Biodegradable conducting polymeric materials for biomedical applications: a review
Abstract
The biodegradable conducting polymers have attracted considerable attention for the last two decades, as a unique class of new biomaterials for emerging both the properties of conducting polymers, that is electrical conductivity, and biocompatible polymers, that is biodegradability. These new biomaterials emerged as the best option for immense potential in biomedical applications. Here, we review the recent developments towards biodegradable conducting polymers and their applications in biomedical fields. Firstly, we introduce the biomaterials and their biocompatibility, followed by a short discussion on conducting polymers and their new hybrid biodegradable conducting polymers along with their applications in biomedical fields such as tissue engineering, drug delivery, biosensors and antibacterial activity. At last, we sum up this review with current challenges and future perspectives.
1 INTRODUCTION
Biomaterials are natural or synthetic materials that are biodegradable and interact cordially with biological systems (Qu et al., 2019). These can be well-defined as a material envisioned to interface with biological systems for evaluation, treatment and replacement of organs, tissues or function of the body (Basu & Ghosh, 2017; Reis et al., 2016). They play a key role in the human health system which is attained from nature or the environment (Mir et al., 2018). Generally, biopolymers are the main class of biomaterials as biopolymers are biocompatible, biodegradable and human body-friendly (Rebelo et al., 2017). These biopolymers are further classified as biodegradable and non-biodegradable according to their nature (Andreeßen & Steinbüchel, 2019; Kabir et al., 2020).
Due to their outstanding biocompatibility, biodegradable polymers are known as the best candidate for biomedical applications such as drug delivery systems, vascular grafts, surgical sutures, artificial skin, bone fixation devices, gene delivery systems, tissue engineering and diagnostic applications. Biomedical engineering is an attractive branch which deals with engineering and biology together to put on engineering materials and rudiments in the field of healthcare and medicine. Tissue engineering is also a part of it that fulfils the purposes of recovering or exchange failing or broken body tissue by merging cells, scaffolds and bioactive molecules. Scaffolds should combine various functions such as biodegradability, biocompatibility, ideal mechanical strength, adequate porosity for small molecule transportation, controllability, implantation and sterilization. Hence, natural polymers such as gelatin and chitosan are the perfect match for scaffold fabrication and synthetic aliphatic polyesters too. However, these revealed poor hydrophilicity which limits the cell attachment on surfaces and lack of sites for covalent bonding. While having their biodegradable nature, biopolymers or conventional polymers are insulators in nature so that limits the use in some biomedical applications where conducting properties of biomaterials is indispensable (George et al., 2020). In many applications of implants in the body, such as in bone fixing materials, dental materials and bone substitution materials, there is a need for such type of material showing long-term stable performance. In other applications such as controlled drug delivery, regenerative medicine, tissue engineering and gene therapy, there is a need for some new properties in biomaterials along with biodegradability (Cha et al., 2019; Prakasam et al., 2017; Ribas et al., 2019).
Since the early 20th century, a new class of materials has been widely used as new biomaterials such as synthetic polymers, composites, ceramics, alloys, metals and carbons (Kunčická et al., 2017; Teo et al., 2016). These new biomaterials revealed enhanced and outstanding mechanical properties, high reproducibility along with improved biological and chemical performance, and functionality. These have diverse application fields such as tissue engineering, drug delivery, biosensors and artificial muscles according to the living organism's component with which these new biomaterials do interaction for the medical diagnosis and treatment (Montero de Espinosa et al., 2017).
Researchers showed extensive interest in the biomedical field to fabricate such polymer systems having both electrically conducting and biodegradable properties. Such type of systems has been considered as an auspicious biomaterial due to its compatibility with the biological environment which is the essential requirement for biomedical applications and electrical conductivity too (Boni et al., 2018). Along with biodegradability, these materials revealed some outstanding advantages such as good processability, chemical stability, low production cost and electrical conductivity, which allowed these materials to be used in numerous fields of engineering (Hackett et al., 2017; Puiggalí-Jou et al., 2019). In the current review, we will discuss such types of biodegradable conducting polymers, their advantages over conventional polymers and their applications in the biomedical field.
