” computerized light-beam system (TSE, Homburg/Ts, Germany). Additionally, a functional observational battery of tests 25, 26 was utilized to assess the effects of treatment along with the determination of forelimb grip strength 27. At the end of the study the animals fasted overnight, were anesthetized with CO2, blood samples were collected, and the animals were exsanguinated and necropsied. The organs were weighed at termination and histopathology was performed on the wide array of tissues.
2.3 Trans-resveratrol-fed rats
This animal trial was originally conducted to quantify trans-resveratrol metabolites in plasma and tissues after daily administration of either 50 or 300 mg resveratrol/kg bw and the experimental details were previously described 28. Briefly, male Wistar rats (Unilever HsdCpb:WU) were obtained from Harlan-Winkelmann (Borchen, Germany), randomised into experimental groups of 10 rats each and fed a specially formulated diet deficient in fat but supplemented with 12% canola oil (C1056, Altromin International). The control group received a diet without resveratrol while the experimental group studied here received a diet with 300 mg/kg bw/day resveratrol for 8 wk. Although a recent safety study with trans-resveratol in rats showed that the no-observed-adverse-effect level was 700 mg/kg bw/day 29, our safety studies in rats showed some minor adverse effects (see Section 3) with 150 mg/kg bw/day which were more pronounced at 450 mg/kg bw/day. Therefore, we decided to feed a dose of 300 mg/kg bw/day in order to reach high plasma concentrations of resveratrol and metabolites, without inducing any known side effects. After 8 wk, the animals were anesthetised with a CO2/air mixture and decapitated. Blood was collected immediately and the plasma was separated by centrifugation. The abdominal cavity was opened and the livers were rapidly removed, weighed and washed in ice-cold 0.9% NaCl. All samples were stored at −80°C until further analysis. The experimental protocols and procedures were approved by the Animal Care and Use Committee at the University of Kiel, Germany.
2.4 Glucose and insulin concentration in plasma
For the determination of plasma glucose levels, plasma proteins were precipitated by adding 0.3 mol/L perchloric acid and centrifugation at 800×g for 10 min. The clear supernatant was used for the hexokinase/G6P-DH assay from R-Biopharm (Darmstadt, Germany) according to the manufacturers' protocol. Insulin concentration in plasma was determined by ELISA (Linco Research, St. Charles, MO, USA) according to the manufacturers' protocol.
2.5 HOMA-index
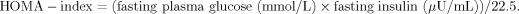
2.6 Determination of HbA1c-concentrations in erythrocytes
The determination of HbA1c values in erythrocytes has been described previously 31. Briefly, erythrocytes were haemolysed with an appropriate volume of distilled ice-cooled water. The suspension was centrifuged; the supernatant was removed and lyophilised. The resulting sediment was resuspended in buffer and trypsin (proteomics grade, Sigma-Aldrich, Taufkirchen, Germany) was added. The mixture was incubated for 12 h at 37°C and filtered. The filtrate was centrifuged and the clear solution was analysed by LC/MS. Data are expressed as the percent HbA1c of the total haemoglobin.
2.7 Plasma concentrations of FFA, cholesterol and triglycerides
Plasma FFA concentrations were determined spectrophotometrically using the NEFA C Kit from Wako Chemicals (Neuss, Germany) according to the manufacturers' protocol. Liver cholesterol was determined using the Chol Kit from Randox Laboratories (Krefeld, Germany) and liver triglycerides were measured using the Trigs Kit from Randox Laboratories.
2.8 Liver concentrations of FFA, cholesterol and triglycerides
Liver tissue was ground in a mortar using liquid nitrogen. Liver FFA, cholesterol and triglycerides were extracted as previously described 32. Briefly, the powdered tissue was extracted with a chloroform/methanol mixture (2:1). Later, the suspension was dried overnight. The pellet was resuspended in a solution of 60% butanol and 40% Triton X100/methanol (2:1). The resulting solution was used for the spectrophotometric quantification of FFA, cholesterol and triglycerides. Liver FFA were measured using the NEFA C Kit (Wako Chemicals). Liver cholesterol was determined using the Chol Kit (Randox Laboratories) and liver triglycerides were measured using the Trigs Kit (Randox Laboratories).
