Free-style technique versus computed tomographic angiography-guided perforator selection in deep inferior epigastric perforator flap harvest: A prospective clinical study
Abstract
Background
Computed tomographic angiography (CTA) is the preferred diagnostic tool in preoperative deep inferior epigastric perforator (DIEP) flap assessment, though some surgeons prefer approaching perforator selection with intraoperative findings alone.
Methods
This prospective observational study conducted between 2015 and 2020 assessed our intraoperative decision-making “free-style” technique for DIEP flap harvest. Any patient with indication for immediate or delayed breast reconstruction using abdominally based flaps and who received preoperative CTA was enrolled. Only unilateral cases performed by the same surgeon were considered. Allergy to iodine-based contrast media, renal impairment and claustrophobia were other exclusion criteria. Primary endpoint consisted in comparing operative times and complication rates between free-style technique and CTA-guided approach. Secondary endpoints included evaluation of agreement rate between intraoperative findings and CTA, and identification of variables affecting operative time and complication rate. Demographics, surgical information, agreement versus non-agreement and complications were collected.
Results
Starting from 206 patients, 100 were enrolled. Fifty were assigned to Group A, receiving DIEP flap with free-style technique. The other 50 were assigned to Group B, receiving DIEP flap with CTA-guided perforators selection. Study groups' demographics were homogenous. Operative time was statistically lower (p = .036) in free-style group (252.4 ± 44.77 min vs. 265.6 ± 31.67 min). Complication rates were higher in CTA-guided group (10% vs. 2%) though this was not significant (p = .092). Overall agreement rate in dominant perforator selection between intraoperatively and CTA-based assessment was 81%. Multiple regression analysis showed no variable increased complication rate, though CTA-guided approach, BMI > 30 and harvesting more than one perforator were respectively associated with B-coefficient of 17.391 (2.430–32.351, 95% CI) [p = .023], 3.50 (0.640–6.379, 95% CI) [p = .017] and 18.887 (6.232–31.542, 95% CI) [p = .004], predicting increased operative time.
Conclusions
The free-style technique proved to be a useful tool for guiding DIEP flap harvest with good sensibility in detecting the dominant perforator suggested by CTA without statistically increasing surgery duration and complications.
1 INTRODUCTION
Since its inception, the deep inferior epigastric perforator (DIEP) flap has been the technique of choice for autologous breast reconstruction (BR), gaining popularity for its low morbidity, superior cosmetic outcomes, and aesthetically pleasing donor site defects (Allen & Treece, 1994; Schaverien & Butler, 2017; Wu et al., 2008).
Performing this procedure requires a significant learning curve in terms of flap design, perforator selection and dissection (Laporta, Longo, Sorotos, Farcomeni, et al., 2017a; Rozen & Ashton, 2009; Santanelli et al., 2015). Diagnostic imaging has long been used for preoperative perforator assessment to guide the surgeon and reduce operative times (Blondeel et al., 1998; Hallock, 2003; Pratt et al., 2012; Rozen, Garcia-Tutor, et al., 2010a). Computed tomographic angiography (CTA) in particular has been recognized as the possible gold standard for perforator selection as it provides high-resolution images with 3D reconstructions and a grid system to localize them (Hummelink et al., 2015; Lee & Mun, 2016a; Masia et al., 2006; Renzulli et al., 2020; Rozen, Ashton, Grinsell, Stella, Phillips, & Taylor, 2008b; Rozen, Ashton, Stella, Phillips, Grinsell, & Taylor, 2008a). On the other hand, concerns have been raised regarding CTA utility and efficacy, since several methodological biases in previous studies and technological procedure limitations have been described over time (Boer et al., 2017; Wade et al., 2018). In fact, some surgeons still prefer approaching flap harvest and perforator selection with intraoperative findings alone, while limiting the use of CTA to patients with previous abdominal surgery (Keys et al., 2013; Klasson et al., 2015).