2 BIOMATERIALS
Biomaterials are the substances, highly used in the multidisciplinary field having a wide area of applications such as diagnostics (biosensors, gene arrays), medical materials (surgical tools, blood bags), emerging regenerative medicine (tissue engineering) and therapeutic purposes (medical devices, implants) (Chen & Zou, 2019; Yousaf et al., 2020). Biodegradability is one of the chief features of a biomaterial. This property is generally present in polymeric materials by breaking the polymeric chain and loss of their weight by some environmental conditions, enzymes, living organisms or simple water molecules (Larrañaga & Lizundia, 2019). Biodegradability in materials is highly desirable for using them in biomedical applications and devices where they complete their given task and automatically fade away from the body or eliminated itself.
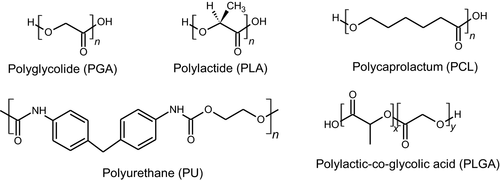
In biomedical applications, synthetic polymers such as aliphatic polyesters are commonly used as biodegradable material due to its biocompatibility with the biological system. Generally, ester groups present on their backbone hydrolysed during degradation. Tailoring of degradation products and degradation rates can be tuned according to structure, composition and molar mass (Englert et al., 2018; Hacker et al., 2019). Some most common biodegradable polymers are widely studied such as polyglycolide (PGA) (Liu et al., 2019), polylactide (PLA) (Bergström & Hayman, 2016; Chen et al., 2018), polycaprolactam (PCL) (Fakhri et al., 2018) and polyurethane (PU) (Javaid et al., 2020) (Figure 1).
3 CONDUCTING POLYMERS
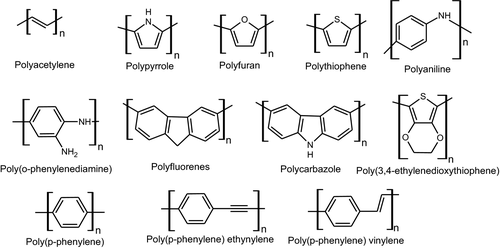
Conducting polymers are well known for outstanding electrical and optical properties such as inorganic semiconductors and metals (Pal et al., 2020). Also, they possess properties of common polymers such as their easy preparation, functionalization and processability (Anantha-Iyengar et al., 2019; Jadoun & Riaz, ; Park et al., 2019). Some common conducting polymers are classified as polyacetylene (PAc) (Wang, Sun, et al., 2019), polypyrrole (PPY) (Jadoun et al., 2018), polyaniline (PANI) (Jangid et al., 2020), poly(o-phenylenediamine) (POPD) (Riaz et al., 2017)), polythiophene (PTH) (Jadoun & Riaz, 2019), polyfuran (PF) (Ozkazanc & Ozkazanc, 2020), polycarbazole (PCz) (Jadoun et al., 2017), poly(3,4-ethylenedioxythiophene) (PEDOT) (Chen et al., 2020), polynaphthylamine (PNA) (Jadoun et al., 2017 b; Jadoun et al., 2017c) and polyanisidine (PANIS) (Jadoun et al., 2018), (Figure 2).
Conducting polymers were found to have great potential in biomedical applications such as tissue engineering during the 1990 s due to its behaviour to modulate cellular activities like proliferation, cell adhesion and differentiation via electrical stimuli (Boni et al., 2018; Guimard et al., 2007; Stenger-Smith, 1998; Wong et al., 1994). Due to their electrical properties and soft interface, conducting polymers have replaced traditional electrodes made up of conductors/semiconductors such as platinum, gold or glassy carbon (Le et al., 2017).
Studies of conducting polymers were performed on bones, muscles, nerves, mesenchymal cells, cardiac cells, etc., due to the sensitive nature of these cells or tissues with electrical stimulation (Alegret et al., 2018; Ferrigno et al., 2020; Petty et al., 2020; Tomczykowa & Plonska-Brzezinska, 2019), though brittle nature, non-biodegradability, non-processability in common organic solvents as well as weak molecular interaction with cells, topography and mechanics limit its use in biomedical applications as biomaterials (Iqbal & Ahmad, 2018).
