Accelerated epigenetic age and shortened telomere length based on DNA methylation in Nicolaides–Baraitser syndrome
Abstract
Background
Nicolaides–Baraitser syndrome (NCBRS) is a rare disorder characterized by neurodevelopmental delays, seizures, and diverse physical characteristics. The DNA methylation (DNAm) profile in NCBRS is significantly different. DNAm is linked to the biological aging of cells and the health risks associated with biological aging. In this study, we examined changes in biological ages in NCBRS to provide insights into the prognosis and health risks of NCBRS.
Methods
We used a publicly available dataset to examine biological aging in NCBRS using DNAm-based epigenetic ages, such as PhenoAge and GrimAge, as well as DNAm-based estimator of telomere length (DNAmTL). We investigated 12 cases, clinically diagnosed as NCBRS, and 27 controls.
Results
Compared to controls, NCBRS cases exhibited significantly accelerated PhenoAge and GrimAge as well as significantly shortened DNAmTL.
Conclusion
These results suggest an acceleration of biological aging in NCBRS and provide insights into the prognosis and health risks of NCBRS.
1 INTRODUCTION
Nicolaides–Baraitser syndrome (NCBRS) is a rare disorder (prevalence < 1/1,000,000) characterized by neurodevelopmental delays (intellectual and speech disability), seizures, and diverse physical characteristics (such as sparse hair, short stature, microcephaly, distinctive facial features, prominent interphalangeal joints, and unusually short fingers and toes; Sousa et al., 2009). This syndrome was first reported in 1993 as being caused by de novo mutations in the SMARCA2 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 2; OMIM number, 600014) gene (Nicolaides & Baraitser, 1993; Sousa et al., 2014; Van Houdt et al., 2012). SMARCA2 constitutes a subunit of a protein complex known as SWI/SWF (SWItch/Sucrose Non-Fermentable), which plays a role in chromatin remodeling and is involved in neurological development and regulation (Sokpor et al., 2017; Son, & Crabtree, 2014).
Epigenetic machinery disorders, including chromatin-remodeling abnormalities as seen in NCBRS, show specific profiles of DNA methylation (DNAm) (Aref-Eshghi et al., 2018; Butcher et al., 2017; Choufani et al., 2015; Siu et al., 2019; Sobreira et al., 2017). Chater-Diehl et al investigated genome-wide DNAm of whole-blood samples from eight NCBRS cases and 23 controls and identified 429 methylated cytosine-phosphate-guanine (CpG) sites that showed significantly different methylation (Chater-Diehl et al., 2019). These CpG sites were located on genes that potentially contribute to NCBRS pathology, such as genes related to neuronal function, cell differentiation, and calcium signaling.
DNAm is linked to biological age, which is used to predict functional capability, mortality, or other health risks more accurately than chronological age. Various methods have been developed to detect biological age that correlates with aging using molecular markers. Among them, DNAm age, also known as the epigenetic clock, has been particularly noted for its usefulness (Jylhävä et al., 2017). Horvath and Hannum independently developed DNAm ages, denoted as HorvathAge and HannumAge, respectively (Hannum et al., 2013; Horvath, 2013). HorvathAge uses 353 CpGs from multiple tissue samples, while HannumAge uses 71 CpGs from blood samples.
Chronological age was used as a surrogate measure of biological age in first-generation DNAm ages (Hannum et al., 2013; Horvath, 2013). Subsequently, PhenoAge and GrimAge have been developed as DNAm ages that are more sensitive to physiological indicators. PhenoAge and GrimAge are better correlated with aging, and they outperform the first-generation tools in terms of predicting mortality and various health risks (Levine et al., 2018; Lu, Quach, et al., 2019). In particular, GrimAge has been reported to outperform other epigenetic clocks in its correlation with age-related phenotype and mortality (McCrory et al., 2021). PhenoAge considers nine physiological measures of phenotypic age (chronological age, albumin, creatinine, glucose, C-reactive protein levels, lymphocyte percentage, mean cell volume, red blood cell distribution width, alkaline phosphatase, and white blood cell count) and relates them to 513 CpG sites on the DNA obtained from blood. GrimAge uses 1030 CpG sites, which are selected based on physiological and stress risk factors (age, sex, DNAm-based smoking pack-years, adrenomedullin [ADM], beta-2-microglobulin [B2M], cystatin C, growth differentiation factor 15 [GDF-15], leptin, plasminogen activation inhibitor-1 [PAI-1], and tissue inhibitor of metalloproteinases-1 [TIMP-1]) as surrogate biomarkers. ADM, B2M, cystatin C, GDF-15, leptine, PAI-1, and TIMP-1 are known to be associated with mortality and health risks, and there are models that allow these to be estimated from DNAm (Lu, Quach, et al., 2019).