2.9 Western Blot analysis of SIRT1 in liver tissue
For the analysis of SIRT1 levels, nuclear extracts from liver cells were used. The extraction of nuclear proteins was performed as previously described 32. Briefly, 20 μg of liver nuclear extracts were run on 12% SDS-PAGE and probed with a SIRT1 antibody (Abcam, Cambridge, UK). α-Actin (Sigma-Aldrich) was used as a loading control. α-Rabbit IgG, HRP-linked (New England Biolabs, Frankfurt am Main, Germany) was used as a secondary antibody.
2.10 Cell culture experiments
Human hepatocellular carcinoma cells (HepG2) were cultured in 6-well plates (Sarstedt, Nümbrecht, Germany) at 37°C (with a 5% CO2 atmosphere) in RPMI 1640 medium with 20% fetal calf serum, 4 mmol/L L-glutamine and 2% penicillin and streptomycin (PAA Laboratories, Pasching, Austria). Prior to each experiment, the cells were starved for 24 h with serum-free medium containing 4 mmol/L of L-glutamine and 2% penicillin and streptomycin.
After synchronization, cells were exposed to trans-resveratrol (Sigma-Aldrich) at concentrations of 0.1, 1.0, 10 and 100 μmol/L. The resveratrol conjugates resveratrol-3-sulfate, resveratrol-4′-sulfate, resveratrol-disulfates, resveratrol-3-glucuronide, resveratrol-4′-glucuronide and resveratrol-diglucuronides (trans-resveratrol-2-C-β-D-/4′-O-β-D-diglucuronide and trans-resveratrol-2-C-β-D-/5-O-β-D-diglucuronide as equimolar solution, synthesised following previously described protocols 15, 28) and were used in concentrations of 10 μmol/L. After 1-h incubation, cells were lysed and harvested for the analysis of transcript levels.
2.11 Real-time PCR assay
In the context of the real-time PCR assay validation, 12 reference genes were tested for their most constant expression levels under different conditions with GeNorm (University of Gent, Gent, Belgium). The human endogenous reference gene panel (Bioline, Luckenwalde, Germany) was used in combination with the Brilliant SYBR Green Kit (Stratagene, Amsterdam, The Netherlands) on an Mx3000p cycler (Stratagene). Cycling conditions were chosen according to the manufacturer's protocol. The GeNorm software suggested peptidyl-prolyl isomerase A (PPIA) and β-2-microglobulin (B2M) as the two most stable genes in this assay and PPIA was chosen as the reference gene.
For expression assays, the primers for SIRT1 and PPIA (Table 1) were designed with Beacon Designer 5.0 (Premier Biosoft, Palo Alto, CA, USA), synthesized by Metabion (Martinsried, Munich, Germany) and validated by standard and melting curve analysis. Sequence analysis of the PCR products was performed by Medigenomix (Martinsried, Munich, Germany) and resulted in amplicon sequences specific to SIRT1 and PPIA which was verified by an NCBI database alignment with the entries NT 008583.16/Hs10 8740 and NT 007819.16/Hs7 7976, respectively. The real-time PCR assays were performed using the Brilliant SYBR Green Kit (Stratagene) on an Mx3000p cycler (Stratagene) with cycling conditions according to the manufacturer's protocol.
| Gene | Primer sequence | |
|---|---|---|
| SIRT1 | Sense | 5′-TAG TAG GCG GCT TGA TGG-3′ |
| SIRT1 | Antisense | 5′-TTC TTC TAA ACT TGG ACT CTG G-3′ |
| PPIA | Sense | 5′-CCA CCA GAT CAT TCC TTC TGT AGC-3′ |
| PPIA | Antisense | 5′-CTG CAA TCC AGC TAG GCA TGG-3′ |
For all PCR assays, total RNA was isolated using the RNeasy-Mini Kit followed by an on-column DNase I digest performed with the RNase-free DNase Kit (Qiagen, Hilden, Germany). The total RNA contents of the samples were quantified by photometric analysis at a wavelength of λ=260 nm before the cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Darmstadt, Germany). Cycling conditions were chosen according to the manufacturer's protocol.
2.12 Statistical analysis
Data were analysed by the SigmaStat version 3.5 (Sysstat Software GmbH, Erkrath, Germany). Data were tested for normal distribution using the Kolmogorov–Smirnov test. All data are expressed as mean values±SEM. Differences in the rat blood marker concentrations were analyzed by ANOVA and Dunnett's two-sided test. Differences in plasma and liver parameters between the control group and the resveratrol group were analysed using two-tailed Student's t-test for non-paired samples. A p-value of <0.05 was considered statistically significant (*p<0.05; **p<0.01; ***p<0.001).
