Effect of pre-existing diseases on COVID-19 infection and role of new sensors and biomaterials for its detection and treatment
Abstract
The entire world is suffering from a new type of viral disease, occurred by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The present article briefly discussed the genome sequencing and interaction of host cells with SARS-CoV-2. The influence of pre-existing diseases such as diabetes, heart disease and age of the patients on COVID-19 infection is reviewed. The possible treatments of SARS-CoV-2 including antiviral drugs, Chinese traditional treatment and plasma therapy are elaborately discussed. The proper vaccine for COVID-19 is not available till date. However, the trials of pre-existing antiviral vaccines such as, chloroquine/hydroxychloroquine, remdesivir, ritonavir and lopinavir and their consequences are briefly presented. Further, the importance of new materials and devices for the detection and treatment of COVID-19 has also been reviewed. The polymerase chain reaction (PCR)-based, and non-PCR-based devices are used for the detection of COVID-19 infection. The non-PCR-based devices provide rapid results as compared to PCR-based devices.
1 INTRODUCTION
Coronavirus is a member of family Coronaviridae and subfamily Coronavirinae, which is large and enveloped RNA virus having the largest genomes (25–32 kb) among RNA viruses (Richman et al., 2016). The genome sequence of human coronavirus (HCoV) has four genera as Alphacoronavirus (α), Betacoronavirus (β), Gamacoronavirus (γ) and Deltacoronavirus (δ). Among all four HCoV, α and β coronaviruses are pathogenic (Woo et al., 2012). The domestic mammals (swine, mice, bat, etc.) and humans are suggested to be the origin and host for α and β coronaviruses, respectively (Lim et al., 2016). However, γ and δ coronaviruses are originated from birds and beluga whale, respectively (Geng et al., 2013). There are six β coronaviruses that infect the human respiratory system. Severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome coronaviruses (MERS-CoV) are very infectious among the six existing HCoV [HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1 SARS-CoV, and MERS-CoV] (Li et al., 2005). The SARS-CoV was originated from bats and birds in China, in 2002, with the common cold as a general symptom (Kuiken et al., 2003). A report suggested that approximately 8000 patients were infected by SARC-CoV, worldwide (Gual et al., 2003). However, the mortality rate due to SARS-CoV infection was found to be ~10% (Wu, et al., 2020a). In 2014, in Saudi Arabia, new HCoV, MERS-CoV, similar to SARC-CoV was reported with a mortality rate of 35% (Morra et al., 2018; Terada et al., 2014). The MERS-CoV was originated from bats similar to SARS-CoV, later camels become an intermediate host (Bermingham et al., 2012; Crossley et al., 2003). In December 2019, a new respiratory viral infection has stated in Wuhan, China, which has been recognized by the family of HCoV. The HCoV family named this novel virus as SARC-COV-2, later World health organization (WHO) termed it as coronavirus disease-2019 (COVID-19).
The generalized symptoms of SARS-CoV-2 infected patients are fever, tiredness, dry cough, nausea, and trouble in breathing, as in more extreme cases. The source and intermediate animal reservoirs of COVID-19 and the mechanism to spread between individuals are not clearly understood. Few reports indicated that COVID-19 infection began from bats and firstly transmitted through an intermediate host (yet to be determined). However, the pangolin has been reported as a potential intermediate host in one of the studies (Zhou et al., 2020). The transmissibility of novel SARS-CoV-2 is quite higher than that of SARS-CoV and MERS-CoV, which can be one of the reasons for the rapid spreading of COVID-19 worldwide. On March 12, 2020, WHO declares COVID-19 as a ‘pandemic’.
The development of various biomedical devices and biomaterials are in continuous thrust for the detection and treatment of coronavirus (Chakhalian et al., 2020). The early identification of COVID-19 infection is very important to reduce the transmission as well as treatment of the infected patient (Pokhrel et al., 2020). The SARS-COV-2 infection can be broadly detected using various existing biomedical devices such as polymerase chain reaction (PCR)-based, and non-PCR-based methods. The detection of SARS-COV-2 infection is dependent on sample size and analyte present in the sample. It also depends on the source of sample collection, for example urine, blood, anal swab and oropharyngeal swabs have been used for the detection of SARS-COV-2 virus (Peng et al., 2020). The delivery of drugs plays a significant role in wiping out the pandemic (Kumar et al., 2020a). The development of nano biomaterials facilitates the delivery of proteins and RNA. Recently, chitosan nanoparticles were used to deliver the drugs in the corona virus-infected patients (Fomenko et al., 2020).
The present article summarizes the interaction of host cells with SARS-CoV-2 and effect of pre-existing diseases on infection of COVID-19. This review also elaborated the genome sequence of SARS-CoV-2 infected patients from different geographical areas. The possible treatment methods including the use of antiviral drugs, plasma therapy and traditional treatment have been also discussed. Further, this article explores the importance of biomedical devices in the detection of COVID-19 infection as well as the recent development of biomaterials for its treatment.
2 GENOMIC STUDY OF COVID-19
The genome of novel SARS-CoV-2 has ~79% and 51.1% similarity with the SARS-CoV and MERS-CoV, respectively (Zhou et al., 2020). However, it demonstrates approximately 96.2% similarity with RaTG13 CoV. In another study, it has been reported that the genome of SARS-COV-2 is ~87.6–87.7% similar to ZC45 and ZXC21 (Woo et al., 2005). The bats are suggested to be the natural reservoir of SARS-CoV-2. The genome size of SARS-CoV-2 has been reported to be 29.9 kb (Wu, et al., 2020a). However, the values for SARS-CoV and MERS-CoV are 27.9 and 30.1 kb, respectively (Wit et al., 2016). The genome of COVID-19 has more similarity with SARS-CoV with a few changes. The amino acid substitution is not observed in COVID-19, which is there in NSP7 and NSP13 (Lam et al., 2020). The infection and transportation of COVID-19 depend on the mutation of NSP2 and NSP3 (Li et al., 2016). Both SARC-CoV and SARS-CoV-2 induce inflammatory cytokines which is the main cause of the damage of human organs (Fang, et al., 2020a).