Our team has performed DIEP-based BR since 2004 (Laporta et al., 2015; Laporta, Longo, Sorotos, & Santanelli di Pompeo, 2017b). We translated this experience in our “free-style” technique, used for dominant perforator selection with intraoperative visual feedback alone, without any guidance imaging.
We conducted a prospective observational study where the primary endpoint consisted in comparing operative times and complication rates between the free-style algorithm and the CTA-guided approach. The study was conducted hypothesizing that the free-style approach had OT and CR similar to those in a CTA-based approach. Secondary endpoints included the evaluation of agreement rate between the intraoperative findings and the CTA, and the identification of the variables affecting operative times and complication rates.
2 PATIENTS AND METHODS
Between April 2015 and March 2020, we prospectively enrolled all patients scheduled for immediate or delayed DIEP flap BR at our facility for a prospective observational study, in accordance with the tenets of the Declaration of Helsinki. The study received Institutional Review Board approval by Sapienza University's Ethical Committee (Ref. CE 6881/2022), and was conducted in accordance with the Strengthening The Reporting of OBservational studies in Epidemiology (STROBE) checklist for cohort studies. All patients received and signed an informed consent for undergoing surgery and for participation in the study upon admission, which was on the day before surgery.
2.1 Inclusion criteria
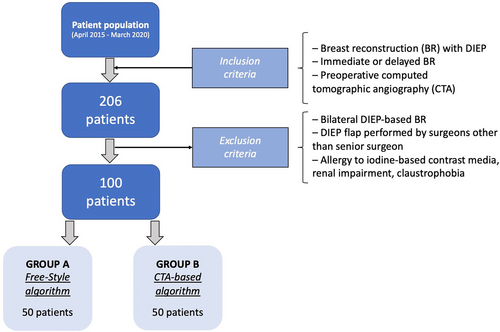
Recruited patients had an indication for undergoing unilateral BR with DIEP flap, that was either performed immediately at the time of mastectomy or that was delayed. All recruited patients received CTA scanning requested for completing oncologic staging or follow-up, but was subsequently used for pre-operative perforator assessment as well.
2.2 Exclusion criteria
To standardize the sample population, we excluded patients requiring bilateral BR. We minimized the impact of operator-based differences, by excluding any patient whose BR was not performed by the senior surgeon (FSdP). Other exclusion criteria included allergy to iodine-based contrast media, renal impairment and claustrophobia.
2.3 Surgical technique
Our unilateral DIEP flap technique consisted in harvesting the flap contralaterally to the mastectomy side, and rotating it by 180° to the chest wall. When available, the superficial inferior epigastric vein (SIEV) was always harvested and anastomosed to enhance venous outflow. The design always extended over the ipsilateral hemiabdomen, including Holm's perfusion zone III in part or in full, but never to zone IV. In immediate BRs, the procedure was carried-out by two teams simultaneously: a team of plastic surgeons and one of breast surgeons.
No limitations were posed in regard to age, BMI, parity, smoking habit, BR timing or need for immediate contralateral balancing. Data collected were: demographics, surgical information (flap weight, timing of reconstruction, type of mastectomy, flap weight, number of perforators) and complications. Two series of 50 patients were enrolled in the study in two consecutive groups: Group A included 50 patients who underwent pre-operative CTA which was blinded to the senior surgeon but was assessed by a second surgeon only to appreciate the presence and patency of deep inferior epigastric artery (DIEA) and vein (DIEV). In this group, DIEP flap was harvested using the free-style technique and all patients signed an informed consent form which asked them to undergo a pre-operative CTA scan that would be blinded to the surgeon. Following the surgery, the senior surgeon reported all intraoperatively visualized perforators across the anterior fascia of the rectus abdominis muscle, using a Cartesian coordinate system used by the radiologist, specifying which one was the dominant. Six months after surgery, the surgeon blindly selected the best perforators from the CTA scans for each enrolled patient in this group. On the other hand, the Group B included 50 patients who underwent CTA-guided perforator selection of DIEP flap.