3 Results
3.1 Acute oral toxicity of trans-resveratrol in rats
An acute oral toxicity study using 2000 mg/kg trans-resveratrol in Wistar rats was done according to OECD Test Guideline 423. Fourteen days after bolus administration of trans-resveratrol, no clinical signs of intoxication or changes in bw were observed (results not shown).
3.2 Three-month repeated dose study in rats
The three-month daily dosing of trans-resveratrol by oral gavage in male and female Wistar rats was done according to OECD Test Guideline 408. The administration of 0, 50, 150 and 450 mg/kg bw/day of trans-resveratrol resulted in neither clinical signs of intoxication nor was there any influence on the bw of the animals. As shown in Table 2, there was a statistically significant decrease in the total white blood cells (leukocytes) of males which received 450 mg resveratrol/kg bw/day (p<0.05) compared with control males but no other significant changes were seen in the haematological tests. In the functional observational battery of tests, there was a significant decrease in grip strength in males which were administered 150 and 450 mg resveratrol/kg bw/day (p<0.01) compared with the control group (data not shown). There were no significant differences observed with the female animals (data not shown). Additionally, the locomoter activity test using the “Motitest” computerised light-beam system showed no observable changes with the male animals. There was a statistically significant decrease in rearing time in females which were administered 450 mg resveratrol/kg bw/day (p<0.05) compared with control animals during the first 15-min interval. However, this effect did not occur in subsequent intervals (data not shown). The gross pathology and histopathology findings showed no significant changes in control versus treated groups for both male and female animals.
| Males | Resveratrol dose/kg bw | |||
|---|---|---|---|---|
| 0 mg/kg | 50 mg/kg | 150 mg/kg | 450 mg/kg | |
| n=11 | n=9 | n=10 | n=9 | |
| Red blood cells (T/L) | 8.32±0.49 | 8.03±0.64 | 8.05±0.46 | 7.88±0.4 |
| Haemoglobin (mmol/L) | 9.9±0.4 | 9.5±0.6 | 9.6±0.5 | 9.4±0.3 |
| Haematocrit (%) | 41.8±2.1 | 40.6±2.9 | 40.9±2.3 | 40.6±1.3 |
| MCV (fl) | 50.4±1.3 | 50.6±1.3 | 50.9±0.9 | 51.6±1.5 |
| MCHC (mmol/L) | 23.6±0.3 | 23.5±0.5 | 23.4±0.4 | 23.2±0.3 |
| Platelets (G/L) | 988±99 | 936±84 | 912±91 | 885±71 |
| Leukocytes, WBC (G/L) | 12.67±3.36 | 12.70±2.65 | 10.88±3.17 | 9.32±2.30* |
| Lymphocytes (G/L) | 10.18±3.09 | 10.04±2.32 | 8.80±2.95 | 7.11±1.95 |
| Segmented neutrophils (G/L) | 1.78±0.59 | 1.96±0.71 | 1.54±0.51 | 1.71±1.01 |
| Banded neutrophils (G/L) | 0.17±0.09 | 0.19±0.10 | 0.14±0.10 | 0.14±0.09 |
| Eosinophils (G/L) | 0.12±0.11 | 0.12±0.13 | 0.16±0.09 | 0.11±0.10 |
| Basophils (G/L) | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Monocytes (G/L) | 0.40±0.15 | 0.40±0.18 | 0.24±0.17 | 0.25±0.12 |
| Females | n=10 | n=10 | n=9 | n=7 |
| Red blood cells (T/L) | 7.26±0.41 | 7.35±0.37 | 7.24±0.24 | 6.99±0.64 |
| Haemoglobin (mmol/L) | 9.4±0.6 | 9.4±0.6 | 9.2±0.5 | 9.2±0.7 |
| Haematocrit (%) | 39.1±2.0 | 39.6±2.0 | 38.6±1.4 | 38.5±2.2 |
| MCV (fl) | 53.8±1.2 | 53.8±1.2 | 53.3±1.0 | 55.3±2.7 |
| MCHC (mmol/L) | 24.0±0.6 | 23.7±0.8 | 23.9±0.7 | 23.8±0.8 |
| Platelets (G/L) | 836±132 | 771±231 | 772±165 | 818±214 |
| Leukocytes, WBC (G/L) | 7.58±1.52 | 8.32±2.38 | 7.41±1.47 | 8.23±1.53 |
| Lymphocytes (G/L) | 6.04±1.36 | 6.83±2.08 | 5.58±1.20 | 6.61±1.02 |
| Segmented neutrophils (G/L) | 1.10±0.24 | 1.13±0.38 | 1.41±0.68 | 1.27±0.57 |
| Banded neutrophils (G/L) | 0.13±0.08 | 0.10±0.07 | 0.09±0.07 | 0.09±0.08 |
| Eosinophils (G/L) | 0.12±0.12 | 0.07±0.06 | 0.14±0.14 | 0.09±0.05 |
| Basophils (G/L) | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Monocytes (G/L) | 0.20±0.13 | 0.19±0.13 | 0.19±0.12 | 0.16±0.08 |
- a Statistics: ANOVA+Dunnett's test (two-sided): *p<0.05
3.3 Plasma glucose and insulin concentrations in trans-resveratrol-fed rats
Compared with the control group, fasting plasma glucose and insulin levels in the resveratrol group were significantly decreased by 35% (Fig. 1A) and 41% (Fig. 1B) compared with the control group, respectively.