The SARS-CoV-2 has 6-11 open read frames (ORFs) (Paraskevis et al., 2020). Two-third of the viral RNA is found in the first ORF (ORF1a/b), which encode 16 non-structural proteins (NSP) and also convert two polyproteins, pp1a and pp1ab (Li et al., 2016). However, the structural proteins such as spike glycoprotein, envelope protein, matrix protein, and nucleocapsid protein are translated by the rest of ORFs (Wu, et al., 2020c). These four structural proteins are responsible for the functioning of COVID-19. The spike glycoprotein binds the virus with receptor angiotensin-converting enzyme (ACE-2). The transport of nutrients and the development of envelope are associated with membrane proteins (Wu, et al., 2020d). However, nucleocapsid and envelope protein is responsible for the host immune function (Cui et al., 2019). Figure 1 demonstrates the origin, host and structural layout of SARC-CoV-2 (Guo et al., 2020a). The genomes of SARS-CoV-2 from the USA, Italy and India are ~99% similar to Chinese SARS-CoV-2, while Nepal SARS-CoV-2 genomes are 100% similar to that of the Wuhan (Sardar et al., 2020). The genome mutation of SARS-CoV-2 from the USA, Italy, India, China and Nepal has been carried out using the real-time (rRT-PCR) method to understand the effect of genome sequence on the transmission of SARS-CoV-2. The sample for gene sequencing was taken from SARC-CoV-2 infected patients of Jin Yintan Hospital (Wuhan, China) for analyses of open read frame ORF1b and N genes (Thompson et al., 1994). The sequence of both, ORF1b and N genes are represented in Table 1 (Chu et al., 2020). The expected amplicon sizes for ORF1b and N genes were calculated to be 132 and 110 bp, respectively. The amplification efficiency of both, ORF1b and N genes were calculated to 99.6% and 95.4%, respectively. The reported gene was a perfect match with the available standard data for gene sequencing (Accession number: MN908947) (Kumar et al., 2018).

| Gene | Sequencing | |
|---|---|---|
| ORF1b | Forward | 5’-TGGGGYTTTACRGGTAACCT−3’ |
| Reverse | 5’-AACRCGCTTAACAAAGCACTC−3’ | |
| Probe | 5’-TAGTTGTGATGCWATCATGACTAG−3’b | |
| N | Forward | 5’-TAATCAGACAAGGAACTGATTA−3’ |
| Reverse | 5’-CGAAGGTGTGACTTCCATG−3’ | |
| Probe | 5’-GCAAATTGTGCAATTTGCGG−3’b | |
The 5’ UTR and 3’ UTR are responsible for inter and intramolecular interaction which is important for RNA-RNA interaction and binding of cellular proteins with the virus. At 5’ end, the genome length, encoded by non-structural proteins, for SARC-CoV, MERS-CoV and SARC-CoV-2 are 29,751, 30,119, and 29,844 bp, respectively. However, at 3’ end, it was encoded by spike proteins and reported to be 21,493aa, 1270aa and 1273aa, for SARS-CoV, MERS-CoV and SARC-CoV-2, respectively. The Indian Council of Medical Research (ICMR) has reported the mutation of the Indian SAES-CoV-2 genome. The Indian SARS-CoV-2 has a mutation in ORF1ab, nsp2, nsp3, helicase, ORF8 protein and S protein. The Indian SARS-CoV-2 patient's genomic analyses revealed that it targets only a unique micro RNA (hsa micro-RNA27b). It is well known that micro RNA is present in humans. The RNA analyses of Indian population demonstrated that Indian human being has a unique RNA termed as hsa micro RNA27b (Guo et al., 2020a). The hsa micro RNA27b is one of the antiviral RNA among 6 antiviral RNAs. The presence of hsa micro RNA27b can be one of the reasons for the lower COVID-19 infection rate which may neutralize the SARS-CoV-2 infection (Guo et al., 2020a).
3 Interaction of SARS-CoV-2 with the host cells and replication
The novel SARS-CoV-2 has a similar interaction with host cells as observed in SARS-CoV. The contact of host cells initiates the attachment of the cellular receptor (ACE-2) with spike proteins (Zhou et al., 2020). The virus comes into contact with the host cell and stimulates endocytosis (Kumar et al., 2018). The S-glycoprotein is divided into two subunits S1 and S2. The main function of S1 subunit is to determine the host range of virus and cellular tropism (by RBD). However, the membrane fusion occurs in the endosome, which is determined by S2 subunit (Zhang et al., 2014). Following this, the genomic RNA (gRNA) released into the cytoplasm. However, the main function of uncoated RNA is to convert ORF1a and ORF1ab into polyproteins pp1a and pp1ab (Xia et al., 2020). The 16 non- structural proteins are produced by ORF1a, which form replication–transcription complex (RTC) in double-membrane vesicle (DMV). Besides, the gRNA started replication and form nucleocapsid (Sawicki et al., 2005). It is collected in Endoplasmic Reticulum Golgi Intermediate Compartment (ERGIC) and budded into the lumens. Finally, the plasma membrane combines with virion and releases the HCoV virus (Hussain et al., 2005). The interaction and replication of SRS-CoV-2 are schematically represented in Figure 2.

Reproduced with permission from
Shereen et al.,2020; Copyright (2020) Elsevier].The attachment of S protein with ACE-2 is one of the major factors for the interaction of SARS-CoV-2 (Aniket et al., 2020). There are several host factors such as, interferon-inducible transmembrane proteins (IFITMs), which inhibit the attachment of HCoV. The attachment with previously existing HCoV such as SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by IFITMs protein due to its antiviral resistance against RNA viruses (Wang, et al., 2020a). Recently, the binding efficiency of SAR-CoV-2 is reported to be approximately 10–20 times higher than that of SARS-CoV (Letko et al., 2020). The research outcome suggested that SARS-CoV-2 attach more easily than other HCoV (Shereen et al., 2020).
