Advances and current challenges in non-invasive wearable sensors and wearable biosensors—A mini-review
The present research was financially supported by Brazilian agencies CAPES, Finep, FAPESP and CNPq.
Abstract
Flexible wearable sensors and wearable biosensors have attracted considerable interest in the scientific community. They have shown relevant potential for continuous monitoring of physiological functions in real-time and non-invasively using biofluids. Recent nanotechnology advances in sensor miniaturization and lower costs have driven the development of new flexible sensors. These new materials make sensors and biosensors promising and will cause a substantial impact on society by providing an improvement in the quality of life, as well as in a faster diagnosis and early treatment. However, considerable challenges still need to be overcome for these sensors to reach their totality, as such as improving available power supplies, low power data transmission mode, problems with device deformation and contaminated, continuous wear, understanding the correlations between analytes in biofluids and blood to validate results, and issues of security and privacy. Overcoming these challenges will allow, through the diffusion of this technology, to modify how the disease can be monitored in the future. Due to this, systematic studies should be conducted to elucidate and enhance the understanding of the current challenges for the development and implementation of wearable sensors (WS) and wearable biosensors (WBS). In this mini-review, we discuss the state of the art of these devices, critically addressing the advances by debating the current gaps and the challenges that need to overcome this science area.
Abbreviations
-
- ERG
-
- electroretinography
-
- ISF
-
- interstitial fluid
-
- NFC
-
- near-field communication
-
- PET
-
- polyethylene terephthalate
-
- RFID
-
- radio-frequency identification
-
- T
-
- temperature
-
- WBS
-
- wearable biosensors
-
- WS
-
- wearable sensors
Highlights
- This paper reviews recent advances, state of the art, and challenges towards the development of non-invasive wearable sensors (WS) and wearable biosensors (WBS).
- The WS and WBS were classified, and three kinds of biosensors were evaluated: epidermal, oral and ocular.
- These sensors offer promise for healthy advanced management of diseases with improved control.
1 INTRODUCTION
Technological advancement in wearable sensors (WS) and wearable biosensors (WBS) received much attention due to their ability to collect useful real-time information about the health of individuals and their high specificity, portability, data acquisition speed, low costs and low power consumption increasingly improved in the last years (Bandodkar et al., 2016; Kim, Campbell, et al., 2019; Qiao et al., 2020; Ray, Choi, Bandodkar, et al., 2019). Due to its potential to provide continuous and real-time physiological data through constant and non-invasive measurements of various biochemical markers in the most varied body biofluids, the area of the wearable devices has advanced exponentially over the past decade (Kim, Campbell, et al., 2019; Salim & Lim, 2019; Vigneshvar & Senthilkumaran, 2018). Many innovative tools have been presented in a wide range of applications (Chang et al., 2019; Gray et al., 2018; Noah et al., 2017; Purohit et al., 2020; Scognamiglio & Arduini, 2019). For example, a simple search for patents on the orbit platform with the words wearables and biosensors returns patent numbers of 74141 and 6180, respectively, with 408 patent deposits of biosensors only in 2019.
They are focused on the detection and quantification of physical and chemical properties of metabolites, electrolytes in biological fluids (Mahato et al., 2017; Purohit et al., 2020; Scognamiglio & Arduini, 2019). The principal biological fluids evaluate sweat, interstitial fluid (ISF), tears or saliva through enzymatic, electrochemical or colorimetric (optics) reactions (Ashley et al., 2019; Bariya et al., 2018; Bussan & Robertson, 2019; Chao et al., 2017; Eftekhari et al., 2018; Ghrera et al., 2018; Kaur et al., 2018; Legner et al., 2019; Parrilla, Cuartero, & Crespo, 2019; Ray, Choi, Reeder, et al., 2019; Tasca et al., 2019). In this context, WS can be used to monitor a wide range of targets, such as body movements, or signals from the environment outside the body, such as exposure to vapours or environmental toxins. WBS, in turn, are even more specific, defined as biological recognition sensor devices, which allow the detection of a specific target, such as ion, molecule, enzyme, cell receptor, antibody or organelle (Gray et al., 2018; Reid & Mahbub, 2020; Vigneshvar & Senthilkumaran, 2018). A biosensor contains at least two fundamental units: a specific biological receptor responsible for selective analyte recognition and a transducer accountable for making the detected signal a useful signal (Gray et al., 2018; Kim, Campbell, et al., 2019). Figure 1a shows in more detail the schematic representation of a WBS.
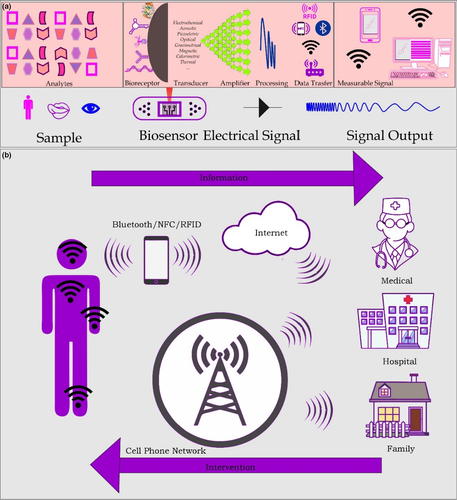
Real-time monitoring enables one to collect health and welfare information from users, control and manage chronic disease monitoring (Mccaul et al., 2018; Qiao et al., 2020; Someya & Amagai, 2019), early detection of unforeseen or abnormal situations, which may contribute to a better understanding of various diseases (Akkilic et al., 2020; Ye et al., 2020; Figure 1b).