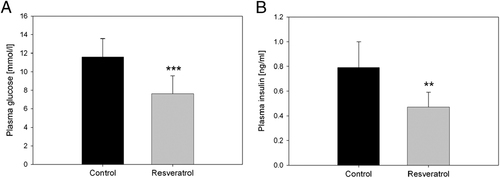
Mean fasting glucose (A) and insulin (B) levels in the plasma of animals of control and the resveratrol group after 8 wk of administrating 300 mg resveratrol/kg bw/day. Data are given as mean values±SEM. Asterisks indicate a significant difference of the values of the control versus the resveratrol group. **p≤0.01, ***p≤0.001.
3.4 HOMA-index
The HOMA-index has been shown to represent the degree of insulin resistance in mammals and in animals. As expected, the HOMA-index was significantly (p=0.02) lower in the resveratrol group compared with the control group, with this index reaching a value of 2.9±0.7 in resveratrol-fed animals, while in the control group, the mean HOMA-index was 10.1±3.9. Overall, insulin sensitivity was 70% higher in resveratrol-treated animals compared with controls.
3.5 HbA c content in erythrocytes
c content in erythrocytes
HbA1c is the stable glucose adduct to the N-terminal group of the β-chain of haemoglobin. The HbA1c content is a useful indicator for the determination of the long-term glycemic state. The level of glycated haemoglobin in the resveratrol group (2.6±0.07%) was significantly decreased (p=0.03) compared with the control group (3.0±0.14%).
3.6 Plasma concentration of FFA, trigylcerides and cholesterol
The concentrations of FFA in the plasma of the animals fed the resveratrol diet tended to be higher than the level of FFA in the plasma of the animals which received the control diet (Table 3), although this difference did not reach the level of significance (p=0.4). The levels of triglycerides as well as cholesterol were comparable in both groups.
| Plasma | Liver | |||||
|---|---|---|---|---|---|---|
| FFA (mmol/L) | Triglycerides (mmol/L) | Cholesterol (mmol/L) | FFA (mmol/L) | Triglycerides (mmol/L) | Cholesterol (mmol/L) | |
| Control group | 0.50±0.07 | 0.3±0.1 | 1.7±0.2 | 41.0±1.7 | 9.3±0.7 | 2.7±0.2 |
| Resveratrol group | 0.57±0.05 | 0.5±0.1 | 1.5±0.2 | 53.8±7.1 | 9.4±0.6 | 2.7±0.1 |
3.7 Liver concentration of FFA, trigylcerides and cholesterol
No differences were observed concerning the contents of either cholesterol or triglycerides in liver tissue of the respective animals (Table 3). In contrast, the FFA content in liver tissue tended to be higher in the resveratrol group compared with the control group. However, this difference was not statistically significant (p=0.066) (Table 3).
3.8 Western Blot analysis of the SIRT1 content in liver tissue
In order to test whether the administration of 300 mg resveratrol/kg bw/day leads to enhanced protein levels of SIRT1 in liver tissue, nuclear extracts were prepared and the SIRT1 levels detected by Western Blot analysis using a SIRT1 antibody. The results show that SIRT1 protein levels were unaltered in the resveratrol group compared with the control group (Fig. 2).