To overcome these problems, common conducting polymers were studied and developed in form of copolymers, composites and blends with some biodegradable and biocompatible polymers which were natural or synthetic. Therefore, to improve their biocompatibility, chemical modification of monomers with various biomolecules or doping the conducting polymers with precise counter ions, which are known as biodopants, are key tactics (Kenry & Liu, 2018). These newly tailored materials were named as biodegradable conducting polymeric materials having mixed advantageous properties of both conducting polymers and biodegradable polymers (da Silva & Córdoba de Torresi, 2019).
4 NEW BIODEGRADABLE CONDUCTING POLYMERS
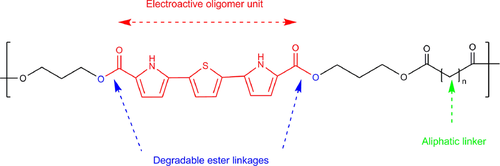
Biodegradable conducting polymers are new generation electroactive biomaterials that are based on intrinsically conducting polymers and biodegradable materials. Conducting polymers possesses outstanding electrical and optical properties which are excellent for biomedical applications. On the other hand, biodegradability and biocompatibility are a desirable and essential parameter for biomedical applications. Hence, conducting polymers such as polyaniline, polypyrrole and polythiophene and biodegradable polymers such as poly (D, L-lactic acid) (PDLLA) and polycaprolactone (PCL) combined by ester linkages, appeared as a promising candidate due to tailored properties of both the polymers and attracted the attention of researchers as sustainable alternatives for application in medical fields (Figure 3). Some approaches which have been applied to overcome the problem of biodegradability and electroactive performances are (i) block copolymers in which modified electroactive oligomers of conducting polymers were connected with degradable ester linkages and (ii) copolymerization of altered biodegradable and electroactive macromonomers based on polyesters prepared in the first step, with conducting polymers (da Silva & Córdoba de Torresi, 2019).
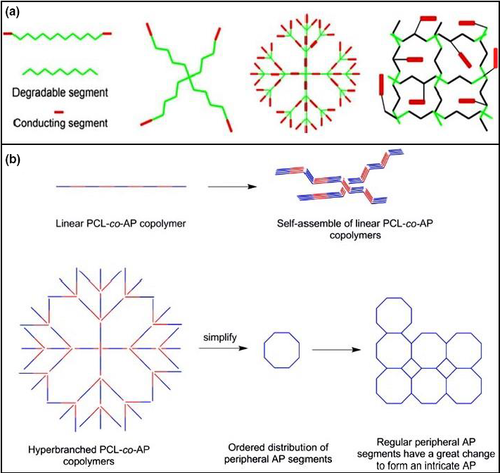
On the other hand, in the performance of any copolymers or functionalized polymers, architectures also play a key role in its functionality and properties. Architecture diversity is a must for achieving the desired properties such as degradation, optimal, mechanical, thermal and biological properties for any specific biomedical application (Wang, Lu, et al., 2019). Several linear (Guo et al., 2011), star-shaped (Guo et al., 2010), hyperbranched (Guo et al., 2010) and cross-linked (Guo et al., 2011) biodegradable polymers have been synthesized by combining the degradability of aliphatic polyesters and electroactivity of conducting polymers based on PCL, PLA and aniline oligomers (Figure 4 (a) and (b)).
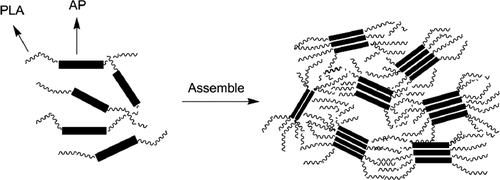
In the sense of well-defined architecture, self-assembly of polymers is a newly emerging field having great potential in biomedical sciences (Sarode et al., 2020; Tian & Zhang, 2019). Self-assembled copolymers of diblock oligomers of tetraaniline and poly(L-lactide) were synthesized via using tetraaniline as an initiator for ring-opening polymerization of L-lactide and studied their performance on self-assembly in chloroform solvent (Wang et al., 2006). A good electroactive and biodegradable triblock copolymer, PLA-b-AP-b-PLA was synthesized which was able to endure self-assembly and produce a micro-phase partition. Hence, the hard AP (aniline pentamer) parts created discontinuous fields while the soft PLA (polylactide) parts formed a continuous matrix by aggregating together (Figure 5). In this form, electrical conductivity flow in AP parts and in between the bridging of two AP, it occurred through the PLA matrix by the tunnel effect, resulting in the decrement of conductivity in PLA-b-AP-b-PLA (Huang et al., 2007).