The first wearable devices spread mainly in physical activity monitoring. They acquired data based on physical sensors that were mostly allowed to monitor mobility (step counter/calorie burning) and vital signs (heart rate; Kim, Campbell, et al., 2019; Noah et al., 2017; Salim & Lim, 2019; Someya & Amagai, 2019).
Recently, devices have focused primarily on real-time insulin monitoring, being less invasive for diabetic patients. They can avoid painful and blood sampling procedures risk, allowing these devices to be more readily accepted, improved and incorporated into the daily lives of users (Neelam, Chhillar, & Rana, 2019).
The convergence of technologies associated with the current development of nanotechnology has driven further progress and fostered design innovation and enhancement of WS and WBS. It provides cost-effective, more straightforward, disposable, pathogen monitoring, testing, and evaluation versions, analytes in various clinical conditions. Such advances have demonstrated the enormous potential WBS present for real-world applications (Zaibudeen & Philip, 2018).
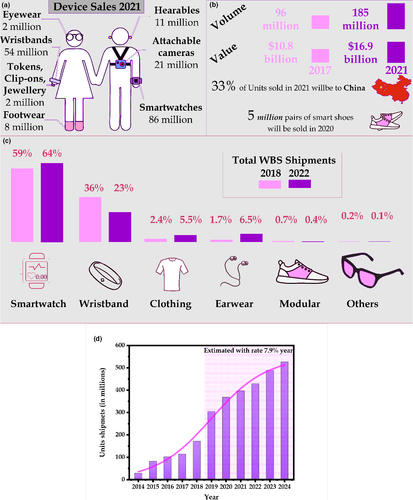
Figure 2a,c shows the growing potential of the sector and is based on different market assessment reports, which show that the incorporation of gadgets and wearable devices in society is expected to grow even more in the coming years. Figure 2b shows the CCS Insight report for 2021 predicts growth from 10.8 billion dollars in 2017 to 16.9 billion in 2021, with approximately 33% of the units to be produced by China (Arora n.d.). Similarly, Figure 2d, adapted from statista (Richter, 2018), forecasts growth of approximately 7.9% per year in unit shipments. Although many are not biosensors, these devices are the vanguard for incorporating future WBS into society. We observed that the vast majority is related to smart watches. Figure 3 shows some of the current wearables devices available for sale in 2020.

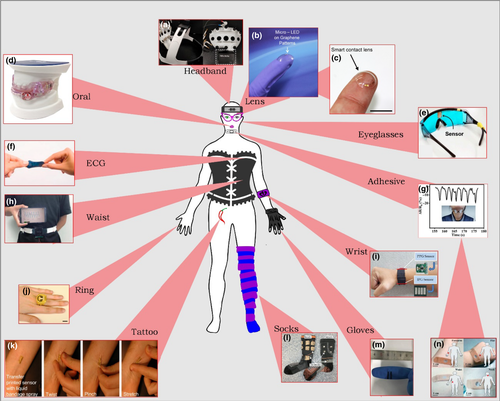
As shown in Figure 4, these devices can be integrated with various accessories for everyday use such as glasses, lenses, mouth guards, bandages, bracelets, bandanas and smart clothes such as bras, socks and gloves, yet can be integrated with tattoos, stickers, among others (Lisak et al., 2015; Ray, Choi, Reeder, et al., 2019; Scognamiglio & Arduini, 2019; Stojanovi & Srdi, 2019; Tasca et al., 2019; Zamora et al., 2018).
The technological progress enabled the enhancement of a variety of platforms as well as device integration for the creation of multiplexed detection systems, more reliable analyte records, improved device resistance, flexibility, acquisition, the transmission of wireless data and increasing reliability in the analyses performed by these devices (Kenry & Lim, 2016).
Although several WBS types have been reported, producing multiple biomarkers sensors is still a significant challenge. Also, different problems have limited and hindered even substantial progress in the area. The main challenges are related to the necessity of the development of flexible, wear-resistant, oxidation and biofouling sensors, biosensor performance and sensitivity, durability, correlation with blood results, integration of multiplexed circuitry, data security and costs for the construction of biosensors (Someya & Amagai, 2019). Overcoming such difficulties is needed to make these promising materials innovative products with massive business impact in our daily lives.
For the improvement and development of a functional WS and WBS, this is the desired flexibility of the substrates that will be used. Furthermore, the substrate thickness is crucial for its application (Salim & Lim, 2019). For most motion detectors such as heart rate monitors, these must be extremely thin substrates to enhance the sensitivity of the detector response to skin contact during analysis (Someya & Amagai, 2019). In this sense, the use of nanomaterials allows the production of devices with flexible electrodes for flexible substrates, as well as improving the mechanical properties, stabilization and oxidation rate and increasing the biosensor sensitivity (Akkilic et al., 2020; Scognamiglio & Arduini, 2019).
Moreover, just thin and flexible devices are not enough. For a more significant performance, a long life must also be improved. An alternative to prolong the lifespan device is the use of the low-power device communication modes, which are incredibly beneficial, such as near-field communication (NFC) that enables biosensor synchronization with smartphones. Short-range communication devices such as Bluetooth communication, NFC readers and radio-frequency identification (RFID) have been the most commonly used not only for allowing an excellent battery economy, and therefore longer monitoring of biosensors, but also due to the optimized mode of operation and a decrease in device size (Purohit et al., 2020; Reid & Mahbub, 2020). Although it has been little discussed, it is also essential to consider the effects caused by these new continuous sources of the exposure of electromagnetic radiation to the user.
Lastly, successful implementation in the market is still required. For this, it is necessary a greater composition understanding of the complex biological fluids that will be analysed and its relationship with blood. WBS must be capable of continuous and reliable detection of a specific analyte. As it is subject to contamination, the devices must deal with possible bio-incrustations, deformations and wear, plus inefficient sample transport to the sensor and limited stability of many bioreceptors. Consideration should also be given to receiver regeneration and calibration for use in the body.