4 INNATE IMMUNE RESPONSE TO HCOV
The innate immunity of human beings is one of the influencing factors to transmit the HCoV. The innate immune system of the host has the main function to use to sense the virus (Akira et al., 2011). Pathogen associated molecular patterns are identified using Pattern recognition receptors (PRRs). Currently, toll-like receptor (TLR), C-type lectin-like receptors (CLmin), and free-molecule receptors in the cytoplasm, such as IFI16, DAI, are recognized PRRs (Li et al., 2020).
In extracellular interaction, macrophages are infected by HCoV, followed by T cells, present in macrophages by CoV antigens (Shereen et al., 2020). The activation and differentiation of T cells generate cytokines from different T cell subunits. These cytokines are responsible for the stimulation of immune response (Akira et al., 2011). However, higher production of these cytokines can damage the CD8 T and NK cells (Ermolaeva et al., 2008). Also, in the intracellular host, HCoV interacts with DDP4R on host cell via spike proteins. This attachment shows the genomic RNA in the cytoplasm, which can partially generate the immune response to dsRNA at the time of HCoV replication (Kawai et al., 2010). The toll-like receptor (TLR) contains nucleic acid, lipids, lipoproteins of virus and bacteria (Kell et al., 2015). The TLR-3 is stimulated by deRNA, while signalling pathways like IRFs and NF-κB produce type-I IFNs and cytokines. The type-I IFNs increases the production of antiviral proteins which help to protect the healthy cells (uninfected) (Shereen et al., 2020). Also, accessory proteins can affect the production of antiviral proteins at the time of dsRNA replication. Therefore, TLR-4 is responsible for the production of cytokines via MyD88 signalling pathways. The higher amount of produced cytokines and chemokines can enhance the infected cells in HCoV infection (Akira et al., 2011) (Figure 3).
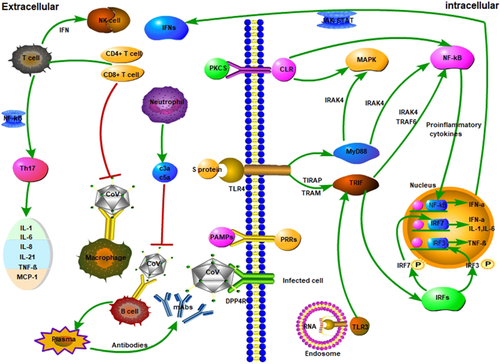
Reproduced with permission from
Li et al.,2020; Copyright (2020). John Wiley and Sons].The extracellular cytokines are produced through the interaction of HCoV with macrophage, followed by T cells which produce the cytokines. In the intercellular region, spike proteins interact with HCoV which shows genomic RNA in the cytoplasm. The drRNA interacts with toll-like receptors (TLR-3 and TLR-4) which produce the antiviral proteins. The antiviral proteins enhance the immunity of host cells via protecting the uninfected cells.
5 COVID-19 INFECTION
The patients of COVID-19 increases very rapidly throughout the world. The SARC-CoV-2 is highly transmissible among previously existing HCoV. The latent and infection periods of SARC-CoV-2 have been reported to be 4.6 and 5 days, respectively (Ferguson et al., 2020). The COVID-19 has an impact on pre-existing diseases such as cardio, diabetes, as well as age.
5.1 COVID-19 infection in old patients
WHO Regional Director for Europe stated that the ‘Older people are at highest risk from COVID-19, but all must act to prevent community spread’. The worldwide COVID-19 patient report suggested that old age people have more chances to infect as compared to the youngster (Yang et al., 2020). According to the available reports from federal centres for disease control in China, approximately 80% of died patients are more than 60 years old. However, in Italy, it was ~81 years old (Aronson et al., 2020). It has been reported that one-third of died American patients (COVID-19) are about 65 years old. In Italy, a higher fatality rate (~20%) has been reported for older (<80 years) patients; however, China has ~14% for the same (Grasselli et al., 2020). Elderly people are more likely to experience a condition of acute respiratory failure, an acute lung infection that causes many deaths (Gerst et al., 2015). However, it also appears that the virus is more likely to damage the heart, and people may die from heart attacks with COVID-19.
5.2 COVID-19 infection on cardiovascular patients
The cardiovascular patients have a higher risk of SARS-CoV-2 infection as reported for SARS-CoV and MERS-CoV. The heart patients have reported more cases with MERS-CoV infection as compared to SARS-CoV (Badawi et al., 2016). In addition, approximately 30% of the patients have heart disease that was infected with MERS-CoV (Wu et al., 2017). Recently, a report on severe COVID-19-infected patients suggested that ~58% of patients had hypertension and 25% of patients had heart diseases (Chan et al., 2020). The initial reports on COVID-19 suggested that approximately 12% of COVID-19 patients have an acute cardiac injury (Zhang et al., 2020a). However, these patients have high risk of death (Huang et al., 2020). The Chinese report on 99 patients suggested that ~40% of COVID-19 patients had cardiovascular disease (CDC, 2020). However, another study reported that approximately 10.5% of died patients have cardiovascular disease (Hi et al., 2020). Several reports indicated that cardiac arrhythmias in critical COVID-19 patients have significantly higher than that of in mild and moderate cases (Hui et al., 2020). (Wang, et al., 2020b) studied 138 COVID-19 patients and reported that ~17% patients have cardiac arrhythmias. Coronavirus can directly harm the heart of high blood pressure patients because high blood pressure damages the arteries and reduces blood flow to the heart. The virus can cause heart muscle inflammation called myocarditis, making it more difficult for the heart to pump (Burrell et al., 2005). The heart attack can be one of the reasons for the deaths of COVID-19 patients (Christopher et al., 2020). Generally, ACE inhibitors are prescribed for hypertension (Ge et al., 2013). Therefore, the dose of ACE has to be stopped for cardio patients infected with COVID-19 (He et al., 2006). Cardiovascular patients need more attention during the treatment of SARS-CoV infection. In another study, it has been recorded that the use of antiviral medicines can cause heart failure and other cardiovascular problems (Sakabe et al., 2013). The SARS-CoV-2 infected patients with the cardiovascular problem have a higher fatality rate as compared to general COVID-19 patients. In another study, it has been reported that the use of ACE for cardio patients increases fatality. The fatality rate for COVID-19 patients using ACE is higher (~36.8%) than that without ACE (25.6%) (Guo et al., 2020a).