5 SYNTHESIS AND PROPERTIES OF BIODEGRADABLE CONDUCTING POLYMERS
Synthesis of biodegradable conducting polymers using conducting materials and degradable materials can be done by various methods such as blending, copolymerization and composite formation. A biodegradable conducting polymer was synthesized by emulsion polymerization followed by precipitation using electrically conducting polypyrrole (PPY) nanomaterials and biodegradable poly(D, L-Lactide) (PDLLA) (Shi et al., 2004). To overcome the problem of excretion through the renal system, Repenko and coworkers introduced the degradable imidazole units into the thiophene backbone by Sonogashira dispersion polymerization and they have also done this reaction using non-degradable benzene unit in the thiophene backbone (Figure 6). This synthesized material with the imidazole unit was found bio-degraded by activated macrophages. The morphology for both was found different for degradable and non-degradable as well as containing side chain of OMe and OEG. The polymeric backbone was scission by reactive oxygen species at the imidazole unit, hence completely degraded into low molecular soluble fragments and was applicable for the fields of theranostics, drug delivery and medical imaging (Repenko et al., 2017).
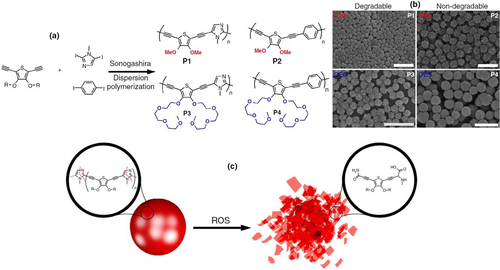
Copyright
2017 Nature).Schmidt et al. (Rivers et al., 2002) synthesized a block copolymer using oligomers of thiophene and pyrrole (conducting) and ester linkage (degradable). They used two hydroxyl groups at each end of conducting oligomers and copolymerized these further with degradable adipoyl chloride. The synthesized copolymer was degraded in PBS (phosphate-buffered saline) with esterase designated the biodegradable nature of these by enzymes found in the body. On these copolymer films, human neuroblastoma cells were developed which unveiled the non-toxic nature of copolymer films. In vivo biocompatibility studies also suggested nontoxicity during the degradation. These biodegradable conducting material's films were conducting in nature and showed 10−4 S/cm conductivity when doped by iodine. To enhance the conductivity of these materials, the new copolymer of adipoyl chloride and quaterthiophen oligomer were synthesized which were able to doped by ferric perchlorate (Fe (ClO4)3) or ferric chloride (FeCl3). In these dopants, counter ions ClO4– and Cl− are biocompatible as well as iron has also the least toxicity. When the material was exposed to cholesterol esterase, the erosion started after 1–2 weeks on the surface. The copolymers were studied with Schwann cell culturing for checking its cytocompatibility for 48 h and revelled no short-term toxicity for copolymer (Guimard et al., 2009).
Further, these authors reported a two-step synthesis of biodegradable conducting copolymers by using various molecular weights of polycaprolactam and tetramers of aniline monomer by ring-opening polymerization followed by oxidative coupling reaction. In this scheme, the ring opening of caprolactam was initiated by aniline dimer with the help of Sn(Oct)2 catalyst. The reason for using an aniline dimer is the low molar mass which was dissolved in caprolactam, hence no need of an organic solvent in polymerization. The conductivity for the above copolymer was found in the range 6.30 × 10−7 −1.03 × 10−5 S/cm depends on the aniline used and can be tuned by appropriate choice of monomer content. In the first step, if a crosslinker 2, 2-bis-(ε-caprolactone-4-yl) propane was added then a good conductive network, as well as degradable moiety, was obtained (Guo et al., 2011).