Patients were further divided according to agreement or non-agreement of the selected perforators, between the intraoperative report and the retrospective (for Group A) or preoperative (for Group B) decision based on CTA scans. We used the findings to assess the sensibility of the free-style technique in detecting the same perforator that would have been deemed appropriate by the same surgeon on CTA evaluation.
We assessed operative times from incision to end of surgery and flap-related complication rate. Flap-related complications were classified in the following manner: fat necrosis, defined as a palpable nodule greater than or equal to 1 cm according to the Rao grading system (Gill et al., 2004; Rao & Saadeh, 2014); partial flap necrosis, defined as fat necrosis >5 cm in diameter or tissue loss >10% of the flap; total flap loss, defined as a total necrosis of the flap, that required its surgical removal. Finally, we studied the impact of demographics, surgical information, CTA versus free-style approach, agreement versus non-agreement on surgery duration and complication rates.
2.4 Free-style technique
Our free-style approach uses direct visualization to guide the perforator selection process, and is based on the following principles: the perforator with the largest vein should be considered as dominant, and thus should be selected. If the perforator with the largest vein is located close to the trans-umbilical line, it should be discarded, regardless of its row, if the dissection of the cranial edge of the flap causes a major bleeding below Scarpa's fascia (Figure 1). If medial and lateral veins are of equal size, the lateral (easy) or the medial (tedious) perforator should be used in case of a small Holm's perfusion zone III. Conversely, the medial perforator should be used in case of large Holm's perfusion zone III. Additionally, in the latter case, the second-best perforator should be included as well. Furthermore, the second-best perforator should be harvested in flaps with a large perforasome III (Rozen, Ashton, et al., 2010b) or in flaps with a design that cross over Holm's perfusion zone III (Holm et al., 2006). If said flaps are based on a dominant medial row perforator (MRP), the second-best perforator can be harvested from lateral row if its dissection requires the incision of less than 1/3 of the rectus abdominis muscle. Otherwise, it can be harvested from the medial row. If the flaps have a dominant lateral row perforator (LRP), the second-best perforator can be harvested from the medial row if its dissection requires the incision of less than 1/3 of the rectus abdominis muscle. Otherwise, from the lateral row instead. In small flaps, the harvesting of a second perforator should be reserved in cases where it entails little to no additional muscle/nerve damage. It should be duly noted that when harvested in series, a type 2 intercostal motor nerve branch might often need to be severed, thus being possibly more invasive than a minor damage only limited to muscle (Rozen, Ashton, Murray, & Taylor, 2008c). A third perforator can be harvested when the conditions allow it, similarly to the harvesting of a second perforator, and when its dissection does not require additional muscle/nerve damage. Small flaps (i.e., that include Holm's zone I and II) should be harvested preferably using a single LRP with the largest vein, or using an MRP if considerably larger. Finally, perforator selection must favor flap perfusion first, even if it comes at the cost of higher abdominal wall morbidity (muscle/nerve damage) and/or worst residual defect.
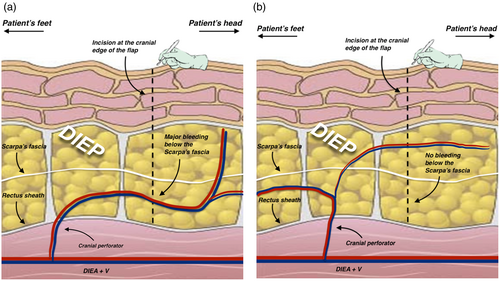
2.5 CTA-guided perforator selection
In the CTA-guided group, the senior author visualized the CTA 1 day prior and immediately before surgery, but not intraoperatively. CTA-guided perforator selection took into consideration the caliber, location and extra/intramuscular course of the perforators. Preference was given to perforators with a large caliber, located not too close to the flap's cranial border and preferably more central, branching into the flap and with a short intramuscular course.