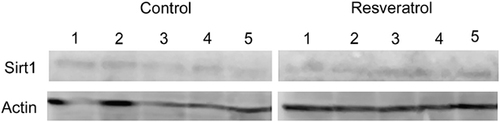
Protein levels of SIRT1 in liver tissue of five animals of the control and five animals of the resveratrol group after 8 wk of administrating 300 mg resveratrol/kg bw/day. Actin levels were used as a loading control.
3.9 Quantification of SIRT1 transcript levels in human HepG2 cells after incubation with ascending resveratrol concentrations
Since it was hypothesised that resveratrol might activate SIRT1 gene expression, the impact of ascending concentrations of resveratrol on the transcript level of SIRT1 was analysed using real-time PCR assays. For these experiments, an incubation time of 1 h was chosen since results of preceding experiments showed the highest impact at this incubation time (data not shown). Resveratrol at concentrations of 0.1 and 1.0 μmol/L did not affect the transcript level of the gene encoding SIRT1. In contrast, a concentration of 10 μmol/L resveratrol led to a significant 1.49-fold increase in the SIRT1 transcript level, while incubation of the cells with a concentration of 100 μmol/L significantly lowered the SIRT1 transcript level (Fig. 3).
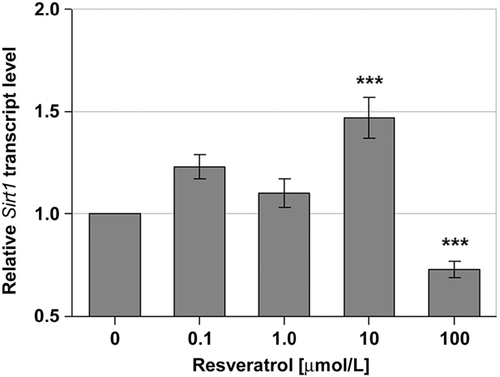
Relative transcript levels of the gene encoding SIRT1 in human HepG2 cells after incubation with 0.1, 1.0, 10 and 100 μmol/L resveratrol for 1 h. The transcript level of the non-treated control cells was set at 1. Statistics: ***p≤0.001 versus control cells (values are presented as the mean (n=5/6)±SEM).
3.10 Quantification of SIRT1 transcript levels in human HepG2 cells after incubation with resveratrol metabolites
It is well known that resveratrol enters the blood circulation predominantly in its conjugated forms. Therefore, we intended to test whether resveratrol metabolites have an impact on the transcript level of the gene encoding SIRT1. The resveratrol metabolites studied were resveratrol-3-sulfate, resveratrol-4′-sulfate, resveratrol-disulfates, resveratrol-3-glucuronide, resveratrol-4′-glucuronide and resveratrol-diglucuronides (trans-resveratrol-2-C-β-D-/4′-O-β-D-diglucuronide and trans-resveratrol-2-C-β-D-/5-O-β-D-diglucuronide in an equimolar solution) at a concentration of 10 μmol/L each. All of the resveratrol metabolites tested are present in human plasma 15 except for resveratrol-4′-sulfate, which was the most abundant metabolite found in the liver of resveratrol-fed rats 28. Again, an incubation time of 1 h was chosen. From the metabolites tested, only the resveratrol-diglucuronides significantly affected the SIRT1 transcript level with a 1.21-fold increase in SIRT1 expression (Fig. 4).
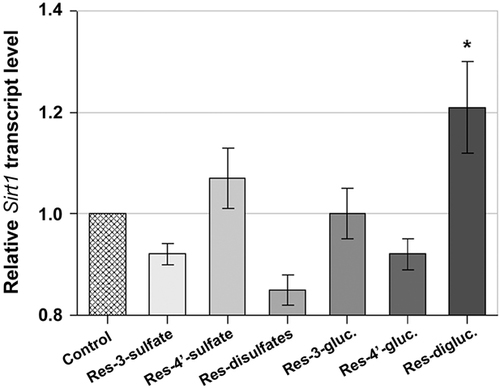
Relative transcript levels of the gene encoding SIRT1 in human HepG2 cells after incubation with 10 μmol/L resveratrol metabolites for 1 h. The transcript level of the non-treated control cells was set at 1. Statistics: *p≤0.05 versus control cells (values are presented as the mean (n=5/6)±SEM).