One-pot synthesis of an electroactive macromonomer was done by using PLLA with EDOT functional end groups via organometallic and enzymatic polymerization approach with stannous octanoate [Sn(oct2)] and Amano lipase Pseudomonas cepacia (PS-IM), Candida antarctica lipase B (CAL-B) catalysts, respectively (Figure 7 (a) and (b)). To initiate the ring opening of lactide to achieve PLLA with EDOT functionalized end group, (2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methanol (EDOT-OH) was used. The structure and representation of copolymer (PEDOT-co-PLLA) with assigned letters of 1H NMR spectroscopy are shown in Figure 7 (c). Analysis results revealed the importance and effectiveness of the biocatalytic synthetic route on environmental impact for short time applications (Silva et al., 2018). Likewise, various other biodegradable conducting polymers were synthesized by using conducting polymers and biomaterials for applications in various biomedical fields (Sharma, Kalia, et al., 2015).
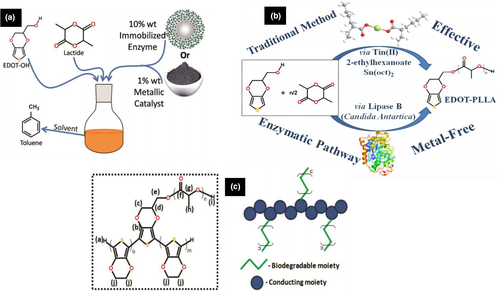
Copyright
2018 Elsevier).6 BIOMEDICAL APPLICATIONS
6.1 Tissue engineering
Tissue engineering is an interdisciplinary field that applies the principles of life sciences and engineering for the development of biological alternatives to reinstate, sustain or improve the tissue function. Biodegradable scaffolds play a critical role in tissue engineering applications (Almouemen et al., 2019). To avoid the removal surgery of implant and to avoid a permanent and chronic immune response, biodegradability is needed. The materials which possess biodegradability, biocompatibility and great processing flexibility are key properties for a scaffolding biomaterial (Armentano et al., 2010). Beside biodegradability, for better tissue repairing, there is a need of electrical conductivity within three-dimensional scaffolds as electrical stimulation provides a favourable impact on the behaviour and function of electroactive tissues. For cell adhesion, their growth and for tissue regeneration, the development of scaffolds needs the chemical, mechanical and electrical properties. All the tissues such as skeletal muscle, cardiac, lung and nerve possesses conductivity in between 0.03 and 0.6 S cm−1. The surface properties of biomaterials play a key role in cell-substrate interactions and cell activities. Hence, it is a challenging task to keep the cells on surface of the material rather than in their scaffolds. By using conducting materials, these targets can be attained (Broda et al., 2011; Mostafavi et al., 2020; Ning et al., 2018).
Rivers et al. (Rivers et al., 2002) synthesized a novel conducting biodegradable polymers via connecting conducting oligomers of thiophene and pyrrole with degradable ester linkages to achieve a new biomaterial which has the capability to intimate biological and electronic interfacing with tissues and cells. These worked fabulously to stimulate tissue regeneration via a temporary scaffold for cell attachment and helps in generating electrical signals which made it perfect in tissue engineering applications. Polyaniline was combined with chitosan to form nanocomposites for use in a potential biomedical application. To induce bioactivity for improved cell–biomaterial interactions, these chemically synthesized PANI/chitosan nanocomposites were modified on surface by glycine N-hydroxysuccinimide (NHS) ester (Figure 8). This surface modification was responsible for enhanced fibroblast adhesion, morphology, proliferation and spreading which was confirmed by scanning electron microscopy and confocal microscopy which unveiled the use of nanocomposites as tissue engineering applications (Borah et al., 2020).