2.6 CTA scanning method and protocol
We specifically chose our equipment to be similar to what has already been described in literature by Phillips et al. (2008), using a Siemens Somatom Sensation 64 tomography scanner (Siemens medical System; Erlangen, Germany) and 100 mL of intravenous Iomeprol 400 (Iomeron®, Bracco UK Ltd.) as contrast medium.
Patients were examined in the supine position, and all angiograms were acquired and reported by the same appointed experienced radiologist. The radiologist identified all perforators below the umbilicus, describing the caliber and branching pattern of the DIEA, according to Moon and Taylor classification (Moon & Taylor, 1988). Using a grid with the umbilicus set as 0-point, the lower abdomen was marked according to a Cartesian coordinate system. X- and Y-coordinates were respectively the horizontal and vertical axes of any point location where each perforator penetrates the anterior fascia of the rectus muscle.
2.7 Statistical analysis
The two groups were analyzed for demographics, flap characteristics, surgery duration and complications using Chi-square for categorical variables and Mann–Whitney U for continuous variables. Logistic multiple regression analysis was used to analyze the impact of all variables on operative time and complication rate. Statistical calculations were performed using SPSS 20 (SPSS, Chicago, IL), considering a p value <.05 as significant.
3 RESULTS
A total of 206 patients underwent DIEP flap-based breast reconstruction between April 2015 and March 2020. Following the exclusion criteria, 100 patients were enrolled in the study, out of which the first 50 were assigned to Group A and the following 50 were assigned to Group B (Figure 2).
The two patient groups were homogenous for age, BMI, smoking history (defined as active smokers if patients partook in the habit within the last year before surgery), parity, flap weight, timing of reconstruction, contralateral balancing, type of mastectomies and number of perforators for flap harvest. Mean follow-up was 57.4 months (range 36.9–73.8) for Group A and 24.0 months (range 13.1–41.6) for Group B. Additional venous drainage was accomplished using SIEV in 33 patients (66%) from Group A and 30 (60%) from Group B.
Group A had a mean operative time of 252.4 ± 44.77 min and flap-related complication rate of 2.0%, consisting of only 1 fat necrosis (2.0%), but no partial (0%) or total flap necrosis (0%). Seventeen flaps (34%) were harvested based on a single perforator, while 27 flaps (54%) were based on two perforators and the remaining 6 flaps (12%) on three perforators. A total of 229 perforators were visualized intraoperatively on the ipsilateral side of the flap pedicle, whereas only 202 were identified using CTA, showing an accuracy of 88.2%.
In Group B, mean operative time was 265.6 ± 31.72 min and flap-related complications were 10.0%, which included one fat necrosis (2.0%), three partial flap necrosis (6.0%) and one case of total flap failure (2.0%). Twenty flaps (40%) were harvested based on one perforator, while 27 flaps (54%) were based on two perforators and 3 flaps (6%) on three perforators. A total of 235 perforators were visualized intraoperatively while only 198 were identified using CTA, showing an accuracy of 84.3%. Patients and flaps characteristics among the two groups are summarized in Table 1.