Therefore, this mini-review addresses vital advances and challenges in the WS and WBS development, been mostly for individual systems and other sorts, such as stability, endurance, accuracy, reliability, validation, communication, security and power. For this purpose, this paper shows a review of the latest WS and WBS developed, discussing the advances made in the area and highlighting the challenges to be overcome.
2 AVAILABLE WEARABLE DEVICES SYSTEMS
Various WS and WBS have been proposed for continuous monitoring, including physical such as vital signs, by assessing breathing, pulse, heart rate, blood pressure, various body movements and temperature (Gray et al., 2018; Someya & Amagai, 2019), or to evaluate different metabolic parameters, such as glucose, electrolytes, lactic acid and pH (Dang et al., 2018; Gray et al., 2018; Purohit et al., 2020). In the next topics, the latest systems developed will be discussed, these grouped into three classes, and according to the type of device, epidermal, oral and ocular.
2.1 Epidermal devices
The skin acts as a physical barrier that protects the internal organs from contact with the environment—having several functions in the physiological maintenance of the human body (Chang et al., 2019; Someya & Amagai, 2019). Through contact with the skin, it is possible to monitor different targets and biomarkers.
The development of devices should consider the selection of favourable substrates using functional, biocompatible materials and low-cost fabrication techniques. For epidermal devices, a critical focus is the elasticity of the systems that must be compatible with skin users (Mishra et al., 2018; Zhang, Chen, et al., 2019). Polymeric materials as polyethylene terephthalate (PET), polyimides, among others, are commonly used. Among the most used materials stands out the Ecoflex due to presenting Young's modulus that approximates that of human skin (Kim et al., 2019; Xu et al., 2019; Yang, Kong, & Fang, 2019). The most frequently used active materials for sensor construction are carbon derivatives and silver-based metal nanomaterials (Arenas et al., 2019; Muralee et al., 2020). These can be used to create devices in the form of stickers, tattoos, bandages or even smart clothes for specific monitoring of the sweat, ISF for human motion.
Compared to other biofluids, sweat has advantages, such as the possibility of analytes detections, it is sampling non-invasively, continuously, comfortably, with better correlation with blood levels about diagnoses of diseases, stress or sports monitoring. Usually, its collection does not prevent or restrict the user from performing natural movements.
Sweat is secreted by the sweat glands to regulate body temperature. They are divided into two types, the eccrine and apocrine sweat glands (Legner et al., 2019). The eccrine secretion is odourless and composed of urea, sodium chloride and immunoglobins. While apocrine sweat is composed of steroids, lipids, proteins and pheromones, typically, eccrine sweat is the most relevant for WBS studies (Legner et al., 2019).
Some authors assume that many of the chemical species found in sweat are transport by-products of ISF or blood vessels, which would explain the similarity of the analytes found in them.
The WBS in the monitoring of athletes is one of the leading applications (Seshadri et al., 2019a, 2019b). Since sodium is the primary electrolyte of the human body, being essential for the regulation of osmotic pressure, pH, and water balance, providing critical information about electrolyte imbalance after activities (Qi et al., 2020), long physical exercises or exposed hot and humid environments may also indicate dehydration. Increased lactate in sweat can be associated with changes from aerobic to anaerobic metabolic conditions during activity, while monitoring of ammonium may give evidence of extreme fatigue (Mccaul et al., 2018).
In this context, Zamorra and co-works performed tests on different conductive tissues modified with electrodeposited iridium oxide (IrO2) to form wearable pH-sensitive sensors. Being these characterized by impedance measurements, the steel mesh, in turn, showed sensitivity to pH changes in addition to biocompatibility regarding the IrO2 coating. The sensor was simple, inexpensive, and washable, allowing it to be reused (Zamora et al., 2018). Dang and co-works presented a flexible system for sweat pH monitoring. They developed graphite and polyurethane composite pH sensor, sensitive in the range of pH 5 to 9, and the sensor showed no interference from the primary ions present in the sweat. The sensor withstood up to 53% deformation and more than 500 cycles. Besides transmitting power-free data through an RFID antenna to a smartphone, the authors say that this system can be manufactured at less than U$ 3.53 (Dang et al., 2018).
McCaul and co-works developed and tested a fully integrated wristwatch-based 3D printing platform to analyse sodium concentration in real-time. The platform showed a delay of about 5 min between skin surface sweat detection and the detector (Mccaul et al., 2018). It is hoped that in the future, similar devices will be able to have an even faster detection. In amputees, these sensors may be placed at the muscle or skin sites to which nerves may be reinnervated (Willand, 2015), allowing the monitoring of physical signals in the region or even specific biomarkers. Sun and co-works developed a large, subtle human motion sensor based on a polyacrylamide composite hydrogel and oxidized multi-walled carbon nanotubes, with high tensile strength 0.71 Mpa, with elasticity major 700%, response 300 ms fast and capable of over 300 cycles (Sun et al., 2019). Similarly, Zhang and co-works presented a new strain sensor based on an ultra-elastic hydrogel conductive polymer composite, which demonstrated stability and high creep sensitivity for detecting far-reaching human movements. The hydrogels showed a durable adhesive behaviour on different surfaces. They showed no allergy or residue in the human skin, which demonstrated its potential as a detector of movement and torsion in the human skin (Zhang, Liu, et al., 2019). Other relevant studies on epidermal devices were summarized in Table 1 that presents the relevant characteristics of the devices studied in these works, such as position in the body, kind of sensor, sensitivity, tests evaluated, kind of substrate and response time.