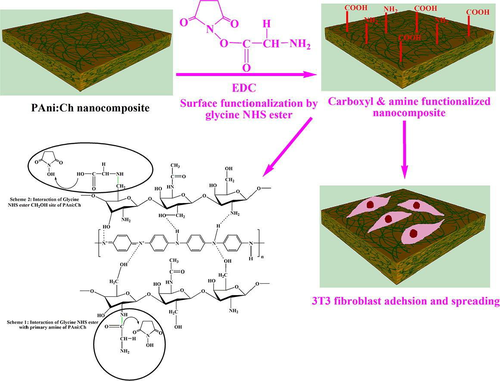
Copyright
2020 Elsevier).A novel, biodegradable and electroactive copolymer (PEDOT-co-PDLLA) was prepared by using persulphate as an oxidant. Based on conductivity measurements, cytotoxicity and in vitro biodegradability, these copolymers exhibited charge density properties and surface chemistry and had great potential for using as scaffold materials in numerous areas of applications, exclusively for neuronal tissue engineering (da Silva et al.., 2018). Schmidt et al. (Schmidt & Rivers, 2004) patented the fabrication of novel biodegradable conducting polymers by using pyrrole and thiophene segments of conducting polymers and ester linkages of aliphatic chains. These functionalized polymers were applicable in various fields of biomedical science such as peripheral nerve regeneration, tissue engineering including wound healing, muscle tissue stimulation, bone repair, spinal cord regeneration, and many other therapeutic purposes. Poly(lactide-co-glycolide) (PLGA) and poly(3-hexylthiophene) (PHT) were modified together for development of electrically conducting nanofibres. In vitro studies of these aligned nanofibres revealed their impact on the adhesion and proliferation of Schwann cells. Hence, PLGA-PHT fibres were applicable in scaffold for neural regeneration (Subramanian et al., 2012). Aniline pentamer and polylactide were used to synthesize a biodegradable and electroactive triblock copolymer, PLA-b-AP-b-PLA, by a condensation reaction. The conductivity of these was determined to be ∼5 × 10−6 S/cm, and this was due to microphase separation of the two-block parts in the PLA-b-AP-b-PLA. This system was biodegradable, biocompatible as well as processable and soluble in most of the organic solvents, hence could be potentially applicable in scaffold materials for cardiovascular or neuronal tissue engineering (Huang et al., 2007). Xie et al. (Xie et al., 2015) presented a polymer network made up of aniline trimer and star-shaped polylactide for applications in bone tissue engineering. An electroactive polymer using polyurethane and aniline pentamer was reported for studying the electrical signals on activities of cells and for tissue engineering applications (Baheiraei et al., 2014).
6.2 Biosensors
Sensors and electronics which can produce flexible interfaces with some biological environment with maintaining biocompatibility and stability are high in demand. The combination of a biocompatible material with conducting polymers offers a wide platform with outstanding properties with advantageous functionality for bioelectronics and biosensors. The reason for the feature of biodegradability in any of the biosensors is the naturally disappearance in respective environments with controllable degradation ability. These biodegradable sensors have been widely used as temporary implants for collecting crucial physiological changes like potential, strain, temperature, oxygen content, blood pressure, pH and fluorescence. Also, these can diminish the possible risks and long-lasting inflammation triggered by conventional implantable devices as well as evade the disturbance and pain which can be begun by removal of device from patients and decrease medical expenses (Chen & Ahn, 2020; Hwang et al., 2015; Rodrigues et al., 2020). Likewise, electrically conducting polymers have various properties which allow them to behave as outstanding material for fast electron transfer and immobilization of any biomolecules for the fabrication of efficient biosensors. These are also useful in diagnostics to measure vital analytes by enhancing the speed, sensitivity and flexibility of biosensors (Borole et al., 2006; Gerard et al., 2002).
In this regard, Pal and lab mates presented a fully organic and biocompatible microfabricated device based on water-based photolithography by using PEDOT: PSS as a conducting polymer and natural silk proteins as a biomaterial in carrier protein form. This flexible biosensor showed brilliant stability and electrochemical activity for about one month and revealed excellent nonspecific recognition of ascorbic acid and dopamine with high sensitivity. After encapsulating glucose oxidase enzyme at ambient temperature and biological pH, it worked as an extremely selective and sensitive glucose biosensor. This biosensor was exceptionally outstanding in retaining their properties with repetitive mechanical deformations and degradable with enzymatic deeds. By tuning the biodegradability and having excellent mechanical properties, these devices were said to be ‘implant and forget’ type device and can be applied in smart skin or implantations (Pal et al., 2016).