| Group A (free-style technique) | Group B (CTA-guided) | p value | |
|---|---|---|---|
| Patients number | 50 | 50 | |
| Mean age (years) | 52.9 ± 8.22 | 52.5 ± 10.33 | .874 |
| Mean BMI (kg/m2) | 24.9 ± 3.56 | 25.15 ± 3.32 | .482 |
| Smoking history | 7 (14.0%) | 10 (20.0%) | .424 |
| Nulliparity | 8 (16.0%) | 5 (10.0%) | .372 |
| Flap weight (grams) | 603.4 ± 254.91 | 609.8 ± 175.49 | .508 |
| Reconstruction timing | Immediate: 42 (84.0%) | Immediate: 36 (72.0%) | .148 |
| Delayed: 8 (16.0%) | Delayed: 14 (28.0%) | ||
| Contralateral symmetrization | Yes: 15 (30.0%) | Yes: 9 (18.0%) | .160 |
| No: 35 (70.0%) | No: 41 (82.0%) | ||
| Mastectomy type | NSM: 11 (22.0%) | NSM: 12 (24.0%) | .490 |
| SSM: 14 (28.0%) | SSM: 9 (18.0%) | ||
| MRM: 25 (50%) | MRM: 29 (58.0%) | ||
| Number of perforators | One perforator: 17 (34%) | One perforator: 20 (40%) | .371 |
| Two perforators: 27 (54%) | Two perforators: 27 (54%) | ||
| Three perforators: 6 (12%) | Three perforators: 3 (6%) | ||
| Operative time (minutes) | 252.4 ± 44.77 | 265.6 ± 31.67 | .036 |
| Total complication rate | 1 (2.0%) | 5 (10.0%) | .092 |
| Fat necrosis | 1 (2.0%) | 1 (2.0%) | |
| Partial flap necrosis | 0 (0%) | 3 (6.0%) | |
| Total flap necrosis | 0 (0%) | 1 (2.0%) |
- Note: Operative time was found to be statistically higher in Group B. Bold values indicates Significant values.
Overall agreement rate between dominant perforators reported intraoperatively or those selected retrospectively on CTA (for Group A) or preoperatively (for Group B) was 81%, and formed the agreement group. The remaining 19% of disagreement rate, formed the non-agreement group (Table 2). Group-specific agreement rate was 82% in Group A (41 flaps), and 80% in Group B (40 flaps). Mean operative time was found to be higher in the agreement group than in the non-agreement group (260.9 vs. 250.8 min), though this was not deemed statistically significant [p = .307]. Conversely, complication rate was significantly higher in non-agreement group compared to the agreement group (15.8% vs. 3.7%) [p = .046]. Binary logistic regression analysis showed that none of the included variables statistically increases the complication rate (Table 3). Multiple regression analysis showed that CTA-guided approach, BMI > 30 kg/m2 and harvesting more than one perforator were respectively associated with B-coefficient of 17.391 (2.430–32.351, 95% CI) [p = .023], 3.50 (0.640–6.379, 95% CI) [p = .017] and 18.887 (6.232–31.542, 95% CI) [p = .004]. This suggested that CTA-guided perforator selection, high BMI and the harvesting of more than one perforator predicted an increase in operative time (Table 4).
| Agreement group | Non-agreement group | p value | |
|---|---|---|---|
| Patients number | 81 (81%) | 19 (19%) | |
| Mean age (years) | 53.22 ± 9.17 | 50.47 ± 5.40 | .152 |
| Mean BMI (kg/m2) | 24.99 ± 3.42 | 24.96 ± 3.44 | .975 |
| Smoking history | 9 (11.1%) | 8 (42.1%) | .003 |
| Nulliparity | 10 (12.35%) | 3 (15.79%) | .688 |
| Flap weight (grams) | 608.17 ± 218.21 | 599.74 ± 241.09 | .979 |
| Reconstruction timing | Immediate: 61 (75.3%) | Immediate: 17 (89.5%) | .180 |
| Delayed: 20 (24.57%) | Delayed: 2 (10.5%) | ||
| Contralateral symmetrization | Yes: 23 (28.4%) | Yes: 1 (5.3%) | .034 |
| No: 58 (71.6%) | No: 18 (94.7%) | ||
| Mastectomy type | NSM: 15 (18.54%) | NSM: 8 (42.1%) | .080 |
| SSM: 19 (23.46%) | SSM: 4 (21.2%) | ||
| MRM: 47 (58%) | MRM: 7 (36.8%) | ||
| Number of perforators | One perforator: 32 (39.5%) | One perforator: 5 (26.3%) | .316 |
| Two perforators: 42 (51.8%) | Two perforators: 12 (63.2%) | ||
| Three perforators: 7 (8.7%) | Three perforators: 2 (10.5%) | ||
| Operative time (minutes) | 260.9 ± 37.30 | 250.8 ± 46.47 | .307 |
| Complication rate | Total 3 (3.7%) | Total 3 (15.8%) | .046 |
| Fat necrosis: 2 (2.47%) | Fat necrosis: 0 (0%) | ||
| Partial flap necrosis: 1 (1.23%) | Partial flap necrosis: 2 (10.5%) | ||
| Total flap necrosis: 0 (0%) | Total flap necrosis: 1 (5.2%) |
- Note: Bold values indicates Significant values.