5.3 COVID-19 infection in diabetes patients
Diabetes is an incurable disease with numerous molecular and vascular anomalies which can change the pathogenic response (Knappet, 2013). It has been reported that the mortality of diabetes patients was significantly higher in previously emerged viruses (Influenza A (H1N1), SARS-CoV and MERS-CoV) (Zhang, et al., 2020b). The diabetes patient has high risk of infection of SARS-CoV-2 as compared to the normal person (Onder et al., 2020). The initial research outcome from 173 positive COVID-19 patients suggested that approximately 16.2% of patients have diabetes mellitus (Onder et al., 2020). The mortality rate of SARS-CoV-2 patients with diabetes is observed to be similar to SARS-CoV and MERS-CoV patients. The fatality rate from 72,314 COVID-19 cases has been reported to be ~2.3%. However, it has increased to 7.3% for the diabetic patient with COVID-19 infection. The rates of serious complications and deaths of COVID-19 patients among people with diabetes are much higher than those without diabetes. The diabetic patients with COVID-19 infection have a higher risk of complications such as high blood sugar, diabetic ketoacidosis, pneumonia and dehydration. These people have higher stress conditions that increase the release of hyperglycaemic hormones which raises the level of blood glucose and abnormal glucose variability (Wang, et al., 2020c). However, approximately 10% of type-2 diabetic patients, infected with SARS-CoV-2, have been observed to suffer from hypoglycaemia (<3.9 mM) (Zhou and Tan 2020). Hyperglycaemia and insulin resistance facilitates enhanced production of end-products of glycosylation and inflammatory cytokines that can change the immune response of T cells (Petrie et al., 2018). Besides, higher level of glucose can inhibit the antiviral activity. Therefore, the control of blood glucose level in diabetes patients can improve the antiviral activity (Michael et al., 2020). Blood sugar level may increase in diabetic patients which reduce the production of insulin, resulting in diabetic ketoacidosis (DKA). The body begins breaking down energy fats, resulting in an accumulation of blood ketones, which make the blood more acidic resulting in the serious heath complications. The diabetic patients have a higher risk of pneumonia such as COVID-19 as it causes due to inflammation of the air sacs of the lungs. Therefore, diabetes patients (above 2 years) have been advised to have pneumococcal and annual influenza vaccines.
ACE-2 has been recognized as the potent receptor of SARS-CoV-2. The ACE-2 has been suggested as a medicine for the treatment of diabetes because it reduces inflammation (Fang, et al., 2020a). Therefore, the diabetes and COVID-19 patients treated with ACE-2 have a higher risk of infection (Zachary, 2020). Dipeptidyl peptidase-4 (DPP-4) has been reported as the receptor for MERS-CoV (Letko et al., 2020). In addition, DPP-4 is used for reducing the blood glucose level, and therefore, DPP-4 can protect the SARS-CoV-2 infection (Fadinil et al., 2020). Another research outcome suggested that diabetes patients are not susceptible towards SARS-CoV-2 infection, while the fatality rate is quite higher than that of normal SARS-CoV-2 patients (Zoppini et al., 2018). In summary, the diabetic patients have a higher risk of complications due to the lower immune system which increases the fatality rate. Several reports suggested that old age diabetes patients have a higher risk of COVID-19 infection as compared to young diabetes patients. It is important to notice that COVID-19 patients display symptoms very similar to flu and in many cases, it results in death of the patients in a very short time. Therefore, better treatment initially requires accurate disease diagnosis. Due to very fast disease progression, non-specific symptoms, and death of the patient in the short duration requires detection scheme sensitive and selective enough to accurately indicate this disease. Importantly, the COVID-19 virus is a single-stranded and fast mutating, which requires sensors to be robust, insensitive to mutation, rapid and very sensitive to viral detection on the onset of the diseases with minimal false-positive results. For this reason, PCR-based detection kits are the best suited.
6 TREATMENT OF COVID-19 INFECTION
The development of a specific vaccine for the treatment of pandemic SARS-CoV-2 is under progress. As COVID-19-infected patient has major problem in the respiratory system. Therefore, the treatment of COVID-19 patients is mainly focused on symptomatic and respiratory support (Colson et al., 2020). Initially, the oxygen therapy is given to all COVID-19 patients (Marmor et al., 2016). Although the specific drug for SARS-CoV-2 is not discovered till date, the medical observers are attempting with different antiviral drugs for the treatment of COVID-19 infection (Dong et al., 2020).
6.1 Antiviral drugs
Various antiviral drugs have been developed for the treatment of viral diseases such as HIV, influenza, Ebola, SARS-CoV and MERS-CoV (Marmor et al., 2016). In past few decades, number of drugs including nucleic acids and viral enzymes have been developed after the break of SARS-CoV and MERS-CoV (McCreary et al., 2020). In the continuation to the development of the specific drug for COVID-19, WHO decided to make a mega trial of pre-existing antiviral drugs [(a) Remdesivir, (b) Chloroquine and hydroxychloroquine, (c) Ritonavir and Lopinavir, (d) Ritonavir and Lopinavir and interferon-beta)] for the treatment of SARS-CoV-2 infection (Kupferschmidt et al., 2020). Remdesivir (GS-5734) is used for the treatment of Ebola in West Africa (Sheahan et al., 2017). The active triphosphate nucleoside in remdesivir binds with RNA. This RNA polymerase and act as RNA chain terminator (Sheahan et al., 2020). The remdesivir (EC50 = 0.07% μM) was applied for medicinal of SARS-CoV and MERS-CoV (Wang, et al., 2020d). In addition, EC50 = 0.77% μM of remdesivir is used for in vitro analyses of SARS-CoV-2 for the duration of 48 h in Vero E6 cell lines (Gordon et al., 2020). Recently, a young COVID-19-infected patient in Snohomish County in Washington was treated with remdesivir and recovered on the next day (Wang, et al., 2020d). However, the Californian COVID-19 patient report suggested that the use of the remdesivir is not helpful (Michelle et al., 2020).