6.3 Antibacterial and antimicrobial application
To overcome the packaging waste, which is caused due to non-biodegradable materials and is a weighty part of municipal solid waste and also harm the environment, biodegradable polymeric materials from renewable sources have been paid much attention (Zhong et al., 2020). The degradation of these materials can be induced by microorganisms. Due to the high cellular response, conducting polymers are recently used for antibacterial/antimicrobial application (Das & Prusty, 2012; Maráková et al., 2017). Nanocomposites of polyaniline, cellulose and cobalt ferrite were found to show good antimicrobial activity against Bacillus subtilis and Escherichia coli, along with Candida albicans as unicellular fungi. These nanocomposites were conducting (3.5 × 10−3 S/cm) in nature having biodegradability too. With increasing the content of cobalt ferrite in nanocomposites, biodegradability was found to decrease but antimicrobial nature was increased (Abou Hammad et al., 2019). In other studies, a copolymer of polypyrrole and chitosan was chemically synthesized and characterized by spectral, thermal and morphological analysis. Strong interactions between PPY and CS were observed in the copolymer chains and the electrical conductivity of CS was found to increase due to grafting. Antibacterial activity of PPY-co-CS was enhanced dramatically in comparison with PPY and CS and showed antibacterial activity stronger than trimethoprim (25 mg), rifampicin (5 mg) and penicillin (10 mg) (Figure 9). These copolymers showed equivalent antibacterial activity with erythromycin (15 mg) and amikacin (30 mg) antibiotics (Cabuk et al., 2014).
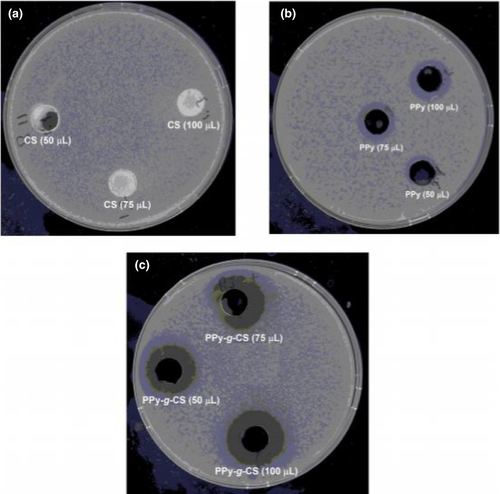
Copyright
2014 Elsevier).Polyaniline-graft-chitosan copolymer was fabricated by using ammonium persulphate (NH4)2S2O8 as initiator and was characterized for checking its conductivity and biocompatibility. These copolymers were tested against Escherichia coli and displayed an enhanced antibacterial activity in comparison with pristine CS and PANI (Cabuk et al., 2014). Likewise, various copolymers with chitosan were synthesized by Cabuk and coworkers by using pyrrole, thiophene, and aniline monomers and FeCl3 or (NH4)2S2O8 as initiators. The grafting efficiency, yield, dielectric constants, densities and particle sizes of the copolymers were determined by various analysis techniques and got the improved thermal stabilities and conductivities of copolymers. The synthesized polythiophene-g-chitosan, polypyrrole-g-chitosan and polyaniline-g-chitosan were tested against Bacillus megaterium,Escherichia coli,Klebsiella pneumoniae,Staphylococcus aureus and Enterococcus faecalis, microorganisms and surprisingly higher or equivalent antibacterial activities were achieved against the microorganisms equated with erythromycin, penicillin, rifampicin amikacin and trimethoprim antibiotics (Cabuk et al., 2015). Biodegradable conducting hydrogels were developed by using polyaniline and guar gum/acrylic acid hydrogel network which revealed outstanding applications against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus (Sharma, Kaith, et al., 2015).
6.4 Drug delivery
Drug delivery is an aggressive field with requirement for extra effective biomaterials for drug delivery. Biodegradability is a key feature in drug carrier applications where temporary implant in only needed to evade the removal surgery and breakdown of material within the body to control drug release, and to improve cell integration. Biodegradable materials provide a harmless framework for delivering medicine safely to the body (Hivechi et al., 2019; Kopeček, 1984). On the other hand, conducting polymers are widely used in drug delivery for targeting a tissue through charge mediated control of drug motion. The electroactive materials offer a strong technology stage by merging the electrical feature with other processing methods. Through electrostatic interactions, conducting polymers revealed the capacity to move charged molecules to their backbone and is controllable by the oxidation and reduction state of the conducting polymers. Hence, upon electrical or electrochemical stimuli, this mechanism helps in vigorously control drug release (Svirskis et al., 2010; Uppalapati et al., 2016).