| Odds ratio | 95% C.I. | p value | |
|---|---|---|---|
| Age | 1.024 | 0.898–1.168 | .724 |
| Group B | 2.886 | 0.192–43.460 | .444 |
| Non-agreement | 1.091 | 0.070–17.020 | .950 |
| BMI (kg/m2) | 0.776 | 0.483–1.247 | .295 |
| Smoking history | 11.785 | 0.756–183.760 | .078 |
| Nulliparity | 0.744 | 0.589–1.456 | .354 |
| Flap weight (grams) | 1.004 | 0.997–1.012 | .242 |
| Delayed reconstruction | 1.220 | 0.054–27.753 | .901 |
| Mastectomy type | |||
| SSM | 1.310 | 0.047–36.830 | .874 |
| NSM | 2.320 | 0.077–70.040 | .628 |
| Number of perforators | 0.330 | 0.034–3.245 | .342 |
| Operative time (minutes) | 1.029 | 0.995–1.063 | .094 |
| B coefficient | 95% C.I. | p value | |
|---|---|---|---|
| Age | −.109 | −.997–0.778 | .807 |
| Group B | 17.391 | 2.430–32.351 | .023 |
| Agreement | 13.752 | −6.189–33.695 | .174 |
| BMI (kg/m2) | 3.509 | 0.640–6.379 | .017 |
| Smoking history | −7.446 | −28.021–13.130 | .474 |
| Nulliparity | 11.112 | −11.119–33.342 | .323 |
| Flap weight (grams) | 0.005 | −0.043–0.053 | .836 |
| Timing of reconstruction | −4.415 | −25.771–16.942 | .682 |
| Mastectomy type | 5.034 | −6.506–16.574 | .388 |
| Number of perforators | 18.887 | 6.232–31.542 | .004 |
- Note: Bold values indicates Significant values.
4 DISCUSSION
In the last 15 years, several studies have focused on DIEP flap perfusion, attempting to correlate perforator selection with fewer flap-related complications (Hembd et al., 2018; Lee & Mun, 2018; Mohan et al., 2016; Rozen, Ashton, Stella, Phillips, & Taylor, 2008d). Some authors found MRP to be overall more reliable, not interfering with the course of intercostal nerves, and providing a better blood supply to Holm's zone III (Bailey et al., 2010; Lee et al., 2015; Rozen et al., 2019; Rozen, Ashton, Murray, & Taylor, 2008c) while other authors suggested using LRP, since they usually have an easier dissection through shorter intramuscular courses (Kamali et al., 2017; Lee et al., 2010; Munhoz et al., 2004; Uda et al., 2015; Wong et al., 2010). In our opinion, selecting perforators should be evaluated on a case-by-case scenario, evaluating the many facets correlated to a clinical case. We have crystallized our experience in the form of an algorithm as an alternative to using preoperative CTA, except for cases where deemed strictly necessary (i.e., previous abdominal surgery). This could be particularly helpful in developing areas of the world where preoperative CTA is not used on a routine basis for undeniable costs and infrastructure requirements (Kaviani et al., 2020; Vania et al., 2020).
