Formation mechanisms of zinc, molybdenum, sulfur and phosphorus containing reaction layers on a diamond-like carbon (DLC) coating
Bildungsmechanismen von zink-, molybdän-, schwefel- und phosphorhaltigen tribochemischen Reaktionsschichten auf einer diamantähnlichen Kohlenstoffbeschichtung (DLC)
Abstract
enDiamond-like carbon (DLC) coatings are nowadays successfully applied on industrial components like pistons, piston rings and bearings in lubricated tribological contacts due to friction and wear reducing effects. In contradiction thereto, todays lubricants and additives are designed for tribological steel/steel contacts, whereby the knowledge on tribochemical layer formation on steel surfaces is comprehensive in contrast to the physical-chemical interactions between diamond-like carbon coatings, lubricants and additives. Therefore the formation mechanisms of zinc, molybdenum, sulfur and phosphorus containing reaction layers on a zirconium modified diamond-like carbon coating a-C : H : Zr (ZrCg) in lubricated tribological contacts were analyzed by means of pin-on-disc (PoD) tribometer by varying the distances from s = 200 m–3,000 m under boundary and mixed friction conditions at T = 90 °C and a contact pressure p = 1,300 MPa regarding the application of diamond-like carbon coatings on gears. The base lubricant poly-alpha-olefin (PAO) was formulated using the anti-wear (AW) and extreme pressure (EP) additive zinc dialkyldithiophosphate (ZnDTP) and the friction modifier (FM) additive molybdenum dialkyldithiophosphate (MoDTP). The chemical composition of the tribochemical reaction layers by means of and Raman spectroscopy and x-ray photoelectron spectroscopy (XPS) as well as for the thickness differ significantly by varying the additivation.
Translation abstract
deDiamantähnliche Kohlenstoffbeschichtungen (DLC) werden heute aufgrund von reibungs- und verschleißmindernden Effekten erfolgreich auf Bauteilen wie Kolben, Kolbenringen und Lagern in geschmierten tribologischen Kontakten eingesetzt. Im Gegensatz dazu sind heutige Schmierstoffe und Additive für tribologische Stahl/Stahl-Kontakte ausgelegt, wobei das Wissen über die Bildung tribochemischer Schichten auf Stahloberflächen im Gegensatz zu den physikalisch-chemischen Wechselwirkungen zwischen diamantähnlichen Kohlenstoffbeschichtungen, Schmierstoffen und Additiven umfassend ist. Daher wurden die Bildungsmechanismen von Zink-, Molybdän-, Schwefel- und Phosphor-haltigen tribochemischen Reaktionsschichten auf einer Zirkonium modifizierten diamantähnlichen Kohlenstoffbeschichtung a-C : H : Zr (ZrCg) in geschmierten tribologischen Kontakten mittels Pin-on-Disc (PoD)-Tribometer analysiert. Die Parameterauswahl basiert auf der Anwendung von diamantähnlichen Kohlenstoffbeschichtungen für Getriebeverzahnungen, sodass die Analysen unter Grenz- und Mischreibungsbedingungen bei T = 90 °C und einer Hertzschen Pressung p = 1.300 MPa durchgeführt wurden. Zur Analyse der tribochemischen Reaktionsschichtbildung wurde die Laufstrecke von s = 200 m–3.000 m variiert. Der eingesetzte Basisschmierstoff Poly-alpha-Olefin (PAO) mittels des Anti-Wear- (AW) und Extreme Pressure- (EP) Additivs Zinkdialkyldithiophosphat (ZnDTP) und des Friction Modifier- (FM) Additivs Molybdändialkyldithiophosphat (MoDTP) formuliert. Die chemische Zusammensetzung der tribochemischen Reaktionsschichten mittels Raman- und Röntgenphotoelektronenspektroskopie (XPS) sowie die Dicke unterscheiden sich durch Variation der Additivierung deutlich.
1 Introduction
Environmental compatibility and the reduction of the greenhouse gas carbon dioxide are decisive determinants in the current political, social and scientific discussion. Existing guidelines and laws with regard to environmental requirements are increasingly criticized and adjusted by tightening them. In particular, the fields of mobility and transport are of high interest and thus are subjects with a high research and development pressure, to meet the restrictive requirements and expectations. A successful approach is the increase of the powertrain efficiency in vehicles by reducing frictional losses. Besides the development of alternative technical solutions, e. g. e-mobility, water-based lubricants, also diamond-like carbon (DLC) coatings are developed and industrially used on bearings, tappets and pistons in lubricated as well as in dry-running tribological systems due to the significant reduction of friction and wear 1-18.
1.1 Structure and chemical composition of tribochemical reactive formed films on steel
Lubricants and additives nowadays are designed for tribological steel/steel contacts, whereby the knowledge on tribochemical layer formation on steel surfaces is comprehensive 9, 19-31. Herein, the commonly used anti-wear (AW) and extreme pressure (EP) additive zinc dialkyldithiophosphate, also called ZnDTP, and the friction modifier (FM) molybdenum ditihocarbamate (MoDTC) are analyzed as well as the synergistic combination of zinc dialkyldithiophosphate and molybdenum ditihocarbamate in various tribological systems under different contact conditions 24, 32-34. The molecules of the additives are always bonded via Sulphur 24, 32. The protective effect of the additives is due to the formation of a tribochemical reactive formed film (TRFF) on the steel surface in tribological contacts. Thereby the interaction between the surfaces of the tribological contact partners is determined by the tribochemical reactive formed film. In order to understand their behavior in tribological contacts, the structure and properties of zinc dialkyldithiophosphate films were analyzed in 19, 24, 28, 31, 33, 35-38, Figure 1. The interlayer of the zinc dialkyldithiophosphate tribochemical reactive formed film is composed of ferrous, zinc and sulfurous (Fe, S/Zn, S) bindings. This interlayer is t ≈10–20 nm thick, whereas the substantial part of the t ≈120 nm thick 24, 38. A tribochemical reactive formed film is composed of various glassy ferrous and zinc containing phosphates changing from short chain structure to a long chain structure 19, 24, 31, 30, 35, 38. It was found that higher temperatures lead predominantly to the formation of short chain polyphosphates in contrast to low temperatures which lead to the formation of long chain polyphosphates 35. Finally, a thin t ≈10 nm toplayer is built out of organic sulfides and zinc polyphosphates 30.
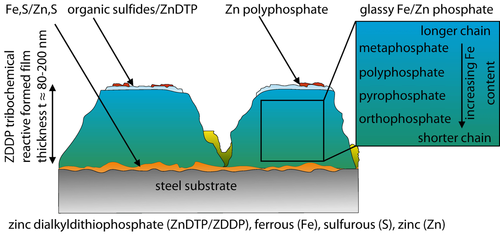
Schematic representation of the chemical composition of zinc dialkyldithiophosphate tribochemical reactive layer on steel surface [19, 24, 35, 39].