Some researchers adopted the route of formation of biodegradable conducting polymers by combining some natural/synthetic biodegradable polymers and conducting polymers for controlled drug release (Guo et al., 2013). Biodegradable conducting polymers have shown great potential in drug delivery systems such as polypyrrole–cellulose-based composite films were designed by electrochemical deposition of a drug comprising polypyrrole film on the outer and inner surface of cellulose film. This film owned high surface area, 1.58 S/cm conductivity and porous structure of cellulose, which are key components for high drug-releasing efficacy. The film was coated with a thin layer of magnesium metal, and the drug was released after the exposure to an electrolyte solution followed by cell mechanism. The drug release rate and its efficiency were dependent on the type and thickness of metal used. This film was lightweight and flexible along with biodegradable, hence could be applied in vivo applications too (Ge et al., 2010). Chiker et al. (Chikar et al., 2012) prepared a dual-component cochlear implant coating with the combination of conducting polymer PEDOT and arginine–glycine–aspartic acid (RGD) functionalized alginate hydrogel to enhance biological stability and the electrical function of the inner ear to enable the long-lasting acuity of sound via this cochlear implant. This coating improved the charge delivery, reduced the electrode impedance and delivered trophic factor into cochlear fluids, hence has the application in improving the user experience of the cochlear implant.
Some biodegradable and electrically conducting polysaccharide hydrogels were prepared by the functionalization of aniline oligomers with polysaccharides via a one-pot synthesis reaction. These hydrogels revealed good pH-sensitive swelling behaviour, electrical conductivity and film-forming properties, therefore provided new possibilities in biomedical applications for drug delivery systems (Guo et al., 2011). The same author prepared a hydrogel network by mixing electroactive aniline trimer with dextran, and as a crosslinker, they used hexamethylene diisocyanate. These hydrogels revealed good conductivity and stable rheological properties along with required stimuli capability which can efficiently control drug delivery. This system was based on an ‘on–off’ kind accurate drug delivery system, which delivered more drugs when more voltage was applied and without any external stimuli, it released less drug. Therefore, these dextran-based biocompatible conductive hydrogels had potential applications in the smart drug delivery system (Qu, Liang, et al., 2019).
7 ADVANTAGES OF BIODEGRADABLE CONDUCTING POLYMERS OVER CONVENTIONAL NATURAL/SYNTHETIC POLYMERS OR BIOMATERIALS
These new biomaterials have many advantages over conventional biomaterials such as repairing of spinal and peripheral nerves, damaged cranial, connective tissues and skin, as well as they provide the relief from long-term health risks too (Javaid et al., 2020). Due to the electrical response of conducting polymers, feasibility for stimulation of chosen tissue, time-controlled drug release and inspiration of propagation or differentiation of numerous cell categories occurs (Kenry & Liu, 2018). Furthermore, there are many more applications such as biodegradable nanocondenser fabrication for implantation in the body.
8 CONCLUSION
Biodegradable conducting polymers have emerged as a new class of biomaterials in the field of material sciences, chemistry and life sciences. During the past decades, various electrically conductive and degradable polymers have been synthesized and applied in diverse biomedical fields such as tissue engineering, drug delivery, vascular grafts, surgical sutures, artificial skin, bone fixation devices, gene delivery systems and other diagnostic applications.
However, this list should be increased for meeting the demands of more versatile and specific applications. It is always a challenging task to design and prepare an ideal electroactive polymer that justifies the fundamentals of biodegradability and biocompatibility to diminish the inflammatory reaction in the host tissue which can occur by non-degradable particles in the body. Optimization of conductivity is also a challenging task by using the least quantity of conducting polymer, but possess adequate conductivity is high in demand and is a challenging task. Processability by using conducting polymers is another challenging task due to the uses of toxic and environmentally harmful organic solvent for dissolving the biodegradable conducting polymers, hence to synthesize hydrophilic and processable materials is still desirable in biomedical fields. Still, this class of new biomaterial has been evolved as an explosive material, will add on further contributions and great opportunities to biomedical science in the eras to arise.
ACKNOWLEDGEMENTS
The authors are thankful to the Elsevier, Springer, Taylor & Francis, and MDPI for copyright permission.
CONFLICT OF INTEREST
There is no conflict of interest.