An acceleration of DNAm age has been reported in various congenital diseases (such as Down’s syndrome, Huntington's disease, Werner syndrome, and Hutchinson–Gilford progeria syndrome) and neurological diseases (such as Alzheimer’s disease, bipolar disorder, major depressive disorder, schizophrenia, and alcohol use disorder or suicide) (Fries et al., 2017; Fries, Bauer, et al., 2020, Fries, Zamzow, et al., 2020; Han et al., 2018; Horvath et al., 2015, 2016, 2018; Levine et al., 2015; Luo et al., 2020; Maierhofer et al., 2017; Okazaki et al., 2019; Okazaki, Numata, Otsuka, et al., 2020; Okazaki, Otsuka, Horai, et al., 2020; Okazaki, Otsuka, Shinko, et al., 2021).
Telomeres are tandem arrays that preserve the terminals of chromosomes, and they are shortened with each DNA replication in human somatic cells. Therefore, telomere shortening is a candidate biomarker for biological aging (Armanios, & Blackburn, 2012; Fasching, 2018; Mather et al., 2011). Lu et al examined the relationship between DNAm levels and telomere length and created a DNA methylation-based estimator of telomere length (DNAmTL) that calculates the expected telomere length from the methylation level at 140 CpG sites (Lu, Seeboth, et al., 2019). DNAmTL is strongly associated with the measured telomere length, and it is better than using only the telomere length in predicting time-to-death, coronary heart disease risk, and congestive heart failure risk (Lu, Seeboth, et al., 2019). Some reports have indicated that telomere length is associated with age-related diseases (von Zglinicki & Martin-Ruiz, 2005).
Changes in the methylation of CpG sites in NCBRS may indicate acceleration in DNAm ages. If this hypothesis is correct, it may provide insights into the prognosis and health risks of NCBRS. In this study, we examined whether PhenoAge and GrimAge, which are more strongly associated with mortality and disease risk than the other DNAm ages, indicated significant acceleration, and whether DNAmTL indicated significant differences in length in NCBRS cases, using published DNAm datasets (Chater-Diehl et al., 2019).
2 MATERIAL AND METHODS
2.1 Datasets and samples
We obtained the GSE125367 dataset, from the Gene Expression Omnibus (GEO), in which genome-wide DNAm profiles were assessed in whole blood samples using the Illumina MethylationEPIC array (Chater-Diehl et al., 2019). This dataset includes 17 cases with variations in SMARCA2 and 27 neurotypical controls. Of the 17 cases, 12 were clinically diagnosed as NCBRS. They assessed genome-wide DNAm profiles of eight cases, who had pathogenic variations in SMARCA2 and a clinical diagnosis of NCBRS, and 23 controls, selected to be age and sex matched to cases, and generated a model to determine whether the variation in SMARCA2 is benign or pathogenic. This model was applied to the remaining nine cases: four cases with uncertain variants and a clinical diagnose of NCBRS, one case with uncertain variants but not a clinical diagnose of NCBRS, and four cases with benign variants but not a clinical diagnose of NCBRS. As a result, the four cases with uncertain variants and a clinical diagnose of NCBRS were determined to be pathogenic, and the one case with uncertain variants but not a clinical diagnose of NCBRS was determined to be marginal. All of the four patients who were not diagnosed with NCBRS were determined to be benign. Therefore, we analyzed 12 cases with a clinical diagnose of NCBRS and 27 controls.
2.2 Calculation of DNAm-based epigenetic age and telomere length
HorvathAge, HannumAge, PhenoAge, GrimAge, and DNAmTL were used. Measurements were performed using an online calculator (https://horvath.genetics.ucla.edu/html/dnamage/) (Horvath, 2013). Epigenetic age acceleration is defined as the residual calculated by regressing DNAm age on chronological age (AgeAccelHorvath, AgeAccelHannum, AgeAccelPheno, and AgeAccelGrim). The value of the residual can be positive or negative, and it indicates that the DNAm age predicted from the chronological age is either accelerating or decelerating, respectively.