4 Discussion
Recent reports have shown that resveratrol improves insulin sensitivity and increased glucose uptake through SIRT1-dependent and SIRT1-independent pathways 5, 6, 21, 22, leading to a beneficial response to the metabolic syndrome. In our previous study, the long-term feeding of resveratrol to rats showed the presence of resveratrol and the resveratrol metabolites in the plasma, liver and kidneys 28. The purpose of this study was to explore the link between resveratrol and SIRT1 expression both in vivo and in vitro. We hypothesized that dietary administration of a high dose of 300 mg resveratrol/kg bw/day would improve insulin sensitivity and increase SIRT1 protein expression in the liver, while the resveratrol metabolites tested in plasma representative concentrations reached in our feeding trial would also increase SIRT1 expression in HepG2 cells.
Prior to the feeding experiment, toxicity studies were undertaken to determine the amount of resveratrol which could be safely added to the rat chow and not lead to signs of intoxication of the animals. The 14-day acute toxicity study showed that administration of 2000 mg/kg resveratrol in one dose did not harm or show clinical signs of intoxication in healthy male and female rats. In fact, this dose of 2000 mg/kg resveratrol was very high considering that the average weight at dosing was 264 g for the males and 197 g for females (data not shown). This result was similar to the recent data on the administration of 5 g resveratrol to healthy men and women aged 18–61 years 9 that suggested that a large dose of resveratrol was safe although this dose was substantially lower than the 2 g/kg administered in the here presented acute toxicity study. With this knowledge, a three-month repeated dose toxicity study was done with healthy male and female Wistar rats to determine the effect of long-term resveratrol exposure. In the male 150 mg/kg bw/day and 450 mg/kg bw/day treatment groups, there was a significant decrease in forelimb grip strength suggesting that there was an adverse effect of long-term exposure to resveratrol. Since this effect was seen in male rats only and was the only significant change in the functional and locomotor tests, additional experiments are needed to determine the general significance of this result. In the 450 mg/kg bw/day treatment groups, a significant decrease in male white blood cell counts (Table 2) and a significant decrease in the female rearing time at the 15-min interval were observed. This adverse result was not observed in a recent repeated dose study in rats fed 750 mg/kg bw/day for 3 months 29. Interestingly, a recent human intervention trial, where subjects were dosed with 2.5 and 5 g resveratrol for 29 days, reported incidences of moderately severe nausea and diarrhea in about 10% of the patients during the study 13. From these results, we concluded that a daily dose of 300 mg resveratrol/kg bw is within the safe range for rats.
Analysis of glucose and insulin levels revealed that administration of this high dose of resveratrol led to a 35% and a 41% decrease in the glucose and insulin level, respectively, in the fasting state compared with animals which did not receive resveratrol (Fig. 1). Consequently, the insulin resistance, as evidenced by calculation of the homeostatic model assessment, was lower in the resveratrol-treated animals compared with controls. This finding of a strong decrease in plasma glucose levels is supported by a recent report showing a decrease in the plasma glucose level in Zucker obese rats fed 5 mg/kg bw/day resveratrol for 2 wk and also in streptozotocin-induced diabetic rats fed 2.5 mg/kg bw/day resveratrol for 2 wk 33, 34. The increased expression of the glucose transporter GLUT-4 and an increased translocation of GLUT-4 to caveolar lipid raft fractions in the streptozotocin-induced diabetic rat heart led to an increased uptake of glucose 34. The results in Fig. 1 also follow other previously reported data where the oral administration of 22.4 mg/kg bw/day resveratrol to 1-y-old mice for 6 months on a high-caloric diet led to increased insulin sensitivity 6. A similar result was also seen in 5-wk-old mice fed 2.5 mg/kg bw/day resveratrol for 16 wk 5. The results from our study suggest resveratrol has a comparable effect in rats after only 8 wk of intervention, although after administration of a much higher dose of 300 mg/kg bw/day. This might indicate a time-dependent effect of resveratrol on insulin sensitivity, which can only be overcome by an increased dose. Although this hypothesis needs further testing, the observation that HbA1c concentrations were significantly decreased in the resveratrol group compared with the control group supports the view that resveratrol increases insulin sensitivity. The decreased HbA1c values of resveratrol-treated animals also indicate that glucose levels were lower during the study since the HbA1c concentration is a useful indicator for the determination of the long-term glycemic state.