Schematische Darstellung der chemischen Zusammensetzung der tribochemischen Reaktionsschicht aus Zinkdialkyldithiophosphat auf einer Stahloberfläche [19, 24, 35, 39]
This leads to a thickness of t ≈80 nm–200 nm of the zinc dialkyldithiophosphate tribochemical reactive formed film on steel in total 24, 39. Furthermore, these zinc dialkyldithiophosphate films possess a high thermal stability up to T≤180 °C 27. Scaling the mechanical properties from toplayer to interlayer, changes of the indentation hardness from HIT(glassy Fe/Zn phosphate) ≈1 GPa–2 GPa to HIT(Fe, S/Zn, S) ≈4 GPa–5 GPa and a modulus of indentation from EIT(glassy Fe/Zn phosphate) ≈15 GPa–40 GPa to EIT(Fe, S/Zn, S) ≈90 GPa were measured 25, 38, 40-42. Hereby, the chemical composition out of long and short polyphosphate bindings as well as the substrate material are decisive. A differentiated consideration has to be conducted for the additives molybdenum ditihocarbamate and molybdenum dialkyl dithiophosphate (MoDTP) regarding the structure of tribochemical reactive formed film on a steel surface 22, 23, 29, 32, 40. Hereby, the molybdenum ditihocarbamate is composed of a carbon containing bonding structure whereby for molybdenum dialkyl dithiophosphate carbon is substituted by phosphorous 32. The tribochemical reactive formed film structure of the molybdenum-based additives is composed of molybdenum disulfide single sheets in a carbon based matrix (MoDTC) or respectively in a molybdenum phosphate glass matrix (MoDTP) 29, 32, 43. Furthermore, the structure and chemical composition of the tribochemical reactive formed film on a steel surface was analyzed by using the synergistic combination of molybdenum ditihocarbamate and zinc dialkyldithiophosphate 34, 44.
1.2 Structure and chemical composition of tribochemical reactive layers on diamond-like carbon
Due to the increased use of diamond-like carbon coatings in industrial applications, research has to orientate onto tribological contacts containing coated contact partners. Hereby, the tribochemical interaction between lubricant, additive and steel surface is more and more substituted by the partners lubricant, additive and diamond-like carbon coating. Therefore, analyses need to be focused on the structure and formation process of tribochemical reactive formed layers (TFRL) on amorphous carbon coatings in order to understand the effects on friction and wear behavior. This topic is controversially discussed in the literature due to the high variety of differing results based on the factors
-
Composition of diamond-like carbon coatings a-C, ta-C, a-C : H, ta-C : H, a-C : M, ta-C : Me, a-C : H : Me, ta-C : H : Me, a-C : X, ta-C : X, a-C : H : X, ta-C : H : X,
-
Amount of additive/s in base lubricant
-
Tribological contact conditions (Temperature T, pressure p, velocity v, environment).
Hydrogenated diamond-like carbon coatings, a-C : H, and non-metallic modified a-C : H : X diamond-like carbon coatings are characterized to be chemically inert resulting in no tribochemical reactive film formation 45-47. This is in contradiction to where tribochemical reactive formed layers were reported for on (t)a-C(:H) coatings 15, 18, 20, 48-59. In general, the formation of tribochemical reactive formed layers on metal or non-metal modified diamond-like carbon coatings (t)a-C(:H)(:Me/X) seems to be driven by the interaction between the metal or non-metal and the lubricant and its additives 18, 50, 51, 57, 60-65. The effects of different amount of additives as well as the tribological conditions are discussed in 47, 53, 60, 62, 66.
The structure and chemical composition of tribochemical reactive formed layers on diamond-like carbon coatings differs in terms of chemical composition, thickness, mechanical properties and wear behavior from the tribochemical reactive formed film on steel. The structure of a zinc dialkyldithiophosphate tribochemical reactive formed layers on diamond-like carbon coatings was analyzed 61, Figure 2. A polyphosphate film is built on an amorphous carbon coating without a sulfurous containing layer like on steel, Figure 1. The polyphosphate is composed of long chain phosphates which change to shorter chain phosphates during sliding in the tribological contact 19.
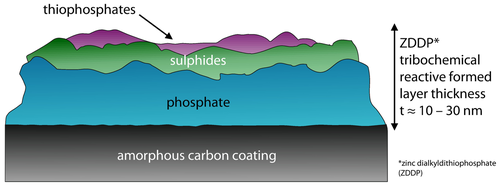
Schematic representation of the chemical composition of zinc dialkyldithiophosphate tribochemical reactive layer on amorphous carbon coating [20, 61].
Schematische Darstellung der chemischen Zusammensetzung der tribochemischen Reaktionsschicht aus Zinkdialkyldithiophosphat auf einer amorphen Kohlenstoffbeschichtung [20, 61]
Hereupon a thin sulfurous layer is located followed by a thiophosphate layer at the top, Figure 2. The thickness of tribochemical reactive formed layers on diamond-like carbon was found to be t ≈10 nm–30 nm for zinc dialkyldithiophosphate additivation 61. Considering the mechanical properties of the zinc dialkyldithiophosphate anti wear tribochemical reactive formed layers, a dependence on the chemical composition of a metal-free amorphous carbon coating was shown resulting in an indentation hardness of HIT(a-C : H, ta-C) ≈0.1 GPa–1 GPa and a modulus of indentation EIT(a-C : H, ta-C) ≈10 GPa–100 GPa in comparison to HIT(a-C : H:WC) ≈8 GPa–10 GPa and a modulus of indentation EIT(a-C : H:WC) ≈400 GPa–500 GPa for a metal modified diamond-like carbon coating 61. The formation process, structure and chemical composition of molybdenum ditihocarbamate or molybdenum dialkyl dithiophosphate induced tribochemical reactive formed layers is similar to the formation process on steel 50. Both consist of molybdenum disulfide nanosheets formed by decomposition of molybdenum ditihocarbamate or molybdenum dialkyl dithiophosphate, respectively, which builds up on several pads. The formation of molybdenum disulfide takes place solely within the tribological contact 19. The thickness and mechanical properties are assumed to be ranging in the same area as on steel 50, 67. The knowledge about the chemical composition, structure, thickness and mechanical properties of tribochemical reactive formed layers in tribological contacts with diamond-like carbon coated components under molybdenum ditihocarbamate/zinc dialkyldithiophosphate additivation is low 19, 56. The tribochemical reactive formed layers is composed of molybdenum disulfide, zincoxide (ZnO) and zincsulfide (ZnS) due to the decomposition of molybdenum ditihocarbamate and zinc dialkyldithiophosphate 24, 32.