The age-adjusted estimate of DNAmTL (DNAmTLadjAge) is defined as the residual calculated by regressing DNAmTL on chronological age. If the value of the residual was positive or negative, it indicated that DNAmTL was either longer or shorter, respectively, than that expected based on the chronological age.
2.3 Statistical analysis
Statistical analyses were performed using R version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria) and EZR version 1.40 (Saitama Medical Center, Jichi Medical University, Saitama, Japan; Kanda, 2013). The correlation between HorvathAge/HannumAge/PhenoAge/GrimAge/DNAmTL and the chronological age was investigated using the Pearson's correlation coefficient. Mann–Whitney U-test was used to test for differences in AgeAccelHorvath, AgeAccelHannum, AgeAccelPheno, AgeAccelGrim, and DNAmTLadjAge between the case and control groups. Multiple regression analysis was used to adjust for the confounding variables (sex and age). We defined two-tailed p < .05 as statistically significant. Dummy variables were used as necessary.
3 RESULTS
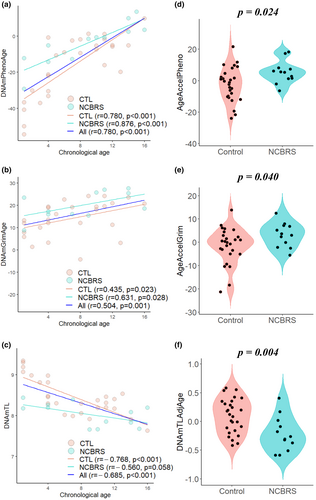
The demographic data of cases and controls are shown in Table 1. The correlations between PhenoAge/GrimAge/DNAmTL and chronological age were significant except for a negative correlation trend in the NCBRS group of DNAmTL (Figure 1). Mann–Whitney U-tests showed that AgeAccelPheno was significantly increased and that DNAmTLadjAge was significantly decreased in NCBRS (Table 1). In multiple linear regression analyses, AgeAccelPheno (estimate = 8.326, p = .024) and AgeAccelGrim (estimate = 5.185, p = .040) were significantly increased in NCBRS. DNAmTLadjAge (estimate = −0.330, p = .004) was significantly decreased in NCBRS (Table 2).
| NCBRS median (IQR) | CTL median (IQR) | p-value | |
|---|---|---|---|
| n | 12 | 27 | |
| Sex (Male/Female) | 5/7 | 14/13 | .731a |
| Age, median (IQR) | 8.5 (4, 14) | 7.0 (4, 11) | .351b |
| AgeAccelHorvath, median (IQR) | 0.314 (−1.704, 2.006) | −0.176 (−1.495, 0.877) | .663b |
| AgeAccelHannum, median (IQR) | 1.149 (−1.326, 2.330) | −0.096 (−4.396, 3.091) | .708b |
| AgeAccelPheno, median (IQR) | 5.439 (1.494, 7.591) | −1.134 (−9.993, 3.400) | .024 b |
| AgeAccelGrim, median (IQR) | 3.345 (−0.628, 6.966) | −0.046 (−4.235, 2.632) | .065b |
| DNAmTLadjAge, median (IQR) | −0.283 (−0.408, −0.061) | 0.122 (−0.133, 0.329) | .007 b |
- Note: p-values shown in bold are significant at < 0.05.
- Abbreviations: CTL, control; IQR, interquartile range; NCBRS, Nicolaides–Baraitser syndrome.
- a p-value was calculated with Fisher’s exact test.
- b p-value was calculated with Mann–Whitney U-test.
| Phenotype | Sex | Age | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p-value | Estimate | SE | p-value | Estimate | SE | p-value | |
| AgeAccelHorvath | 0.867 | 0.926 | .356 | −1.737 | 0.843 | .047 | −0.016 | 0.090 | .861 |
| AgeAccelHannum | 1.168 | 1.809 | .523 | −1.399 | 1.646 | .401 | −0.021 | 0.176 | .908 |
| AgeAccelPheno | 8.326 | 3.539 | .024 | −2.089 | 3.222 | .521 | −0.139 | 0.345 | .689 |
| AgeAccelGrim | 5.185 | 2.430 | .040 | −3.904 | 2.212 | .086 | −0.077 | 0.237 | .709 |
| DNAmTLadjAge | −0.330 | 0.108 | .004 | 0.059 | 0.098 | .549 | 0.005 | 0.011 | .604 |
- Note: p-values shown in bold are significant at < .05.