The free-style technique is based on clinical judgment, and choosing the dominant perforator by visual assessment of the veins' size in the perforator complex. Venous outflow is the major contributing factor for perfusion-related complication in DIEP flaps. This is supported by studies from Rubino et al. who emphasized the correlation between flap weight and vein capacitance, and postulated that although any artery could provide blood supply to a flap, not all veins could drain it (Figus et al., 2012; Rubino et al., 2009). Therefore, larger perforator veins should always be favored, and SIEV should be added when possible.
Regarding our flap design, markings are placed on the hemiabdomen contralaterally to the mastectomy side. Flap is rotated by 180° to place the thickest part of the flap at the lower breast pole, giving it a more natural look without needing to trim the upper pole, which may increase surgery duration and bleeding. In standard cases, we have always found sufficient perforators to perfuse the flap contralaterally and never needed to switch to the ipsilateral hemiabdomen. We did not use couplers to perform anastomoses in our patient population. Regarding recipient vessels, axillary vessels were used in all cases, as we found them to be the ideal recipient site because of reduced operative time and increased possibility to perform a second vein anastomosis. Our recipient vessels of choice are the circumflex scapular vessels, due to following advantages: easy dissection, larger vessel caliber, and optimal flap in-setting (Santanelli Di Pompeo et al., 2015).
The senior surgeon used CTA prior to beginning this study, and surgery duration did not decrease from using CTA after the initial learning curve. Using the free-style technique, the senior surgeon selected intraoperatively the same perforator which was deemed appropriate from CTA assessment in 41 instances for group A, forming the agreement group and showing a sensibility of 82% in detecting the dominant perforator. We reported a similar agreement rate of 80% when using the CTA-guided selection, which confirmed the validity of the free-style technique. In the CTA-guided group, the harvested perforator differed from the one selected preoperatively on CTA in 10 out of 50 cases (20%). Particularly, the plan was changed in seven instances due to intraoperative evidence of a larger perforator which differed from the one recognized on the CTA scan, and in three instances for intraoperative finding of two lateral perforators in series which were deemed appropriate for flap perfusion, without the need for muscle resection. This disagreement between preoperative CTA and intraoperative findings could be attributed to notorious CTA limitations in venous imaging, namely the difficulty in preoperatively identifying “vein-only” or inconsistent venous perforators (Gravvanis et al., 2014). There are some discrepancies in literature concerning the agreement rate of CTA versus intraoperative perforator selection. Some authors report as high as 95.2% (Casares Santiago et al., 2014), 74% (Haddock et al., 2020), or 67.3% (Boer et al., 2017) while others revealed nearly half of DIEP flaps had been harvested with changes from the surgical planning due to insufficient information from preoperative CTA (Keys et al., 2013). Haddock et al. (2020) found in their study that when used for preoperative planning, CTA reduced operative time by over 1 h. However their study only includes bilateral DIEP patients, which is relevant since there is no concern for fat necrosis of zone III. Additionally, the authors mention that no flap was lost in the course of the study although complications were not an endpoint. Finally, all procedures were performed by two senior attending surgeons who routinely use preoperative CTA. The authors speculate that in the blinded group, surgery duration might have been longer because the surgeons felt less confident raising a flap based on a single perforator, tending to isolate more perforators. We believe that experienced surgeons will feel more comfortable approaching the procedure based on whatever learning curve they faced, and their results will improve using a technique they are accustomed to. In our study we did not record individual surgical steps and this can potentially limit our ability to compare our results to that from similar studies. Another potential limitation is how the individual who elaborated the free-style technique is the same individual who performed all surgeries and this may be a bias. Instead, we believe that an experienced surgeon will not taint the results with the effects of a learning curve. Finally, another element from Haddock's study worth reflecting upon is the patients' difference in BMI: mean BMI of 30, while our Italian patients were closer to 25. While it was possible to raise a hefty proportion of our flaps using a single perforator (34% of group A, 40% of group B), that might have been harder to achieve in a population with larger BMI since using a single perforator in these cases might have led to higher complication rate, especially fat necrosis or partial flap necrosis (Lee & Mun, 2016b). However, multivariate analysis from our study showed that BMI did not affect complications. Nevertheless, the relatively small sample population hinders us from drawing conclusions regarding the effect of BMI on complication rate, which has been previously addressed in literature as a potential risk factor for higher morbidity (Patterson et al., 2022).