1.3 Analysis of structure and morphology of tribochemical reactive formed layers on diamond-like carbon coatings in lubricated tribological contacts
Comparing the state of the art in the field of tribochemical interactions between lubricant, additive and steel surface or diamond-like carbon coating, it can be seen that there is little knowledge on the interaction with diamond-like carbon. Therefore, the formation mechanisms of zinc, molybdenum, sulfur and phosphorus containing reaction layers on a zirconium modified and hydrogen containing diamond-like carbon coating a-C : H : Zr (ZrCg) in lubricated tribological diamond-like carbon/diamond-like carbon contacts were studied under pure sliding conditions by means of pin on disc (PoD) tribometer. The zirconium modified and hydrogen containing diamond-like carbon coating was chosen for this study due to promising tribological results on tappets, gears and bearings 7, 8, 11, 68, 69. The mechanical and chemical properties of the zirconium modified and hydrogen containing diamond-like carbon coating can be found elsewhere 68, 69. The synthetic base lubricant poly-alpha-olefin (PAO) was formulated using the anti-wear (AW) and extreme pressure (EP) additive zinc dialkyldithiophosphate as well as the friction modifier (FM) additive molybdenum dialkyl dithiophosphate. After tribological testing the chemical and structural formation process of the tribochemical reactive formed layers were analyzed by means of confocal laser scanning microscopy (CLSM), scanning electron microcopy (SEM), Raman spectroscopy and x-ray photoelectron spectroscopy (XPS).
2 Experimental details
2.1 Coating deposition
The metal and hydrogen containing amorphous carbon coating graded zirconium carbide a-C : H : Zr (ZrCg) was deposited in an industrial scale coating unit CC800/9 Custom, CemeCon AG, Wuerselen, Germany, by using a low temperature coating process T≤200 °C. The coating unit was equipped with two direct current (dc) magnetron sputtering (MS) cathodes operating in middle frequency (mf) pulsed mode. For the deposition of the amorphous carbon coating, two Zr targets with a purity Zr 99.5 % were used. Argon (Ar) and acetylene (C2H2) served as process and reactive gases. The process parameters for the deposition of the diamond-like carbon coatings can be found elsewhere 69. Case-hardened AISI 5115 steel samples (Ø = 25 mm, H = 8 mm) polished to a roughness Ra = 0.02 μm as well as Ø = 6 mm diameter AISI 52100 heat-treated and annealed steel balls were chosen as substrate material. The case-hardened steel AISI 5115 had a surface hardness of H = (60±2) HRC with a case-hardened depth of 55 HRC > (0.9+0.2) mm to ensure a sufficient load carrying capacity for the amorphous carbon coating 38.
2.2 Tribological analysis
The samples were tested under pure sliding conditions in a pin-on-disc tribometer, CSM Instruments, Peseux, Switzerland. Tribological tests were conducted under boundary and mixed lubrication regime by using an initial Hertzian contact pressure p0 = 1,300 MPa, constant normal force FN = 8 N, velocity v = 10 cm/s, radius r = 2.5 mm, oil temperature T = 90 °C and differing distances s = 200 m, s = 400 m, s = 600 m, s = 800 m, s = 1,000 m and s = 3,000 m. A repetition of each test and lubricant was not carried out, since varying only the distance, except s = 3,000 m, repeats the tribological measurement of the lower distance s under constant conditions, p0, FN, v, r and T. The case-hardened AISI 5115 steel samples served as base material after depositing the metal and hydrogen containing amorphous carbon coating graded zirconium carbide a-C : H : Zr. The coated steel AISI 52100 was chosen for the counter parts due to non-availability of case-hardened polished steel balls from AISI 5115 steel.
2.3 Topographical analysis
Topographical analysis of the wear tracks and tribochemical reactive formed layers were conducted by means of confocal laser scanning microscopy (CLSM), Keyence VK X210, Tokyo, Japan, after tribological testing and cleaning by using cyclohexane to wash away the remaining and non-adherent lubricant and additives. The tribochemical reactive formed layers on the metal and hydrogen containing amorphous carbon coating a-C : H : Zr resist the chemical cleaning with comparable cleaning gasolines as described elsewhere 19, 57, 70, 71. Furthermore, the morphology of the tribochemical reactive formed layers at different stages of the formation process was investigated by scanning electron microscopy. Scanning electron microcopy analyses, Oxford Link ISIS, Oxford Instruments plc, UK, were conducted at the Central Facility for Electron Microscopy (GfE), RWTH Aachen University, Aachen, Germany.
2.4 Chemical analysis
The Raman spectrometer, Renishaw InVia Reflex, Renishaw GmbH, Pliezhausen, Germany, with a λ = 532 nm laser and a diffraction grating g(λ = 532 nm) = 1,800 l/mm was used to analyze the tribochemical reactive formed layers. Measurement parameters were kept constant. Furthermore, the laser was calibrated before every measurement by using a silicon reference sample. The data was obtained from a mapping measuring method, Figure 3. Hereby, a matrix with 2×5 measurement points with a size of 20 μm×80 μm and a step width of 20 μm was placed at the edge region of the wear track on the base part, Figure 3.
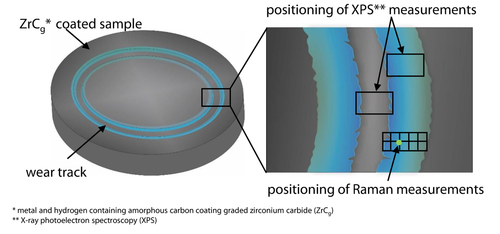
Positioning of the Raman mapping on the edge region of the wear track.
Positionierung des Raman-Mappings im Randbereich der Verschleißspur
Further analysis regarding the chemical composition of the tribochemical reactive formed layers on the diamond-like carbon coating were carried out by X-ray photoelectron spectroscopy (XPS) at DWI Leibniz Institute for Interactive Materials e.V., Aachen, Germany. The penetration depth of the analysing method is limited to x = 10 nm. Three different running distances s = 200 m, s = 600 m, s = 3,000 m at pin-on-disc tribometer were chosen as representative for the observation of the formation process of the tribochemical reactive formed layers inside and at the edge region of the wear track, Figure 3.
2.5 Lubricant
The tribological tests were conducted with formulated lubricants with a viscosity of ISO VG 100 η(T = 100 °C) = 10 cSt. As base lubricant the synthetic oil polyalphaolefin (PAO) was chosen, which is used in different industrial applications like transmissions, hydraulic systems, and aerospace 72. Polyalphaolefin was formulated with the additives molybdenum dialkyl dithiophosphate and zinc dialkyldithiophosphate. Molybdenum dialkyl dithiophosphate is commonly used as friction modifier (FM) whereas zinc dialkyldithiophosphate belongs to the anti-wear (AW) and extreme pressure (EP) additive group 24, 32, 73. The shares of the additivation with molybdenum dialkyl dithiophosphate, PAO1/0, or zinc dialkyldithiophosphate, PAO0/1, were kept at x = 2 wt. %. For the synergistic additivation molybdenum dialkyl dithiophosphate and zinc dialkyldithiophosphate a ratio of 1/3, PAO1/3, was chosen and the shares were adjusted to x = 0.5 wt. % of molybdenum dialkyl dithiophosphate and x = 1.5 wt. % of zinc dialkyldithiophosphate. Important lubricant parameters density ρ, viscosity η and viscosity index VI for all lubricants can be found in Table 1. Furthermore, the chemical composition of the unformulated and formulated lubricants were analyzed at OELCHECK GmbH, Brannenburg, Germany, Table 2. The shares of molybdenum, zinc, phosphorus and sulfur are in accordance with the given amounts in wt. %.