| Indicator | Position | Sensor | Sensitivity | Tests | Substrate | Response time | Ref. | |
|---|---|---|---|---|---|---|---|---|
| – | skin | impedance | – | 10 | acrylic adhesive | N/A | (Guzman et al., 2019) | |
| K+, Na+, pH, and T | head | resistivity |
K+ =57.8 ± 1.2 mV/decade and lower LOD of 4.2 ± 1 µM Na+ =57.7 ± 1.5 mV/decade and lower LOD of 2.5 ± 1 µM |
N/A | polyamide | N/A | (Hanitra et al., 2019) | |
| T | skin/head | resistance | 9.4% extreme 200% strain | the angle from 0 to 180° and a torsion angle from 0 to 720°, the resistances do not change much | polyacrylamide | 30 s | (An et al., 2020) | |
| pH | skin | pH sensors |
−22 ± 9; −61 ± 4 and −61 ± 2 mV pH−1 for the 3 different sensors |
N/A | cotton yarns | 60 ± 20 s | (Smith et al., 2019) | |
| glucose | skin (ISF) | iontophoresis | 0-1 mM | N/A | cellulose | < 3 s | (Kim, 2019) | |
| nucleic acid | skin | human body heat | target DNA was 10 copies/μL within 10 minutes | N/A | Ecoflex | 10 min | (Yang, Kong, & Fang, 2019) | |
| human motion | Headband/gloves | MPU/gyroscope/accelerometer/magnetometer. | N/A | – | N/A | N/A | (Alexandre, 2019) | |
| human motion | socks | voltammetric | 86.4% | N/A | CMC and PVA | N/A | (Leone et al., 2019) | |
| human motion | socks | – | N/A | N/A | N/A | – | (Eguchi et al., 2019) | |
| human motion |
ring/ finger |
magnetic tracking | – | N/A | N/A | – | (Parizi, Whitmire, & Patel, 2019) | |
| human motion | pants | ECG | – | N/A | N/A | – | (Leinonen, 2019) | |
| human motion | head | impedance | –- | N/A | – | N/A | (Interface et al., 2019) | |
| human motion | skin | – | 0.005 to 50.0 kPa−1, a sensitivity approximately −0.107 kPa−1, and high durability (>10,000 cycles) | N/A | PDMS | 6.02 W is ∼180 ms | (Lu et al., 2018) | |
| human motion (respiration diseases) | waist | electrical output signals of the TENG sensor | N/A | N/A | acrylic sheets | N/A | (Zhang, Zhang, et al., 2019) | |
| human motion | skin/arm | electrical conductivity | – | – | PVA | N/A | (Zhang et al., 2020) | |
| human motion (acoustic waves) | skin | piezoresistive | under small strain has been enhanced 10 times by the circular macro-defect of 2 mm diameter/ GrF devices by 10 times in the tiny strain detection (≤2%) | N/A | PET | 98.67 ms | (Xu et al., 2019) | |
| Nitrophenol toxic nerve agents | arm/body | voltammetric | linearity over the 90-300 mg/L range, the sensitivity of 10.7 µA∙cm3∙mg−1 and LOD of 12 mg/L in air | 100 Bending | polyimide | N/A | (Mishra et al., 2018) | |
| energy buffering device | arm | stretchable supercapacitor | the areal capacitance of 167 mF/cm2 at a current density of 0.4 mA/cm2 | 3 times in different angles | silicone rubber | N/A | (Rajendran et al., 2019) | |
- * –, not clear; CMC, carboxymethylcellulose; ECG, electrocardiogram; LOD, limit of detection; MPU, microprocessor unit; ms, milliseconds; N/A, not available; PDMS, Polydimethylsiloxane; PET, polyethylene terephthalate; PVA, poly(vinyl alcohol); W, Watts—temperature (T).
Figure 5 shows some more recent and relevant epidermal device examples. Ahyeon Koh and co-works developed and evaluated different properties of a new microfluidic device to monitor sweat. This device allowed the multivariate colorimetric detection of different types of analytes, such as lactate, glucose, water, chloride and pH (Figure 5a–c).
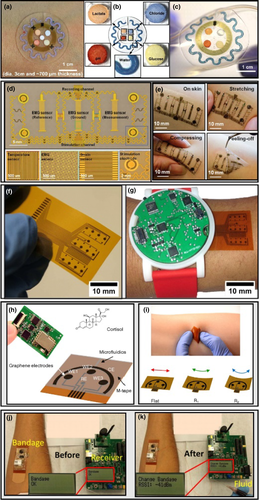
B. Xu and co-works developed multifunctional epidermal devices perfectly integrate sensors for different kinds such as electromyography, temperature and voltage with electrical stimulation of compression electrodes, in a simple architecture that in the future should allow prosthetic control as a sensory response (Figure 5d,e). Yokus and co-works development a new WBS based on multiplexing, hardware, integrated with wireless technology for electrochemical analysis of glucose, lactate, pH and temperature, with continuous detection sensing in simulated and sampled sweat (Figure 5f,g).
Torrente-Rodríguez and co-works evaluated a set of multiplexed adhesive type sensors used for glucose monitoring during exercise, with simultaneous measurement of pH, temperature and humidity to correct the glucose signal (Figure 5h,i). Farooqui and Shamim produced a new bandage used on the forearm, and this sensor informs the presence of blood in the bandage.
2.2 Oral cavity devices
Oral cavity sensor and biosensors, usually based on the electrochemical or optical transducer placed in the oral cavity, has experienced considerable progress and shown advantages faced to other systems, mainly because of the versatility of use saliva as biofluid.