Rozen and Ashton (2012) believe that limitations in CTA imaging are probably due to how challenging it is to obtain optimal timing for image-acquisition (Pellegrin et al., 2013). It is beyond any shadow of doubt that the CTA introduces a bias linked to the radiologist's expertise, which may not always be consistent. The free-style technique overcomes these imaging difficulties because it strictly relies on the surgeon's skill. Additionally, we found that in CTA approach, even after dissecting the dominant perforator, there comes a time where all other perforators need to be exposed in order to clamp then severe them, thus avoiding unnecessary bleeding. From our standpoint, this does not add time to dissection. Conversely, searching for one specific perforator within a multitude and having to make sure it is the right perforator identified on CTA may add extra time to dissection.
To ensure the homogeneity of cohort groups regarding flap weights and operative time, bilateral DIEP flaps BR were excluded from the study since their perforasome only include Holm's zones I and II, and bilateral procedures are notoriously longer. Additionally, to exclude the possible effect of a learning curve on the results we only included patients operated by the senior author. Upon univariate analysis, we found surgery duration to be statistically lower in the free-style group A where the senior surgeon used the free-style technique for perforator selection. Moreover, multiple regression analysis confirmed that other than increased BMI and harvest of more than one perforator, the CTA-guided approach statistically increases operative time.
We found higher complication rate in the CTA-guided group B (10% vs. 2%) and in the non-agreement group, although this was significant only for the latter. Nevertheless, multivariate regression analysis actually showed that no variables, including the non-agreement group, affect the incidence of complications. This result confirms that even changing the selections of perforator recommended by the CTA does not necessarily increase complications.
The second tenet of the free-style technique lies in choosing a second perforator whenever possible. In our series, selection of a second or third perforator occurred in 33 cases on 50 (66%) for Group A and in 30 cases on 50 (60%) for Group B. As reported in previous vascular studies (Wong et al., 2010), we agree that when performing the reconstruction of a large breast, a second perforator should be selected, reducing the risk of partial flap and/or fat necrosis. A third perforator might help vascularity in large flaps, however what is gained in flap viability may be jeopardized by a reduction in the pedicle's arc of rotation and increase in its risk of kinking (Grover et al., 2014). Even if some authors suggested that one of the benefits of using a CTA-based plan include the selection of perforators with shorter intramuscular course (Casey et al., 2009; Teunis et al., 2013), limitations to this kind of information are still present, such as a steep learning curve to intramuscular vessel interpretation, usually influenced by the increased content of water in the muscle, the evaluation of which eventually lies in the hands of the surgeon rather than the radiologist (Keys et al., 2013).
This study attempted to convert experience derived from our learning curve into a standard approach which could be helpful when a preoperative CTA is not strictly mandatory, or when it cannot be used on a routine basis, which applies to areas of the world where the expenses of this diagnostic tool cannot be covered consistently.
5 CONCLUSION
The free-style technique proved to be a useful tool for guiding DIEP flap harvest with good sensibility in detecting the dominant perforator suggested by CTA without statistically increasing complication rate and surgery duration. Even though larger comparative series are necessary, we believe that the current data attest to the reliability of our technique. Hopefully, our free-style approach can serve other breast microsurgeons well and can be reprised in future research efforts for further validation.
CONFLICT OF INTEREST STATEMENT
We, hereby certify, that to the best of our knowledge no financial support or benefits have been received by author or any co-author, by any member of our immediate family or any individual or entity with whom or with which we have a significant relationship from any commercial source which is related directly or indirectly to the scientific work which is reported on in the article. None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