parameter [unit] |
PAO1/0 molybdenum dialkyl dithiophosphate/ zinc dialkyldithiophosphate |
PAO0/1 molybdenum dialkyl dithiophosphate/ zinc dialkyldithiophosphate |
PAO1/3 molybdenum dialkyl dithiophosphate/ zinc dialkyldithiophosphate |
|---|---|---|---|
Add. share [wt. %] |
2 |
2 |
2 |
ρ(T = 15 °C) [kg/dm3] |
0.84 |
0.84 |
0.84 |
η(T = 40 °C) [mm2/s] |
63.33 |
63.60 |
63.33 |
η(T = 100 °C) [mm2/s] |
10.44 |
10.52 |
10.47 |
VI [-] |
155 |
154 |
155 |
element [mg/kg] |
PAO1/0 molybdenum dialkyl dithiophosphate/ zinc dialkyldithiophosphate |
PAO0/1 molybdenum dialkyl dithiophosphate/ zinc dialkyldithiophosphate |
PAO1/3 molybdenum dialkyl dithiophosphate/ zinc dialkyldithiophosphate |
|---|---|---|---|
Zinc Zn |
/ |
1,636 |
1,139 |
Phosphorus P |
1,003 |
1,435 |
1,134 |
Sulphur S |
2,154 |
2,876 |
2,626 |
Molybdenum Mo |
1,517 |
/ |
226 |
Water H2O |
<0.10 |
<0.10 |
<0.10 |
3 Results and discussion
3.1 Tribological analysis at pin-on-disc tribometer
After coating deposition, the samples were tribologically tested by constant parameters p0 = 1,300 MPa, FN = 8 N, v = 10 cm/s, r = 2.5 mm, T = 90 °C and differing distances s = 200 m, s = 400 m, s = 600 m, s = 800 m, s = 1,000 m and s = 3,000 m. Figure 4 shows the coefficient of friction under PAO1/0, PAO0/1 and PAO1/3 lubrication at s = 200 m, s = 800 m and s = 3,000 m representatively. The coefficient of friction for PAO1/0 remains in the area between 0.040 ≲ coefficient of friction (PAO1/0, s = 200 m-3,000 m) ≲ 0.052. The anti-wear and extreme pressure additive zinc dialkyldithiophosphate leads to an increased coefficient of friction (PAO0/1, s = 200 m-3,000 m) ≈0.060–0.065. Moreover, the synergistic additivation of molybdenum dialkyl dithiophosphate and zinc dialkyldithiophosphate results in a lower coefficient of friction compared to PAO0/1 lubrication coefficient of friction (PAO1/0, s = 200 m-3,000 m) ≲ 0.055 by neglecting the running-in phase. This leads to the conclusion, that the friction reducing effect of molybdenum dialkyl dithiophosphate is not suppressed by the presence of zinc dialkyldithiophosphate. Deviations in the coefficient of friction between s = 200 m and s = 3,000 m can be neglected taking into account, that s = 200 m are only 1/15 of the distance of s = 3,000 m. The distance s = 200 m can be seen as a running-in phase at higher distances. The same argument is valid for s = 800 m.
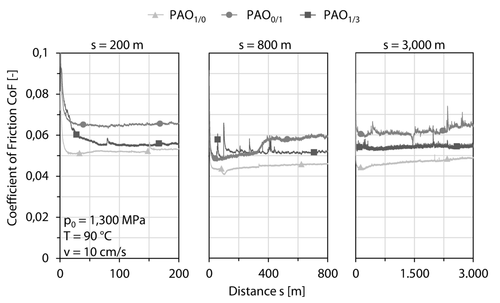
Pin-on-disc tests under PAO1/0, PAO0/1 and PAO1/3 lubrication in an a-C : H : Zr/a-C : H : Zr contact at p0 = 1,300 MPa, v = 10 cm/s and T = 90 °C.
Pin-on-disc Tests unter PAO1/0, PAO0/1 and PAO1/3 Schmierung in einem a-C : H : Zr/a-C : H : Zr Kontakt bei p0 = 1.300 MPa, v = 10 cm/s und T = 90 °C
3.2 Topographical analysis
Topography measurements of the wear tracks and tribochemical reactive formed layers were conducted by means of confocal laser scanning microscopy after tribological testing and cleaning subsequently. The different stages of the tribochemical reactive layer formation process are shown exemplarily for PAO0/1, Figure 5a–f. The scratches crossing the tribochemical reactive formed layers can be attributed to paper wrapping of the samples indicating a low adhesion between diamond-like carbon coating and tribochemical reactive formed layers 58. The distance of s = 200 m, can be regarded as an initial phase for the tribochemical reactive film formation process, Figure 5a. Herein, tribochemical reactive formed layers can be observed at the edge region of the wear track. Regarding the distances from s = 400 m to s = 1,000 m, the color of the tribochemical reactive formed layers edges turns into blue shimmering surfaces, which can be assumed to be phosphorus containing species for PAO0/1, Figure 5b–e. Due to the circumferential speed v the less adherent tribochemical reactive formed layers are transported to the edge region of the wear track, which leads to a blue shimmering accumulation zone, Figure 5f. In addition, brown to black discolorations located further outside the edge region of the wear track, can be found next to the tribochemical reactive formed layers, Figure 5a–f. The tribochemical reactive formed layers thickness is decreasing in the brown and black discolored areas by taking the confocal laser scanning microscopy images into account, Figure 5a, b.

Confocal laser scanning microscopy images of tribochemical reactive formed layers for PAO0/1 on a-C : H : Zr, at p0 = 1,300 MPa, v = 10 cm/s and T = 90 °C.
Konfokale Laserscanning-Mikroskopie-Aufnahmen tribochemisch reaktiv gebildeter Schichten unter PAO0/1 Schmierung a-C : H : Zr, bei p0 = 1.300 MPa, v = 10 cm/s und T = 90 °C.
In order to enable a more detailed analysis of the structure and formation process, scanning electron microcopy images were taken of the tribochemical reactive formed layers. The morphology and initial formation process of tribochemical reactive formed layers at s = 200 m under PAO1/0, PAO0/1 and PAO1/3 lubrication on the a-C : H : Zr (ZrCg) coating as well as the topography of the as-deposited a-C : H : Zr coating are shown in Figure 6a–d. The tribochemical reactive formed layers first accumulate in the interspaces of the cauliflower structure of the amorphous carbon coating for all three lubricants, Figure 6b–d. Afterwards the tribochemical reactive formed layers grows over the elevations of the cauliflower structure. Hereby, a significant difference can be observed between the tribochemical reactive formed layers at PAO1/0 and PAO0/1, Figure 6b, c. The coating thickness seems to be lower for PAO1/0 at s = 200 m in comparison to PAO0/1 at s = 200 m, because the cauliflower structure is still present. The zinc containing presence of the tribochemical reactive formed layers leads to a visual vanishing of the underlying diamond-like carbon coating structure due to overgrowing.