Saliva consists of a complex biofluid constituted by several analytes. They are permeating from the blood in different ways (transcellular or paracellular paths). The analytes (e.g. ions, acids, sugars, enzymes, hormones, peptides, proteins, antibodies, microorganisms, growth factors and so on) have shown high potential for general health surveillance (Bandodkar & Wang, 2014; Lee & Wong, 2010).
To be more readily accessible than the bloody and requiring fewer pretreatments, it has been used to the monitory nutritional, hormonal, emotional and metabolic state of the human body (Aguirre et al., 1993; Ilea et al., 2019; Kim, Campbell, et al., 2019; Malon et al., 2014).
An interesting study was presented by Mannoor and co-works (Mannoor et al., 2012), which developed a dental tattoo based on graphene printed onto water-soluble silk and modified with antimicrobial peptides, for continuous monitoring of pathogenic bacterial on tooth enamel. Despite real time on body applications that have not been described, this oral wearable biosensor showed excellent specificity, response time, with limits detection down to a single bacterium, as well as wirelessly achieving remote powering and readout.
More recently, researches on oral cavity wearables biosensors have resulted in the development of several mouthguard platforms for sensing the biomarkers in saliva. In this context, Ciui et al. (2018) reported an electrochemical oral cavity sensor for glycated end compounds (Nε (carboxymethyl)lysine), a class of biomarkers involved in oxidative stress, which produce long-term damage to proteins in ageing processes, atherosclerosis and diabetes. Briefly, the sensor was fabricated via screen printing method on a standard and cheap, flexible foil, using Ag/AgCl conductive ink for providing reference electrode and carbon ink to give work and counter electrodes, being posteriorly attached to a mouthguard. The sensors exhibited high repeatability, lifetime storage, short timescale measurements, ease of use, and high selectivity and sensitivity with a limit of detection of 166 ng mL−1 in phosphate buffer. Lastly, when tested in raw and untreated human saliva, promising results were observed.
Saliva used as biofluid has not been restricted to the biologic factors monitoring as nutritional, hormonal or metabolic state of the human body but also has been applied to monitoring physical issues as a fracture. In this context, an unusual approach was developed by Hashem and co-workers (Hashem et al., 2020), which demonstrated a wearable bio-tooth sensor to the monitoring of tooth fracture and its metabolites. The idea consists of a multipurpose wireless wearable graphite bio-tooth sensor to monitoring, coaching, drinking, chewing, fracture and infection. Briefly, the biosensor (range of 2 × 3 mm) consists of a human–machine interface responsible by imaging, integrated to biomarkers analysis to diagnose the various motion patterns from the oral cavity. The biosensor showed to be capable of analysing object surface aiming tooth shape reconstruction and, besides, provided a solution to validate the 3D-intraoral images using an image acquisition sensor.
The development of the oral cavity WS and WBS has improved in the last years, and specific issues still have to be carefully approached in the design of an ideal biosensor. Due to complex mouth anatomy, comfort becomes crucial in the choice of adequate substrates. Thus, aiming minimal inconvenience to the user, properties as flexibility, lightness and thickness are encouraged. Also, toxicity assays need to be carried out to investigate the biocompatibility of many electronic components, which shows absent in many reported studies. Lastly, are summarized in Table 2 some critical results about the development and employment of several oral cavity wearables biosensors applied in the analysis of a wide range of analytes.
| Analyte | Platform | Sensor | Response time | Features | Comments | Ref |
|---|---|---|---|---|---|---|
| Lactate | Enzyme modified electrode on a mouthguard | Impedance | 60 s | Lactate range 0.1-1.0 mM | High selectivity, stability and low potential detection | (Kim et al., 2014) |
| Uric acid | Enzyme modified electrode on a mouthguard | Amperometric | 60 s | The sensitivity of 2.45 μA mM−1 | Bluetooth wireless link for data transmission; high sensitivity, selectivity and stability | (Kim et al., 2015) |
| Glucose | Enzyme modified electrode on a mouthguard | Amperometric | - | The detection range of 5-1000 μmol L−1; stable and long-time monitoring up to 5 h | Telemetry system; wireless transmitter high selectivity; continuous monitoring | (Arakawa et al., 2016) |
| Glucose | On-chip disposable biosensor based on enzyme modified electrode | Amperometric | 30 s | Sensitivity of 26.6 μA mM−1 cm−2; detection range of 0.1-20 mg dL−1 | Excellent clinical accuracy; point-of-care use; real-time monitoring; | (Zhang, Yunqing, et al., 2015) |
| pH, t, salinity, glucose and alcohol | Tooth-mounted biosensor layered by a porous silk film and hydrogel | Passive dielectric | - | Small size (down to 4 mm2); Sensitivity around 0.6 MHz in 1 g L−1 of glucose; | Wide range of analytes; opportunity of adding more specifically modified layers for targeting sensing | (Tseng et al., 2018) |
| Sodium | Breathable elastomeric membrane | Potentiometric | - | Sensitivity of 188 12 mV dec−1; | Biocompatibility; stretchable; high sensitivity, real-time monitoring quantification of sodium intake | (Lee, et al., 2018) |
| pH | Polyaniline gold nanostructures | Optical sensor | 3.5 s | Sensitivity of 0.0299 a.u. pH−1 in the range of pH =2-8 and 0.0234 a.u. pH−1 in the range =8-2 | Possibility to be modified by different sensitive materials; more stable than the electrochemical methods; excellent reversibility | (Luo et al., 2017) |
| Cytokines | Antibody modified electrode | Impedance | - | Analysis range of 1-100 pg mL−1; | High selectivity and sensitivity | (Bellagambi et al., 2017) |
| Uric acid | Enzyme modified electrode on a mouthguard | Amperometric | 60 s | The sensitivity of 2.45 μA mM−1 | Bluetooth wireless link for data transmission; high sensitivity, selectivity and stability | (Kim et al., 2014) |
Some other relevant examples were shown in Figure 6. Y. Lee and co-works developed an intraoral electronic device that provides continuous monitoring of sodium through a hybrid electronic system of microchips in a membrane and evaluated the monitoring of sodium in real time in humans (Figure 6a). Similarly, Xuemeng Li and collaborators developed a mouthguard sensor sensitive to the presence of volatile sulphur to evaluate and diagnose oral diseases. The developed sensor presented a highly sensitive and selective response to the sulphur, in addition to biocompatibility, low toxicity and low cost (Figure 6b). Mohamed Hashem and co-works built and evaluated on a laboratory scale a graphite-based WBS capable of detecting different analytes, including saliva, by analysing the surface of devices (Figure 6c). Similarly, Takahiro Arakawa and co-works developed a mouthguard for non-invasive glucose monitoring by saliva with high sensitivity to artificial glucose (Figure 6d).