In past few decades, chloroquine (4-amino-quinoline) and hydroxychloroquine have been adopted as major vaccines to prevent malaria, rheumatoid arthritis and lupus erythematosus as well as SARS-CoV due to its antiviral and anti-inflammatory activity, respectively (Gao et al., 2020). Apart from its antiviral ability, chloroquine has ability to modulate the immunity which enhances in-vivo antiviral impact after oral administration. Therefore, chloroquine can be adopted as a vaccine to reduce SARS-CoV-2 infection (Touret et al., 2020). However, another study conducted on Chinese COVID-19 patients demonstrated that the combination of a new antiviral drug remdesivir and chloroquine slowed down the growth of SARS-CoV-2 (Abdul et al., 2017). The mechanism for the effectiveness of chloroquine against COVID-19 is still unclear. However, few reports suggested that chloroquine increases the pH of endosome which can inhibit the spread of SARS-CoV (Abdul et al., 2017). However, another report suggested that chloroquine and hydroxychloroquine inhibit the generation of sialic acid biosynthesis by inhibiting quinone reductase 2, which affects the ACE-2 glycosylation in human cells. In addition, the immune module of chloroquine and hydroxychloroquine has antiviral property (Tricou et al., 2010). In contrast, high dose of chloroquine increases the toxicity which subsequently, causes the adverse effect to the body (Gautret et al., 2020). To overcome the toxic effect of chloroquine, hydroxychloroquine has been developed with balanced toxicity (Biot et al., 2006). Hydroxychloroquine has been used as a potential alternative of chloroquine for the treatment of malaria, arthritis as well as SARS-CoV infection (Marmor et al., 2016). Another study reported that the application of hydroxychloroquine with azithromycin reduces approximately 50% viral growth of SARS-CoV-2 after 7 days of treatment (Gautret et al., 2020). In another in vitro study, it has been demonstrated that hydroxychloroquine (EC50 = 0.72% μM) is more effective than chloroquine (EC50 = 5.47% μM) (Yao et al., 2020). Recently, worldwide reports on COVID-19 treatment suggested that the application of hydroxychloroquine can be a better alternative to inhibit COVID-19 infection (Rathi et al., 2020).
Figure 4 shows the effect of antiviral drugs on the structure and replication cycle of SARS-CoV-2. The use of antiviral drugs reduces the acidity in endosome cells in which external material to be ingesting and viruses can take over to enter a cell (Alberts et al., 2002). Figure 4 demonstrated that the antiviral drugs enter during the interaction of the virus with the host cell and reduce its replication.
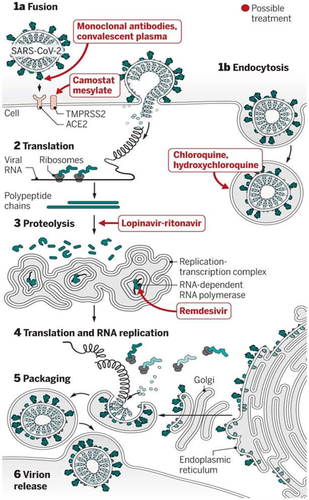
Reproduced from
Kupferschmidt et al.,2020; Open access].The combination of Lopinavir and Ritonavir antiviral drugs were used for the treatment of HIV (Chu et al., 2004). However, these drugs were also helpful to inhibit the infection of SARS-CoV/ MARS-CoV. Recently, the combination of Lopinavir and Ritonavir has been applied to COVID-19-infected patients in China but the results are not satisfactory, as the use of Lopinavir and Ritonavir can damage the liver (Aspiroz et al., 2011). The use of a combination of Ritonavir, Lopinavir and interferon-beta inhibits the MERS-CoV infection (Chan et al., 2015). In another study, nitazoxanide has been reported for its antiviral activity against Ebola, MERS-CoV and Influenza viruses. The nitazoxanide (EC50 doses of 0.92 µM) was used to prevent MERS-CoV infection (Savarino et al., 2006). Recently, EC50 (2.12 µM) is used to treat COVID-19-infected Vero E6 cell lines in vitro. Figure 5 demonstrates the effect of chloroquine and remdesivir on inhibition of COVID-19 infection. The trial of chloroquine and remdesivir has been performed on Vero E6 cells. During the trial, chloroquine and remdesivir were pretreated with the drugs for 1 h (Full time) (Figure 5). Following this, the virus was introduced (2 h). The mixture of the viral drug was extracted and cells were cultured in the medium containing drug. However, for ‘Entry’ treatment, the drugs were applied to cells for 1 h prior to viral attachment. Drugs were introduced for 2 h for the ‘Post-entry’ trial and retained until the end of the experiment (Figure 5).