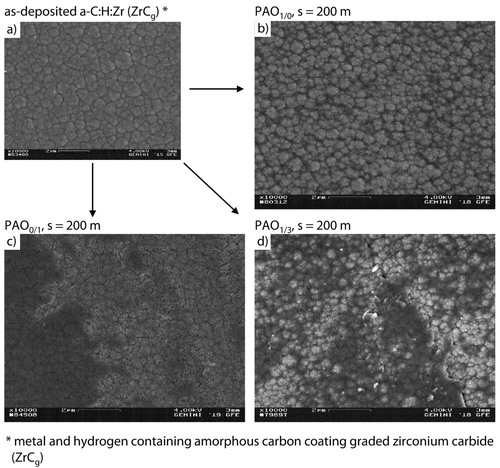
Scanning electron microcopy topography images of as deposited a-C : H : Zr and tribochemically reactive formed layers for PAO1/0, PAO0/1 and PAO1/3 at p0 = 1,300 MPa, v = 10 cm/s and T = 90 °C.
Rasterelektronenmikroskopische Topographieaufnahmen der hergestellten a-C : H : Zr-Beschichtung und tribochemisch reaktiven gebildeten Schichten für PAO1/0, PAO0/1 und PAO1/3 bei p0 = 1.300 MPa, v = 10 cm/s und T = 90 °C
The formation process under PAO1/3 lubrication can be described as a combination of the tribochemical formation processes for PAO1/0 and PAO0/1. Furthermore, the topography of the tribochemical reactive formed layers at PAO1/3 consists of selected areas that are not or rather less overgrown in accordance with PAO1/0. Moreover, isolated areas are overgrown at PAO1/0 lubrication.
The tribochemical reactive formed layers for PAO1/0, PAO0/1 and PAO1/3 at s = 800 m and s = 3,000 m are shown by scanning electron microscopy topography images, Figure 7a–f. At s = 800 m the cauliflower structure is predominantly overgrown by the tribochemical reactive formed layers under PAO1/0 lubrication. The structure is becoming denser and larger interspaces lead to deep valleys within the structure.
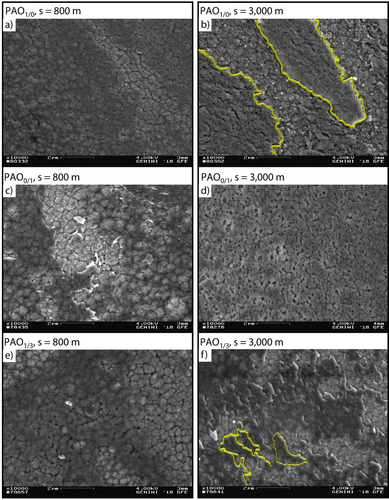
Scanning electron microcopy topography images of tribochemical reactive formed layers under PAO1/0, PAO0/1 and PAO1/3 lubrication at p0 = 1,300 MPa, v = 10 cm/s and T = 90 °C.
Rasterelektronenmikroskopische Topographieaufnahmen von tribochemisch reaktiv gebildeten Schichten unter PAO1/0, PAO0/1 und PAO1/3 Schmierung bei p0 = 1.300 MPa, v = 10 cm/s und T = 90 °C
The tribochemical reactive formed layers at PAO0/1, s = 800 m shows torn open areas in the structure down to the diamond-like carbon coating, Figure 7c. An explanation for the detaching of the tribochemical reactive formed layers could be brittle cracking due to the possible increase of residual stresses within the tribochemical reactive formed layers, respectively to its increasing coating thickness or a low adhesion between tribochemical reactive formed layers and diamond-like carbon coating 9, 58. The tribochemical reactive formed layers at PAO1/3, s = 800 m can be described in accordance with PAO0/1, s = 800 m, but seems to be thinner due to the distinctly visible structure of the cauliflower structure of the diamond-like carbon coating a-C : H : Zr, Figure 7c, e. After s = 3,000 m tribological testing, the chemical interaction between tribochemical reactive formed layers and amorphous carbon coating leads to significant differences in the grown structure under PAO1/0, PAO0/1 and PAO1/3 lubrication, Figure 7b, d, f. The molybdenum dialkyl dithiophosphate containing tribochemical reactive formed layers shows a “coarse scale-like” structure, Figure 7b. Between these scales, the cauliflower structure of the amorphous carbon coating is almost completely overgrown by the tribochemical reactive formed layer, Figure 7b. Regarding the tribochemical reactive formed layers at PAO0/1, s = 3,000 m the morphology appears as a “sponge-like” matrix with small holes inside, which are distributed all over the diamond-like carbon coating, Figure 7d. The tribochemical reactive formed layer at PAO1/3, s = 3,000 m shows a “fine scale-like” structure distributed over the amorphous carbon coating, Figure 7f. Within the fine scale-like structure, the tribochemical reactive formed layer seems to grow on various pads, so that the amorphous carbon coating is not totally overgrown and is partially visible. The tribochemical reactive formed layer at PAO1/3 appears thinner, in comparison to the structures at PAO1/0, s = 3,000 m and PAO0/1, s = 3,000 m, Figure 7b, d. Finally, the formation process of the structure of the tribochemical reactive formed layer at PAO1/3, s = 3,000 m seems to be driven by the molybdenum dialkyl dithiophosphate content due to its scale-like morphology comparable to PAO1/0, s = 3,000 m, Figure 7b. In conclusion, the initial formation process of the tribochemical reactive formed layer is quite similar for the three considered lubricants, Figure 6b, c, d. A difference can be observed regarding the thickness of the tribochemical reactive formed layers and the finally built up morphology, Figure 7b, d, f.
3.3 Chemical analysis
The tribochemical reactive formed layers were analyzed regarding the chemical composition to get deep view inside the formation process due to tribochemical interaction between lubricant, additive and diamond-like carbon coating. For this purpose, Raman spectra were recorded on tribochemical reactive formed layers for PAO1/0, PAO0/1 and PAO1/3 lubrication at s = 200 m, s = 800 m and s = 3,000 m, Figures 3, 8a–c. Furthermore, the reference Raman spectra of the as-deposited a-C : H : Zr coating is also presented to enable a comparison between the results. The effect of the tribochemical reactive formed layer on the diamond-like carbon coating was already analyzed elsewhere and will not be considered in-depth in this study 70. Raman spectra at PAO1/0, s = 200 m-3,000 m, show significant peaks in the area of ῦ = 300–1,000 cm−1 in comparison to the reference spectra of the diamond-like carbon coating, Figure 8a. A modification of the D and G peak due to the tribochemical interaction between tribochemical reactive formed layer and amorphous carbon coating a-C : H : Zr can not be observed as in literature with a different base lubricant 70.
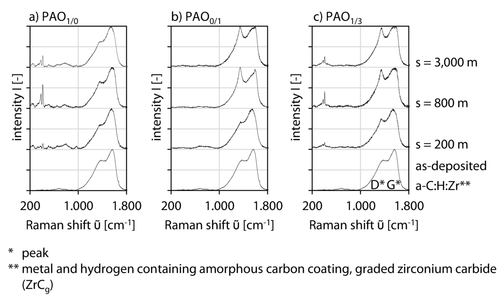
Raman spectra of tribochemical reactive formed layers under PAO1/0, PAO0/1 and PAO1/3 lubrication at p0 = 1,300 MPa, v = 10 cm/s and T = 90 °C and of a-C : H : Zr as-deposited.