- Abbreviation: SE, standard error.
We additionally addressed the first-generation clocks: HorvathAge and HannumAge. AgeAccelHorvath and AgeAccelHannum did not show significant difference between the NCBRS and control groups (Supplementary Figure S1, Tables 1 and 2).
4 DISCUSSION
To the best of our knowledge, this is the first study to report the differences in epigenetic age and telomere length in NCBRS. We found increased AgeAccelPheno and AgeAccelGrim as well as decreased DNAmTLadjAge in the NCBRS group compared to controls. This indicated that the altered DNAm profile is associated with the biological age acceleration in NCBRS.
PhenoAge correlates with all-cause mortality, cancer, cardiovascular disease, and chronic respiratory disease (Levine et al., 2018). GrimAge is superior in predicting time to death and risk for coronary artery disease and cancer (Hillary et al., 2021). DNAmTL also significantly correlates with all-cause mortality and time to onset of coronary artery disease and congestive heart failure (Lu, Seeboth, et al., 2019). Although there is no exhaustive study on the prognosis of NCBRS, the original case reported by Nicolaides and Baraitser died by status epilepticus and respiratory failure at 33 years (Sousa et al., 2014). It has also been suggested that some of the features of NCBRS are progressive (Sousa et al., 2009). Our results suggest accelerated biological aging and associated health risks in NCBRS, and this may provide insights into the prognosis for NCBRS.
We observed significant changes in AgeAccelPheno and AgeAccelGrim, but not in AgeAcceleHorvath and AgeAccelHannum. HorvathAge and HannumAge were developed to estimate the chronological age. In contrast, PhenoAge and GrimAge were developed to capture age-related phenotype and mortality risk, respectively. Our findings suggest the benefit of using multiple epigenetic clocks for a multifaceted understanding of the disease.
Chater-Diehl et al investigated DNAm-based blood cell composition including CD8 + T cells, CD4 + T cells, natural killer cells, B cells, monocytes, and granulocytes, and found a decrease in CD4 + T cells and a more significant increase in monocytes in NCBRS cases compared to controls (Chater-Diehl et al., 2019). We additionally estimated naive CD8 + T cells, exhausted CD8 + T cells, naive CD4 + T cells, and plasma blasts, in addition to the above six cell types, by the online epigenetic calculator (Horvath, 2013; Houseman et al., 2012). We observed a significant increase in monocytes in NCBRS, in accord with the work of Chater-Diehl et al. (2019) (Supplementary Figure S2 and Supplementary Table S1).
Our study has several limitations. First, the data we used were obtained only from whole-blood samples, and samples from other tissues were not examined. Thus, our results need to be reproduced and validated in samples derived from other tissues. Second, we only analyzed 12 cases of NCBRS. This sample size is relatively small. This is a limitation that could be attributed to the rare occurrence of NCBRS; however, it is still necessary to measure the DNAmAge in more cases to validate the results of our study.
In conclusion, we analyzed the biological age and telomere length based on DNAm in NCBRS and found accelerated PhenoAge and GrimAge as well as shortened DNAmTL in NCBRS. These results suggest an acceleration of biological aging in NCBRS, and it may contribute in the prognosis and better understanding of the health risks of NCBRS.
ACKNOWLEDGMENTS
We thank Yasuko Nagashima for technical assistance. This research was partly supported by JSPS KAKENHI, grant numbers 15K19727 and 18K15483.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Satoshi Okazaki designed the research. Akitoyo Hishimoto managed the research. Yutaka Shinko conducted statistical analyses and wrote the first draft of the paper. Ikuo Otsuka, Tadasu Horai, Saehyeon Kim, and Takaki Tanifuji were involved in the analysis, interpretation, and writing. All authors contributed to, reviewed, and approved the final version of the manuscript for publication.
Open Research
DATA AVAILABILITY STATEMENT
The publicly available GSE125367 dataset (Chater-Diehl et al, 2019) was downloaded from the Gene Expression Omnibus database (GEO, https://www.ncbi.nlm.nih.gov/geo).