Reproduced from
Tchesnokov et al.,2019; Open access]Therefore, previously developed antiviral drugs can be used for the treatment of COVID-19. Table 2 summarizes the antiviral drugs, used for the treatment of viral disease.
| Sr. No. | Drug | Status | Target disease | Reference |
|---|---|---|---|---|
| 1. | Ribozyme | Preclinical | Human CoVs | (Tchesnokov et al., 2019) |
| 3. | Lopinavir/ Ritonavir | Approved | HIV/AIDS, SARS, MERS | (Cvetkovic et al., 2003) |
| 4. | Nitazoxanide | Approved, | Human/animal coronaviruses | (Idsa et al., 2018) |
| 5. | Ribavirin | Approved, | HIV/AIDS, SARS, MERS | (Idsa et al., 2018) |
| 7. | Bananins and 5-hydroxychromone derivatives | Preclinical | Human CoVs | (Gautret et al., 2020) |
| 8. | Lopinavir, N3, CE−5 and GRL−001 | Preclinical | SARS-CoV, MERS-CoV, HCoV−229E, HCoV-NL63 and animal CoVs | (Chan et al., 2003) |
| 9. | Griffithsin | Preclinical | SARS-CoV, MERS-CoV, HCoV−229E, HCoV-OC43, HIV, HCV | (O’Keefe et al., 2010) |
| 10. | Favipiravir (T−705) | Investigational | Ebola, influenza A(H1N1) | (Gordon et al., 2020) |
| 11. | Chloroquine | Approved, Investigational, Vet approved | Malaria, autoimmune disease | (Savarino et al., 2003) |
| 12. | Oseltamivir | Approved | Influenza viruses A | (McQuade et al., 2015) |
| 13. | Penciclovir/Acyclovir | Approved | HSV, VZV | (Shiraki, 2018) |
| 14. | Ganciclovir | Approved, Investigational | AIDS-associated cytomegalovirus infections | (Furuta et al., 2013) |
| 15. | Favipiravir (T−705) | Investigational | Favipiravir (T−705) | (Goldhill et al., 2018) |
| 16. | Nitazoxanide | Approved, Investigational, Vet approved | A wide range of viruses including human/animal coronaviruses | (Rossignol, 2014) |
6.2 Conventional Chinese treatment
The use of the antiviral drug causes adverse effect on human health. Therefore, various conventional treatments have been adopted to treat COVID-19 infection. The recent Chinese report suggested that traditional Chinese medicines have been a potential alternative to reduce SARS-CoV-2 infection (Ren et al., 2020). It has been found that the lung is the main infected organ (~90%) for COVID-19 patients. However, damp and toxin plague are the main cause of infection. The traditional Chinese medicines aim to treat the damaged organ through conventional methods (National Health Commission of the People's Republic of China, 2020). This medicine was also used to treat SARS-CoV patients. The SARS-CoV-2 patients were treated with traditional decoctions such as qingfeipaidu decoction (QPD), gancaoganjiang decoction, sheganmahuang decoction, qingfeitouxiefuzheng recipe, etc (Wu, et al., 2020d). Chinese government treated approximately 60,000 COVID-19 patients using these conventional decoctions (Publicity Department of the People's Republic of China, 2020). This treatment is helpful for the treatment of mild cases (Zhao et al., 2020). However, it has been reported that severe cases were recovered 2 days earlier than that of treated with hydroxychloroquine. It has been reported that the oral liquid of Shuanghuanglian can be used to inhibit the SARS-CoV-2 infection (Lin et al., 2002). However, another report suggested that the oral liquid of baicalin, chlorogenic acid and for sythin in Shuanghuanglian is more effective to inhibit a variety of viruses and bacteria (Lu et al., 2000). According to the Chinese research report, both, mild and severe COVID-19-infected patients were treated with traditional medicine. However, it is more suitable for the treatment of mild cases.
6.3 Plasma therapy for COVID-19
In the absence of specific drugs, various antiviral drugs, therapies were applied to recover COVID-19 patients. The use of antiviral drugs has limitation due to its harmful side-effect on the human body. However, conventional treatment was not effective for severely infected patients. Convalescent plasma therapy has been recognized as one of the most effective ways to treat critical patients (Jenkins et al., 2015). The convalescent plasma therapy is a technique, which is applied for the treatment of virus-infected patients using antibodies from the blood of improved patients (Lai, 2015). In 2014, WHO declares convalescent plasma therapy as an empirical treatment during Ebola outbreak (Cheng et al., 2005). The use of convalescent plasma therapy reduces the mortality rate for patients of MERS-CoV, Ebola, and Influenza A H1N1 viruses (Arabi et al., 2015). Convalescent plasma therapy has been observed as a better alternative for the treatment of severely infected COVID-19 patients. A COVID-19 recovered patient's blood contains unique anti coronavirus antibodies. The serum of these blood antibodies are used for the treatment of severely infected patients. These blood antibodies can stop the reproduction of the virus and also improve the immune response of patients. A research report suggested that plasma treatment is more effective at the initial stage (within 14 days of symptoms) of COVID-19 infection. The late use of plasma therapy has less effect and increases the risk of organ damage. The plasma of recovered patients is used to make the plasma globulin, specifically for the SARC-CoV-2. However, this globulin has adverse effect to human health. It has been reported that after testing in 10 adult COVID-19-infected patients, 200 ml of convalescent plasma saturated the patient's condition after 3 days of treatment (Duan et al., 2020). However, after 7 days, high level antibodies were produced which remove the viremia. Another research report suggested that the convalescent plasma treatment improves the breathing of patients and remove the mechanical ventilator after 3 days of treatment (Shen et al., 2020). The condition of plasma donor is an influential factor for this treatment. A generalized criteria has been adopted for the plasma donor which are as follows,
- The body temperature of a plasma donor must be normal for more than 3 days.
- The plasma donor has at least two consecutive negative reports of SARS-CoV-2.
- The plasma has to be collected from the donor who has plasmapheresis in the range of 21 CFR 630.15.
- The donor has a positive serological test report at the time of plasma collection.
The plasma therapy can be an effective treatment for COVID-19 if it is started at the early stage of infection and considering the donor and acceptor eligibility factors. In addition to the success of convalescent plasma therapy, a research report suggested the risks factor associated with plasma treatment. The plasma treatment may fail in a few patients which increases the COVID-19 infection. It can also affect the immune system of the patient, which subsequently, causes the re-infection. Apart from this risk factor, plasma therapy has been potentially used for the treatment of COVID-19 patients worldwide.
7 EFFECT OF LOCKDOWN ON COVID-19 INFECTION
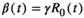
where, γ is the individual recovered rate.