Raman-Spektren von tribochemisch reaktiven gebildeten Schichten unter PAO1/0, PAO0/1 und PAO1/3 Schmierung bei p0 = 1.300 MPa, v = 10 cm/s und T = 90 °C und der hergestellten a-C : H : Zr Beschichtung
Regarding the Raman spectra of PAO0/1, s = 200 m-3,000 m, no peaks can be identified at ῦ = 300–1,000 cm−1, Figure 8b. The verification of the formation of (Zn, S) bindings at ῦ = 262 cm−1, ῦ = 350 cm−1 or a (Zn, S, P) binding at ῦ ∼960 cm−1, depending on the chemical composition, was expected regarding the results of the scanning electron microcopy topography images 74-77. A possible explanation is that the excitation energy of the λ = 532 nm laser is too low, so that an oscillation of the molecules is not achieved. In contradiction to PAO1/0, s = 200 m–3,000 m, the intensity of the D peak increases significantly from s = 200 m–3,000 m and leads to a differing I(D)/I(G) ratio 9, 78-82, Figure 8a. In addition, the Raman spectra of PAO1/3, s = 200 m–3,000 m show a double peak at ῦ = 350 cm−1–430 cm−1, which can be attributed to molybdenum disulfide (MoS2) 62, 83-85, Figure 8c. The intensity of the molybdenum disulfide peak increases from s = 200 m to s = 800 m and slightly decreases from s = 800 m to s = 3,000 m, Figure 8c. This can be explained with regard to Figure 6d, whereby the intensity is low due to the thin tribochemical reactive formed layer at s = 200 m. In addition, Figure 7e, f, represents the transition from a thick overgrown tribochemical reactive formed layer at PAO1/3, s = 800 m to a fine scale-like morphology grown on scattered pads at PAO1/3, s = 3,000 m leading to a not totally overgrown diamond-like carbon coating and subsequently to a reduced intensity of the molybdenum disulfide peak in the Raman spectra. The reduction mechanism of molybdenum disulfide can be found for PAO1/0 from s = 800 m to s = 3,000 m and can be explained by the possible formation of further reaction products reducing the intensity signal of molybdenum disulfide.
Due to the high number of peaks at PAO1/0, s = 200 m-3,000 m a differentiated consideration of Figure 8a is required. The identified peaks of the tribochemical reactive formed layer are marked in the measured Raman spectra via dotted lines for molybdenum disulfide (MoS2), molybdenum carbide (Mo2C), zirconium carbide (ZrC), zirconium dioxide (ZrO2), molybdenum trioxide (MoO3) and an area labeling for molybdenum disulfide, Figure 9. Regarding the observed low tribochemical reactive formed layers thickness of several nanometers in comparison to the penetration depth of the laser d≈1 μm, the backscattered Raman signal is dominated by the D and G peak of the metal and hydrogen containing amorphous carbon coating a-C : H : Zr, leading to low intensities of the reaction products of the tribochemical reactive formed layer. The Raman spectra of the as-deposited a-C : H : Zr amorphous carbon coating shows a slight peak at ῦ = 500–700 cm−1, which was attributed to zirconium carbide and zirconium dioxide, Table 3. The ratio of zirconium to carbon at the toplayer is x(Zr) = 4 at. % to x(C) = 96 at. % measured within the first μm beginning at the toplayer 68. By taking the tribological contact into account, the formation of scattered zirconium dioxide is possible by reaction with oxygen from the lubricant. Zirconium carbide is formed during the coating process.
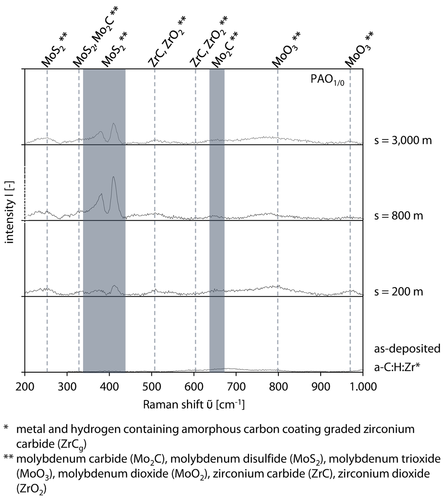
Raman spectra of tribochemical reactive formed layers for PAO1/0, at p0 = 1,300 MPa, v = 10 cm/s and T = 90 °C.
Raman-Spektren tribochemisch reaktiv gebildeter Schichten für PAO1/0, at p0 = 1.300 MPa, v = 10 cm/s und T = 90 °C
identified molecule |
Raman peak position ῦ [cm−1], measured |
Raman peak position ῦ [cm−1], literature |
|---|---|---|
molybdenum carbide |
∼333, ∼650-670 |
|
molybdenum disulfide |
∼240, ∼330, ∼350-430 |
|
zirconium carbide, zirconium dioxide |
∼508-512, ∼608 |
|
molybdenum trioxide |
∼795-805, ∼970-977 |
A small peak appears at ῦ≈333 cm−1 for PAO1/0, s = 200 m–3,000 m and could be assigned to molybdenum carbide or molybdenum disulfide 62, 84, 86, 87, Table 3. Furthermore, a molybdenum disulfide double peak was measured with different intensities as explained above. In addition, zirconium carbide and zirconium dioxide is measured at ῦ≈508 cm−1–512 cm−1 and ῦ ≈608 cm−1 88, 89, Table 3. It can be assumed that due to possible non-stoichiometric bindings of (Zr, C) and (Zr, O) a shifting of the peaks is possible. In addition, a second molybdenum carbide peak is measured at ῦ≈650–670 cm−1 which is in accordance with 62, 86, Table 3. Finally, the formation of molybdenum trioxide is proved by two peaks at two small areas ῦ≈795 cm−1–805 cm−1 and ῦ≈970 cm−1–977 cm−1 62, 76, 87, 90, Table 3.
An explanation for the formation of molybdenum disulfide, and molybdenum trioxide can be the decomposition of molybdenum dialkyl dithiophosphate during the tribological contact in accordance with the decomposition process of molybdenum dithiocarbamate (MoDTC) 32, 43, Figure 10. Hereby the molybdenum dialkyl dithiophosphate molecule decomposes due to the contact temperature and contact pressure to a sulphurous containing polyphosphate, molybdenum disulfide and molybdenum trioxide by adding oxygen. The formation process of molybdenum carbide can be assumed to be a result of worn off carbon bindings of the amorphous carbon coating toplayer and molybdenum from the additive.