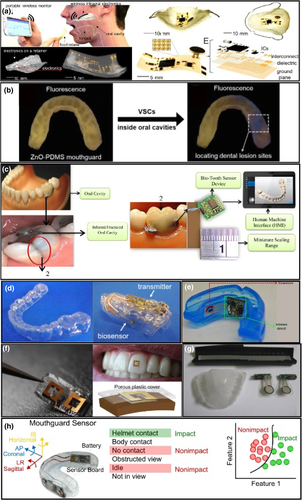
Jayoung Kim and co-works developed a WBS for continuous monitoring of salivary uric acid via electrochemical detection and with transmission via Bluetooth 4.0 (Figure 6e). Peter Tseng and co-works developed a device based on permeable membranes for detecting liquid analytes. In vivo evaluation was demonstrated for detecting food during human ingestion (Figure 6f). Lantada and co-works developed a splint-type sensor to assess intraoral tightness to assess bruxism and other pathologies (Figure 6g). Wu and co-works developed a WS to accurately assess head impacts caused during a football match (Figure 6h).
2.3 Ocular WBS
The seek for vision corrections and aesthetic reasons make the ocular research be widely studied (Korde et al., 2019; Lim et al., 2019; Yuan et al., 2019). The most common material in contact with the eyes is the contact lenses, a thin curved lens placed on the film of tears that covers the surface of the eyes (Horne et al., 2020; Mayers et al., 2019). Due to its presence in the ocular environment and being an inexpensive material with uncomplicated production, it can also be a potential WBS for other applications (Kim et al., 2020; Reid & Mahbub, 2020).
The miniaturization of electronic components facilitates the incorporation of sensors into contact lenses (Yu, Yang, & An, 2019). The diagnostic for healthcare through electronic sensors in contact lenses are more straightforward than those obtained by blood analysis (Farandos et al., 2015). Even though there are differences between the blood and tear composition, the barrier between them allows the migration of some metabolites that can be correlated. Therefore, it is possible to analyse the tear fluids and compare to the condition of the blood fluids without needing an invasive procedure (Kim et al., 2017).
The tear fluids present in the eye have a vital function. Lubrication and cleaning (basal tears), protection (reflex tear), emotional (psychic tear) and its composition has proteins, electrolytes and antibodies, providing quantitative information about potential diseases (Aparna & Shanti Iyer, 2020; Salim & Lim, 2019).
In order to ocular WBS to be useful, it is necessary to overcome some tears analysis challenges related to the type of tear due to its difference in composition, the amount of analyte and the evaporation of fluids (approximately 10 µl of tears can be collected; Farandos et al., 2015).
The main application of contact lenses biosensor is the glucose concentration monitoring in tears fluids for diabetes treatment, where it is more advantageous than the blood test, which causes some physical discomfort (Bussan & Robertson, 2019; Forster & Cumba, 2019). Another monitoring made by ocular sensors is the intraocular pressure, directly related to glaucoma issues, which can be detected and treated early (Aitsebaomo et al., 2019; Campigotto et al., 2019).
In order to succeed the ocular WBS uses, it will be necessary not to interfere with the transparency and flexibility conditions of the contact lenses and nor to affect the optical necessities (Kim, Kukjoo, et al., 2019; Wei et al., 2019). The electronics compounds incorporated must be capable of having their mechanical, chemical and electrical properties for a highly sensitive, selective and wireless response, which needs to be connected in real time with the user by a computer or a smartphone (Takamatsu et al., 2019).
Many materials have been proposed in the ocular devices. Due to the specification of the required condition, the most well succeeded are polymers and its hybrids (Li et al., 2019), graphene-based structures (Lee & Yook, 2019), metal nano and mesh structures (Kodama & Yamada, 2020) and carbon nanotubes (Kahng, 2019). All these materials can provide a cheap contact lens with the biosensor incorporated and allow a non-invasive diagnostic through the human eye.
In this context, Zou and co-works (Zou et al., 2019) developed an intraocular biosensor made by Chitosan functionalized nitrogen/graphene for glucose detection. Due to the sp2-hybridization from graphene structures, it allows a reactive site for electrochemical reactions, and this reactivity is intensified when associated with nitrogen. The well-synthetized ocular WBS was tested with the presence of other reactive materials such as uric acid, ascorbic acid and dopamine, commonly present in tears. No change in the detection of glucose was observed, confirming then the selectivity of the biosensor.