The one-time social distancing (20 weeks) data demonstrated that reproduction rate reduces by approximately 60%. However, longer and rigid temporary social distancing was not more effective to reduce the basic production rate of COVID-19 (Lu et al., 2020). Figure 6 represents the dynamics of COVID-19 infection without considering seasonal effect. The reproduction rate was taken constant (2.2) during the entire scenarios. The success of social distancing ranged from zero to a 60% reduction in R0. The long-term (20 weeks) social distancing has the lowest average peak and total outbreak duration. The more success rate (20–40%) has been recorded in long-term social distancing as compared to temporary distancing scenarios (Lu et al., 2020).
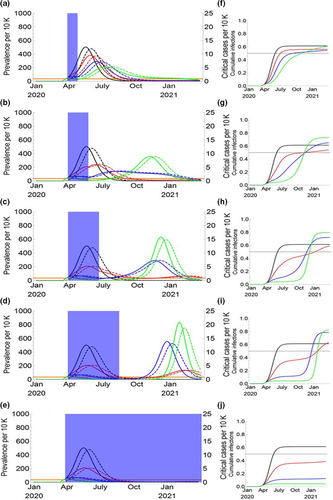
8 MEDICAL DEVICES AND BIOMATERIALS FOR COVID-19
Biomedical devices play an important role in the detection of diseases as well as drug delivery systems. The COVID-19 infections in patients can be examined by pathology, immunology and molecular biological instruments. The higher molecular weight (140 kDa) encoding 3800 DNA bp of COVID-19 suggests that it requires drug delivery system (Zhang, et al., 2020c). These vaccines can be delivered through the DNA, mRNA or protein (Kowalski et al., 2019). The materials used for protective equipment, such as protective suits, masks and gloves are also an important factor to control the COVID-19 infection. A few challenges in developing protective suits are poor anti-toxicity, breathability and heat dissipation. However, low protection against aerosols, reuse of gloves and mask are its main concerns (Itani et al., 2020). As far as the treatment of COVID-19 is concerned, the role of effective detection devices becomes important. There are several sensing devices used to detect the COVID-19 infection, as discussed in the subsequent section.
8.1 COVID-19 sensing
The polymerase chain reaction (PCR)-based and non-PCR-based methods are used to identify the SARS-COV-2 infection. The PCR-based method includes- PCR, real-time PCR (RT-PCR), reverse transcriptase PCR (RT-rPCR), real-time quantitative PCR (RT-qPCR), TaqMan-based real-time RT-PCR and dual TaqMan-probe-based RT-PCR are often used as a gold standard (Wan et al., 2016).
8.1.1 Real-time PCR
In RT-PCR, initially, RNA is converted to cDNA by reverse transcription (Tahamtana et al., 2020). The transcription of RNA to DNA entails primer design in alignment with the desired RNA target sequence, followed by addition of dNT’s, reverse transcriptase (enzyme), and nuclease-free buffer, which results in conversion of RNA to amplified cDNA target (Lu et al., 2020). The transcription and amplification process requires heating and cooling cycles. The produced cDNA is identified either by gel electrophoresis or by gene sequencing (Baelum et al., 2009). In RT-PCR, target amplification can be monitored and quantified with relative ease. However, the application of RT-PCR requires costly RT-PCR machines and its post-analysis is quite complex (Dharavath et al., 2020). To reduce the post-sample handling, specific target amplification and to get a reliable result, TaqMan probes are introduced in the RT-PCR systems.
8.1.2 TaqMan probe-based RT-PCR
It possesses fluorescent dye on 5’ end and a quencher on the 3’ end. The fluorescent signal is reversed when TaqMan probes anneal to the target, separating the quencher and reporter dye (Baelum et al., 2009). In the meantime, the growing primer reaches the 3’ prime end and cleaves the quencher due to endonuclease activity. This detection format allows very specific, sensitive and selective identification of the nucleic acid target. It is important to note that PCR-based nucleic acid detection may require thermocyclers or quantitative PCR machine, which is costly and does not provide onsite detection capability. Besides, droplet digital PCR (dd-PCR) was also adopted as a sensing device for COVID-19 detection.
8.1.3 Droplet digital - PCR
The dd-PCR is a new technology, where TaqMan probes are used (Yu, et al., 2020). The amplification is carried out in a dispersed droplet phase where dispersed phase constitutes of water–oil emulsion (Taylor et al., 2017). Due to high surface area and a large number of droplet steps before amplification, reaction is enhanced significantly. As a case study, 336 samples from 76 patients were analysed using both, RT- PCR and dd-PCR to detect the SARS-COV-2. Out of which, 95 samples were positive, 161 negative, and 67 were single gene positive when RT-PCR was used, whereas the dd-PCR indicated 95 samples to be positive with the Ct values of RT-PCR, which was well correlated with the copy numbers determined by dd-PCR, on targeting ORF1ab segment. Further, it was found that out of 67 single gene-positive samples, dd-PCR samples showed 26 samples as negative and 41 of the samples as SARS-COV-2 positive. The studies concluded that RT-PCR and dd-PCR are both accurate, however, dd-PCR was found to be more accurate when viral load was low.
8.1.4 Non-PCR-based devices
The non-PCR-based method includes self-sustained sequence replication, isothermal multiple displacement amplification, nucleic acid sequence based amplification, the loop-mediated isothermal amplification of DNA (LAMP), real-time LAMP, and real-time quantitative LAMP (RT-q LAMP) (Zhou et al., 2019). Among which, isothermal methods have become popular due to its simplicity. This method uses a single enzyme and does not require thermocyclers, making it easy to use in any lab setup. The process of target amplification can be accomplished at temperature of 60–75°C. Besides, it uses a single enzyme and provides detection sensitivity similar to PCR-based methods.