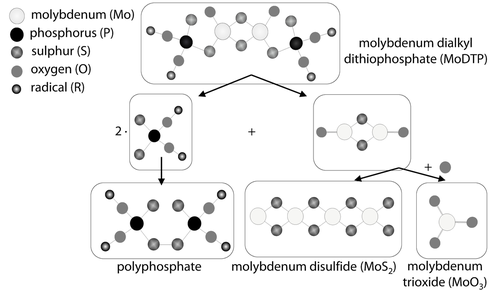
Schematic representation of the degradation of molybdenum dialkyl dithiophosphate in accordance with degradation model of molybdenum ditihocarbamate [32, 43].
Schematische Darstellung des Degradationsprozesses von Molybdändialkyldithiophosphat gemäß dem Degradationsmodell von Molybdän-Ditihocarbamat [32, 43]
In addition, x-ray photoelectron spectroscopy measurements inside and at the edge region of the tribochemical reactive formed layers were conducted on the tribologically tested diamond-like carbon coating under PAO1/0, PAO0/1 and PAO1/3 lubrication at s = 200 m, s = 600 m and s = 3,000 m. In order to keep the analyses in reasonable scope the results of s = 3,000 m under PAO1/0, PAO0/1 and PAO1/3 lubrication are shown representatively. The formation of molybdenum trioxide at E(PAO1/0) = 231.6 eV was proven regarding the molybdenum spectra at s = 3,000 m under PAO1/0 lubrication, Table 4, Figure 11a.
identified molecule* |
Peak positions E [eV], i/e** at s = 3000 m |
XPS peak positions E [eV] |
literature |
||
|---|---|---|---|---|---|
E(PAO1/0) |
E(PAO0/1) |
E(PAO1/3) |
|||
Mo2C |
227.4/- |
-/- |
-/- |
∼227; 227.8; 228.2 |
|
MoS2 |
227.4/229.7 |
-/- |
231.0/230.4 |
∼228,4-229.8 |
[22, 48, 52, 93, 13, 67, 56, 57] |
MoO3 |
231.4/233.1 |
-/- |
231.0/230.4 |
∼232,4-233,2, |
|
MoO2 |
232.5/- |
-/- |
231.0/230.4 |
∼229,1-229,6 |
[94, 22, 48, 52, 13, 67, 56, 57] |
ZnS |
-/- |
1,022.1/1,022.4 |
-/- |
∼1,022.3-1,022.7 |
[56, 57, 13, 92, 46, 93, 15] |
ZnO |
-/- |
-/- |
1,021.3/1,021.3 |
∼1,021.7-1,022.4 |
[56, 57, 13, 92, 46, 93, 15] |
ZnP |
-/- |
1,019.9/1,020.4 |
-/- |
∼1,020.6-1,020.9 |
[56, 57, 13, 93, 53, 15, 22, 49] |
P |
134.0/134.5 |
134.0/133.0 |
132.3/134 |
∼132.1-134.6 |
[53, 46, 57, 15, 49] |
S |
162.5/165.0 |
162.4/162.5 |
163.0/164 |
∼160.2-163.3 |
[56, 57, 13, 93, 53, 15, 22, 49] |
ZrS2 |
-/- |
183.3/183.3 |
181.7/180.9 |
∼181.1; ∼183.5 |
|
- * identified molecule: molybdenum carbide (Mo2C), molybdenum disulfide (MoS2), molybdenum trioxide (MoO3), molybdenum dioxide (MoO2), zincsulfide (ZnS), zincoxide (ZnO), zinc phosphate (ZnP), phosphorus (P), sulfur (S), zirconium disulphide (ZrS2) ** inside the wear track/edge region of the wear track (i/e)
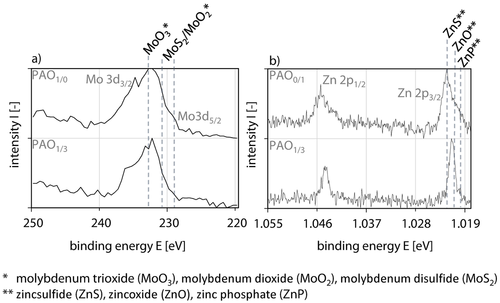
X-ray photoelectron spectroscopy measurements of Mo on tribochemical reactive formed layers for PAO1/0 and PAO1/3 and Zn on tribochemical reactive formed layers for PAO0/1 and PAO1/3 lubrication at s = 3,000 m inside the wear track.
Röntgenphotoelektronenspektroskopische-Messungen von Mo in tribochemisch reaktiv gebildeten Schichten für PAO1/0 und PAO1/3 und Zn in tribochemisch reaktiv gebildeten Schichten PAO0/1 und PAO1/3 Schmierung bei s = 3.000 m innerhalb der Verschleißspur
Furthermore, a second peak E(PAO1/0) = 229.7 eV can be assigned to molybdenum disulfide so that both measurements are in accordance with the results at Raman spectroscopy for PAO1/0, Table 4, Figure 11a. Also metaphosphate at E(PAO1/0) = 134.5 eV and sulfite E(PAO1/0) = 165.0 eV were verified inside as well as at the edge region of the wear track, Table 4. It is assumed, that molybdenum trioxide and molybdenum disulfide are present in a metaphosphate matrix. The peak positions of molybdenum at E(PAO1/3) = 231.0 eV can not be clearly assigned to molybdenum trioxide, molybdenum disulfide or molybdenum dioxide, which is due to a superposition of the peaks. Sulfide and phosphate at PAO1/3, s = 3,000 m inside and at the edge region of the wear track were measured at comparable positions as it was for PAO1/0.
Based on the x-ray photoelectron spectroscopy analysis the zinc containing tribochemical reactive formed layers at PAO0/1, s = 3,000 m is composed of zincsulfide, zinc phosphate, Table 4, Figure 11b. Due to superposition of the zincsulfide, zinc phosphate and zincoxide peaks and the verification at s = 200 m and s = 600 m inside and at the edge region of the wear track, the presence of zincoxide can be assumed. In accordance to the zincsulfide peaks, Sulfide is positioned at E(PAO1/0) = 162.5 eV, which can be assigned to sulphides. Phosphate is present as metaphosphate E(PAO1/0) = 134.0 eV inside and pyrophosphate E(PAO1/0) = 134.5 eV at the edge region of the wear track. Moreover, zirconium disulphide (ZrS2) was measured at E(PAO0/1) = 183.3 eV leading to the assumption that zirconium is released from the coating during the tribological contact and reacts with sulfide, Table 4. The results of PAO1/3 at s = 3,000 m also indicate the formation of zirconium disulphide at E(PAO1/3) = 181.7 eV inside as well as at the edge region of the wear track E(PAO1/3) = 180.9 eV, Table 4. With regard to the zinc spectra zincoxide was primarily found at E(PAO1/3) = 1,021.3 eV, Table 4, Figure 11b. However, zincsulfide and zinc phosphate should also be present due to the results for PAO1/3, s = 600 m inside and at the edge region of the wear track and the peak width in Figure 11b. Phosphate and sulfide for PAO1/3, s = 3,000 m are located in accordance with the findings for PAO0/1, s = 3,000 m, Table 4. In Figure 12 the C spectra on tribochemical reactive formed layers for PAO0/1, PAO0/1 and PAO1/3 lubrication at s = 3,000 m inside the wear track as well as the reference spectrum of a-C : H : Zr is presented. The diamond-like carbon coating is composed of aliphatic bindings at the toplayer, Figure 12. A significant difference can be seen by comparing the Mo spectra of PAO1/0, s = 3,000 m and PAO1/3, s = 3,000 m taking the results for C into account, Figures11a, 12. Thereby carbidic C bindings appear for PAO1/0 lubrication, contrary to PAO0/1 and PAO1/3, as a result of the removal and chemical transformation of the upper layers of the diamond-like carbon coating during the tribological contact leading to the formation of molybdenum carbide or a possible reduction of molybdenum monoxide into molybdenum carbide 20, 59. This leads to the conclusion, that the anti-wear additive has positive suppression effects on the reaction of graphitic bindings and Mo to molybdenum carbide in PAO1/3. Hereby, the amorphous carbon coating is protected against the possible negative effect of tribochemical wear through molybdenum carbide 20, 67.