Xiao et al. (2018) have demonstrated a gold-based for a potential power source in contact lenses where the Au-Ag alloys can support enzymes (lactate oxidase and bilirubin oxidase) for an electrocatalytic response. This biosensor worked for 5.5 hours at 150 mV (artificial tear solution) but lost his density drastically due to the presence of ascorbate present in tears composition, which quickly oxidizes the nanostructured gold electrode. The stability found in this biosensor can be used for one 1-day disposable lenses. In Table 3 are summarized the other critical results about the ocular WBS. More examples are shown in Figure 7. Agaoglu and co-works developed a microfluidic sensor with a transduction mechanism detectable by a smartphone camera, with a low detection limit <0.06% for uniaxial and <0.004% for biaxial strain. This sensor was evaluated measured the intraocular pressure-induced strain in porcine eyes in the physiological range, with continuous monitoring for >19 hr and a lifetime reaching >7 months (Figure 7a,b).
| Analyte | Platform | Sensor | Features | Comments | Ref |
|---|---|---|---|---|---|
| Pathogenic Detection | Contact lenses with antiviral agents | Colorimetric affinity | Minimum detection of interleukin−1α was 1.43 pg mL−1 | Good detection, nontoxic, did not affected the contact lenses normal conditions | (Mak et al., 2015) |
| FeMeOH | Contact lenses with gold hybrid | chronoamperometric | electrochemical system distributed in the four quadrants of the lens | Fast detection, electrochemical videos of the lachrymal fluid | (Donora et al., 2020) |
| pH | Contact lenses functionalized with anthocyanin | Colorimetric | A sensitive contact lenses with pH changes | Good detection, potential application for antimicrobial and oxidative systems | (Elsherif et al., 2019) |
| T | Contact lenses functionalized cholesteric liquid crystals | Colorimetric | Sensitivity range of 29.0–40.0°C | Low cost, wireless response | (Yetisen & Vigolo, 2019) |
| Glucose | cobalt oxide (Co3O4), palladium (Pd), and silver/silver chloride (Ag/AgCl) | cyclic voltammetry | Low concentration of glucose (10 mM) | Non need of alkaline fluid for operation, non-enzyme degradation | (Strakosas et al., 2019) |
| Intraocular pressure | Contacts lenses with graphene woven fabrics | Mechanical deformation | 0–10 mmHg of detection | Sensitive device and do not affect the transparency | (Zhang, Chen, et al., 2019) |
| Intraocular pressure | Contacts lenses with polydimethylsiloxane membrane | Mechanical deformation | Stability after repeated cycles | Good in vivo tests, low cost | (Kouhani et al., 2019) |
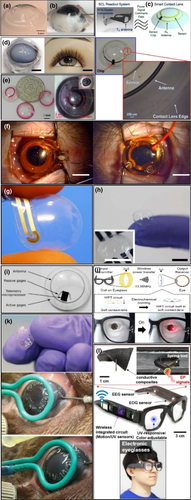
Chiou and co-works developed a biocompatible contact lens sensor wireless with a capacitive circuit interface and a wirelessly powered radio-frequency identification (RFID) addressable system for sensor control and data communication with a low sensibility and low energy consumption, 120pF and 110 μW, respectively (Figure 7c). Joohee Kim and co-works reported the manufacture of sensors in soft and transparent lenses for the detection and monitoring of glucose with wireless energy transfer. Live tests showed that the sensors are suitable for determining the fasting glucose level (Figure 7d,h)).
Hongbin An and co-works developed and evaluated a sensor system to monitor intraocular pressure based on a microfluidic lens. The tests on enucleated porcine eyes show a linear response, and these lenses can track the intraocular pressure changes (Figure 7e). Similarly, Yin and co-works developed lenses with graphene electrodes to register full-cornea recording of electroretinography (ERG) in monkeys. These sensors show a higher signal amplitude than conventional ERG electrodes for in vivo visual ERG (Figure 7f). Chu and co-works developed a biocompatible WBS based on contact lenses to glucose monitoring. This WBS shows a good relationship between output current in different glucose concentrations 0.03–5.0 mM that is commons in normal and diabetics patients (Figure 7g).
Leonardi and co-works reported a wireless sensor with a good for the continuous monitoring of intraocular pressure based on WS lens. This system allows minimally invasive glaucoma monitoring (Figure 7i). More recently, Takamatsu and co-works developed a WS through electrochemically printing a wireless-powered circuit onto a moist, soft contact lens. This new sensor allows an energy transfer between the glasses transmitter and the receiver lens and will allow the integration of more complex circuits in the future (Figure 7j).
Kouhani and co-works reported another excellent example of wireless WS to real-time monitoring of intraocular pressure. In this work, the authors report a WS with design simplicity, high responsivity and low cost (Figure 7k). Finally, Lee and co-works reported a new glass WS not only to monitor various biological phenomena but to coordinate the recorded data for active commands and game operations for human–machine interaction applications and to monitor electroencephalogram and electrooculogram (Figure 7i).
3 DISCUSSION
WS and WBS should be refined to provide faster responses and longer usage times in case of continuous monitoring. For this, it is desirable that they are lightweight, ultra-thin, and equipped with wireless technologies (power and data transmission). Also, a large number of challenges must be overcome to improve these devices, as described below. Some relevant challenges are reported in Figure 8.
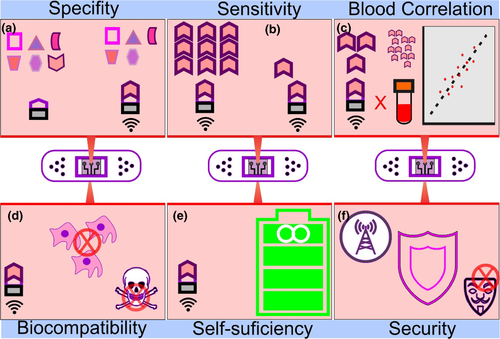
The most critical parameter of WBS qualification is its selectivity since biofluids have a large number of different biomarkers (Figure 8a). It is also necessary that WBS can differentiate fluids between targets and non-targets. Besides that, there is also a risk associated with external contamination, such as cosmetics and foods.