Liu et al. (Liu et al., 2020) investigated 4880 nasal and pharyngeal swab samples using RT-PCR method, out of which 1875 were found to be positive. It was, therefore, concluded that nasal and pharyngeal swabs displayed poor sensitivity. Other study reported the detection of COVID-19 infection using RT-LAMP method, which is a rapid visual detection kit (Yan et al., 2020). The detection employed colour change of reaction mixture, from orange (negative) to green (positive) and also utilized turbidity (650 nm) measurements (Shirato et al., 2020). Primers used for the detection of Orf2ab and spike gene segments are provided in Table 3. The pseudo-virus, ranging from 1 copy/µl to 1 × 108 copies/µl was detected using these primers (Park et al., 2020).
| Sr. No. | Sequence | Segment used for targeting | Reference |
|---|---|---|---|
| 1. | orf1ab−4F3 | GGTATGATTTTGTAGAAAACCCA | (Islam et al., 2015) |
| 2. | orf1ab−4B3 | CAACAGGAACTCCACTACC | |
| 3. | orf1ab−4FIP | GGCATCACAGAATTGTACTGTTTTTGCGTATACGCCAACTTAGG | |
| 4. | orf1ab−4BIP | AATGCTGGTATTGTTGGTGTACTGAGGTTTGTATGAAATCACCGAA | |
| 5. | orf1ab−4LF | AACAAAGCTTGGCGTACACGTTCA | |
| 6. | S−123F3 | TCTATTGCCATACCCACAA | |
| 7. | S−123B3 | GGTGTTTTGTAAATTTGTTTGAC | |
| 8. | S−123FIP | CATTCAGTTGAATCACCACAAATGTGTGTTACCACAGAAATTCTACC | |
| 9. | S−123BIP | GTTGCAATATGGCAGTTTTTGTACATTGGGTGTTTTTGTCTTGTT | |
| 10. | S−123LF | ACTGATGTCTTGGTCATAGACACT | |
| 11. | S−123LB | TAAACCGTGCTTTAACTGGAATAGC | |
| 12. | nCoV N-F3 | TGGACCCCAAAATCAGCG | (Taylor et al., 2017) |
| 13. | nCoV N-B3 | AGCCAATTTGGTCATCTGGA | |
| 14. | nCoV N-FIP | CGTTGTTTTGATCGCGCCCC-ATTACGTTTGGTGGACCCTC | |
| 15. | nCoV N-BIP | ATACTGCGTCTTGGTTCACCGC-ATTGGAACGCCTTGTCCTC | |
| 16. | nCoV N-LF | TGCGTTCTCCATTCTGGTTACT | |
| 17. | nCoV N-LB | TCTCACTCAACATGGCAAGGAA |
In essence, it is observed that RT-LAMP methods have been used in most of the cases for the detection SARS-COV-2, while in a few cases, RT-PCR has been used. RT-LAMP provides a venue for point of care diagnosis, where real-time thermocycler is not available. However, RT-LAMP-based technique has the possibility of sample cross-contamination or aerosol contamination, sufficient enough to cleave the target RNA samples (Kitagawa et al., 2020). Further, primer design needs extra care, as there is chance of non-specific amplification. The designing, hair-pin structure for specificity, or mutations slows down the annealing kinetic, which increases the analysis time. Although, for time being, RT-LAMP provides the solution to the pandemic (Wei et al., 2020).
8.2 Importance of biomaterials for COVID-19
Recently, various polymers such as polypropylene (PP), polyvinylidene fluoride (PVDF), and polytetrafluoroethylene (PTFE) are used to prepare the protecting kits (Correa et al., 2020).The protecting kits include face mask and shields. The polypropylene is used to prepare the certified masks such as N95, FFP2, FFP3 and surgical masks (Cao et al., 2020). However, polycarbonate (PC) and poly (ethylene terephthalate) (PET) are used to prepare the face shields (Piedmont Plastics, 2020). It has been demonstrated that the use of nano biomaterials in the drug delivery system enhances the activity of antiviral drugs (Kumar et al., 2020b). Recently, various organic nanoparticles including lipid and polymer nanoparticles were used for DNA and mRNA delivery (Yu, et al., 2020). Lipid nanoparticles such as liposomes are suitable for drug delivery due to its spherical structure and enhanced biocompatibility (Islam et al., 2015). Recently, negatively charged ionized liposome is used to deliver mRNA for the SARS-CoV-2 spike protein (Cohen et al., 2020). Among polymeric nanoparticles, polyethyleneimine (PEI) with modified fat chains and Poly (b-amino) esters (PABEs) were adapted to deliver the DNA and mRNA (Sonaje et al., 2012). In another study, it has been reported that chitosan nanoparticles were used for delivery of antiviral drugs to the infected patients. Recently, Novochizol (containing chitosan nanoparticles) is used for the treatment of infected areas, especially lungs infection due to COVID-19 (Zhou et al., 2020). Lately, graphene has been recognized as an alternative material for the treatment of COVID-19 infection due to its antibacterial and antiviral efficiency (Palmieri et al., 2020).
9 CONCLUSIONS
COVID-19 infection is continuously increasing worldwide. The present article reviewed the genome sequence as well as host-cell interaction with SARS-CoV-2. The ACE-2 has been identified as the primary receptor of SARS-CoV-2. The influence of pre-existing diseases like diabetes, hypertension, and age factor on infection as well as the fatality rate of SARS-CoV-2 has been discussed. The pre-existing antiviral drugs such as Remdesivir, hydroxychloroquine, Ritonavir and Lopinavir are used for the treatment of COVID-19 patients. The use of hydroxychloroquine and azithromycin (EC50 = 0.72% μM) reduces ~50% growth of SARS-COV-2 after 7 days of the treatment. A number of PCR- and non-PCR-based biomedical devices are widely used for the detection of COVID-19 infection. The non-PCR-based detection device provides rapid results. The biomaterials such as polypropylene, polycarbonate and polyethylene terephthalate are used for the manufacturing of face mask and shields, respectively. The chitosan nanoparticles are used to deliver antiviral drugs to the infected areas.