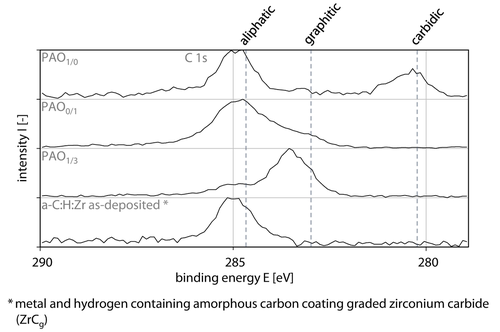
X-ray photoelectron spectroscopy measurements of C on tribochemical reactive formed layers for PAO1/0, PAO0/1 and PAO1/3 lubrication at s = 3,000 m inside the wear track and a-C : H : Zr as-deposited.
Röntgenphotoelektronenspektroskopische-Messungen von C in tribochemisch reaktiv gebildeten Schichten für PAO1/0, PAO0/1 und PAO1/3 Schmierung bei s = 3.000 m innerhalb der Verschleißspur und a-C : H : Zr
The changes from aliphatic bindings into graphitic bindings at PAO0/1 and PAO1/3 can also be seen in the Raman spectra regarding the D peak intensity increase, Figures 8b, c, 12. The explanation for the tribochemical interaction mechanism were given for comparable tribological tests in 70. Based on the scanning electron microcopy images, Raman spectra and x-ray photoelectron spectroscopy results, a schematic structure of the tribochemical reactive formed layers under PAO1/0, PAO0/1 and PAO1/3 lubrication can be assumed, Figure 13. The tribochemical reactive formed layers of molybdenum dialkyl dithiophosphate on a-C : H : Zr is composed of a metaphosphate matrix with embedded molybdenum disulfide, molybdenum trioxide and molybdenum carbide particles inside, Figure 13a. According to the scanning electron microcopy images the tribochemical reactive formed layers should be several t ≈80 nm–150 nm thick. The structure of the tribochemical reactive formed layers under PAO0/1 lubrication could be bonded by a zirconium disulphide/ zinc sulphide layer, Figure 13b. Thereupon a glassy zinc phosphate consisting of metaphosphates and pyrophosphates containing zincoxide is located as a toplayer, which is similar to the structure of tribochemical reactive formed layers by zinc dialkyldithiophosphate on steel 19, 24, 30, 31, 33, 35-37. The structure of the tribochemical reactive formed layers appears “sponge-like” with a thickness between t≈100 nm–180 nm covering the amorphous carbon coating. The morphology of the synergistic additive molybdenum dialkyldithiophosphate/zinc dialkyldithiophosphate seems also to be bonded by a zirconium disulphide/zinc sulphide layer with a phosphate toplayer above. The phosphate is composed of molybdenum trioxide, molybdenum disulfide and zinc phosphate in a metaphosphate matrix. The thickness of the tribochemical reactive formed layers is significantly reduced in comparison to the tribochemical reactive formed layers thicknesses of molybdenum dialkyl dithiophosphate and zinc dialkyldithiophosphate to t ≈40 nm–80 nm.
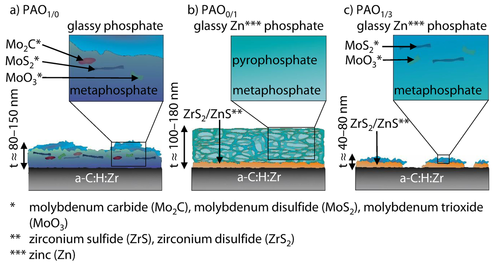
Schematic representation of the chemical composition of molybdenum dialkyl dithiophosphate, zinc dialkyldithiophosphate and molybdenum dialkyl dithiophosphate/zinc dialkyldithiophosphate tribochemical reactive formed layers on diamond-like carbon.
Schematische Darstellung der chemischen Zusammensetzung von Molybdändialkyldithiophosphat, Zinkdialkyldithiophosphat und Molybdändialkyldithiophosphat/Zinkdialkyldithiophosphat tribochemisch reaktiv gebildeter Schichten auf diamantähnlichem Kohlenstoff
4 Summary and conclusion
In this study tribochemical reactive formed layers in tribological contacts under PAO1/0, PAO0/1 and PAO1/3 lubrication on a zirconium and hydrogen modified amorphous carbon coating
a-C : H : Zr (ZrCg) were analyzed. Herein, the focus was set on the formation process, morphology and chemical composition of the tribochemical reactive formed layers.
The morphology differs from a “coarse scale-like”, PAO1/0 to a “sponge-like” matrix at PAO0/1 and a “fine scale-like” structure at PAO1/3 lubrication. The chemical analysis by Raman spectroscopy prove the formation of molybdenum disulfide, molybdenum trioxide, molybdenum carbide at PAO1/0. In addition, the detailed chemical structure was analyzed by X-ray photoelectron spectroscopy and verified the results of Raman spectroscopy and gave a deeper view inside the structure of tribochemical reactive formed layers on an amorphous carbon coating. Furthermore, zinc dialkyldithiophosphate suppresses the formation of molybdenum carbide, which is beneficial for the wear reduction in tribological contacts. Finally, a schematic model of the tribochemical reactive formed layers on an amorphous carbon coating was assumed based on the described results with regard to the formation mechanism.
In a next step transmission electron microscopy (TEM) analysis of the tribochemical reactive formed layers on the amorphous carbon coatings have to be conducted. Hereby, the morphology and structure could be analyzed on atomic scale. Moreover, the mechanical properties could be analyzed by means of nanoindentation to measure the indentation hardness HIT and modulus of indentation EIT of the tribochemical reactive formed layers. In addition, nanoscratches will help to understand the plastic and elastic deformation behavior in tribological contacts. The chemical composition and structure should also be investigated by means of Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS).
Acknowledgements
The authors gratefully acknowledge the financial support of the German Research Foundation, Deutsche Forschungsgemeinschaft (DFG), within the project “Physical and chemical interaction between diamond-like carbon coatings, additives and lubricants” BO1979/46-1. The authors would also like to thank the Gear Research Centre (FZG), TU Munich, Garching b. Munich, Germany, for providing the lubricants.