The sensitivity should be enhanced to track small amounts of the target in the human body, for example, the detection of prostate-specific antigen, whose detection concentration is 4 ng/ml blood (Figure 8b). Furthermore, still necessary to improve the simultaneous monitoring of several analytes using the same device, without cause increases in the size of devices and artefacts during analysis.
The correlation between the concentrations of analytes presents in biofluids to its presence in the blood must be obtained to increase the reliability of the use of the devices (Figure 8c).
Epidermal WBS monitoring requires intense exercise/exertion or exposure to the warm environment to generate a certain amount of sweating and, therefore, may not be useful to those with mobility difficulties. Many works have adopted the iontophoresis technique to circumvent this problem related to sweat generation dependence. The sweat rate should be constant, and sweat performance should not be affected by variations, such as a low sweat rate. Similarly, these challenges occur with tears in ocular WBS, sample evaporation, a higher correlation between analyte and blood levels are also required.
Another questionable factor is the durability of these devices. Usually assessed only by bending and deformation testing and may not represent the actual durability of the devices. They are subject to other deterioration factors such as sensor biofouling and oxidation. Also, a better approach to the analysis of time measurements is required.
Although contact with the body occurs in peripheral and superficial areas of the skin, mouth and eyes, it is essential that the materials used for building WBS are biocompatible and are not allergens (Figure 8d), and avoid possible inflammation and inconvenience during use could result in obtaining artefacts during monitoring.
These devices must provide continuous answers, and different ways of harnessing power can be incorporated. A wide range of media can now power WBS (Bandodkar et al., 2016; Heikenfeld et al., 2018; Parizi, Whitmire, & Patel, 2019; Sarpeshkar, 2012; Wen et al., 2016; Zhang, Zhao, et al., 2015). Energy consumption has three primary sources: wireless communication and data transfer, data collection and the power supply of sensors.
To circumvent such this problem, the use of safe, high energy portable batteries is desirable (Figure 8e). In addition to collection devices and storage of alternative energies such as body movement (piezoelectricity), exposure to sunlight (solar cell), consumption of biomarker itself as a fuel cell, or more likely, the combination of these sources should be alternatives to solve current problems with WS and WBS power supplies (Reid & Mahbub, 2020).
Efficient monitoring modes are also required; for example, power-saving methods when these devices are not communicating to reduce power consumption for data transmission. At the peak, WS and WBS should be capable of efficiently wireless communication and transmit data through long distances enabling better data management. The most widely addressed data transmission modes today are Bluetooth or low energy Bluetooth and RFID, the last two limited by the need for proximity to receiving signals.
A widespread issue about the use of these devices concerns the accessibility and management of acquired data, as it may be subject to the risk of intrusion and tampering. Hacked systems could put the health of patients at risk. These software and hardware must be designed with security and privacy strictly limited to the user so that data encryption can add more security in data transfer (Figure 8f).
Besides, it must be considered that the high data volume resulting from continuous measurements will result in a large amount of data to be processed. It is necessary to use algorithms for filtering and cleaning data.
Another point that has not been addressed in most studies and of paramount importance for the development of efficient WS and WBS is related to the long-term effects of the exposure of electromagnetic waves generated by these devices. However, many studies propose the use of transmission of lower energy data, such as NFC or RFID (as can be seen in Figure 9), there has been no concern with biological problems, which can be caused by continuous exposure to electromagnetic waves in this energy range.
The excellent review published by Pall (2018) reports the various biological damages associated with exposures by electromagnetic waves in the 2,400 MHz, the damage has been observed in several studies such as oxidative stress, damage to sperm and testicles, neuropsychiatric effects, including changes in EEG, apoptosis, damage to cellular DNA, endocrine changes and calcium overload, in addition to the fact that most of the damage has a cumulative effect. What further reinforces the importance of studies to prevent damages that may be caused by continuous monitoring, and that propose ways to overcome such obstacles, such as the use of devices that are capable of accumulating the recorded data and whose transmission is done in safe intervals that minimize possible damage associated with continuous exposure to electromagnetic waves.
The use of data prospecting protocols should enable risk situations to be predicted and establishes better correlations between detected signals and clinical diagnoses. Finally, these devices must be easily calibrated and especially must be mass-produced at low costs to allow greater diffusion and incorporation into the market.
4 CONCLUDING REMARKS AND FUTURE PERSPECTIVES:
Recent advances in technology and materials have made it possible to provide a massive increase in interest in wearable devices for continuous monitoring. Many challenges still need to be overcome for greater trust and diffusion of these devices, such as improve the available power supplies that allow increasing the continuous use of these sensors. Another critical question is about the low power data transmission mode that does not expose the user to problems due to constant electromagnetic radiation exposure. Issues with device deformation, contamination and biofouling are significant challenges, and new materials should be evaluated to overcome these problems. Another alternative may be to by requiring that the devices contain disposable and replaceable components. It is also necessary to understand the correlations between analytes in biofluids and blood to validate results and, finally, the safety and privacy issues that should allow the user to be assisted safely and adequately. It is expected that these devices will become more agile and will be incorporated into fashion accessories to embody the daily lives of its users, thus allowing non-invasive and future monitoring of a wide range of sensors and biomarkers. Also, further validation of the clinical relevance of the information obtained by WBS is still required. The expansion of these devices will have a significant impact on daily life and allow a new interpretation and health management mode of its users.
ACKNOWLEDGMENT
We would like to thank every funding agency that allowed the development of the present work.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors have equally contributed to the review.