In addition, a trend toward higher FFA-levels in Wistar rats was observed in our study. Administration of resveratrol did not lead to a significantly enhanced content of FFA in plasma and liver tissue in the fasting state. In addition, no effects of resveratrol on the concentrations of triglycerides and cholesterol, neither in plasma nor in liver tissue, were observed. This is in contrast to a recent report that showed a decrease in triglycerides and cholesterol in plasma and liver tissue of Sprague–Dawley rats fed 30 or 70 mg/kg bw/day resveratrol on a hyperlipidemic diet for 4 wk 35. Interestingly, in mice, there are some conflicting data on the levels of triglycerides and cholesterol in response to resveratrol. The Baur study detected no effect of a 22.4 mg/kg bw/day dose of resveratrol on the plasma levels of triglycerides or cholesterol in the fasting state 6. However, the Sun study saw a significant decrease in total cholesterol but no changes in triglyercides after a dose of 2.5 mg/kg bw/day of resveratrol 5. Although further study is needed to address these conflicting data in rodents, the different results obtained by Baur et al. 6 and Sun et al. 5 may be due to the different fat contents of the diets used as well as different study lengths.
To understand the mechanism underlying the effect of resveratrol on insulin sensitivity, the expression of SIRT1 was determined both in vivo and in vitro. SIRT1 is a mammalian deacetylase whose function has been linked to metabolic regulation 36. Resveratrol has recently been shown to have SIRT1-dependent effects 5, 23, 37, 38 but can also act through a SIRT1-independent pathway 22. Whether this happens in vivo is still a subject of discussion 24. Therefore, we addressed this question by studying the effect of resveratrol on SIRT1 expression in the liver of the rats fed 300 mg/kg bw/day resveratrol and in vitro using HepG2 cells. In the animal study, no differences in the liver protein SIRT1 levels were found between the animals fed with resveratrol compared with the animals in the control group (Fig. 2). This result is in accordance with previous findings and implies that resveratrol leads to increased insulin sensitivity, but that this effect is not linked to an increased expression of SIRT1 6. However, in HepG2 cells, resveratrol enhanced the SIRT1 transcript level at a concentration of 10 μmol/L while a further increase in resveratrol led to a significantly lower transcript level. This supports the hypothesis that resveratrol acts at different concentrations via different mechanisms possibly through the activation of the transcriptional coactivator PGC-1α 39, 40. Interestingly, the resveratrol-diglucuronides trans-resveratrol-2-C-β-D-/4′-O-β-D-diglucuronide and trans-resveratrol-2-C-β-D-/5-O-β-D-diglucuronide, showed a significant impact on SIRT1 transcript levels. However, the quantitatively dominating metabolite in liver tissue in the present animal study with a concentration of 0.07 μM has been shown to be resveratrol-4′-sulfate 28 and this metabolite had no effect on SIRT1 transcript level when tested in a concentration of 10 μM, which was most effective for resveratrol. This result might explain the unaltered SIRT1 protein level in liver tissue of the resveratrol-fed animals compared with that of control animals. In addition, none of the compounds that did increase the SIRT1 transcript levels in HepG2 cells at a concentration of 10 μM, resveratrol and the resveratrol-diglucuronides, were quantified in the liver samples of animals on the resveratrol diet (limit of quantification 0.036 μg/g) 28. Thus, the mechanisms by which resveratrol and its metabolites act on insulin sensitivity and whether these mechanisms behind are dose-dependent need to be clarified in future studies.
In conclusion, the feeding of 300 mg resveratrol/kg bw/day to Wistar rats for 8 wk positively affected insulin sensitivity but showed no significant impact on the total cholesterol, FFA or triglycerides. Additionally, the increased insulin sensitivity is unlikely due to a change in SIRT1 expression since the effective concentration to induce a change in SIRT1 expression by both, resveratrol or its metabolites, is much higher than the actual concentration present in the animal. Further studies will be needed to directly compare SIRT1 transcript levels with protein expression in the liver of rats fed resveratrol and in HepG2 cells incubated with resveratrol and the resveratrol metabolites. Future studies will also need to identify the molecular mechanism by which resveratrol improves insulin sensitivity.
Acknowledgements
This research was financially supported by the German Federal Ministry of Education and Research (BMBF; project no. 0312252R).
The authors have declared no conflict of interest.




