Highly Stretchable, Transparent, Self-Healing Ion-Conducting Elastomers for Long-Term Reliable Human Motion Detection
Abstract
The flexible electronic sensor is a critical component of wearable devices, generally requiring high stretchability, excellent transmittance, conductivity, self-healing capability, and strong adhesion. However, designing ion-conducting elastomers meeting all these requirements simultaneously remains a challenge. In this study, a novel approach is presented to fabricate highly stretchable, transparent, and self-healing ion-conducting elastomers, which are synthesized via photo-polymerization of two polymerizable deep eutectic solvents (PDESs) monomers, i.e., methacrylic acid (MAA)/choline chloride (ChCl) and itaconic acid (IA)/ChCl. The as-prepared ion-conducting elastomers possess outstanding properties, including high transparency, conductivity, and the capability to adhere to various substrates. The elastomers also demonstrate ultra-stretchability (up to 3900%) owing to a combination of covalent cross-linking and noncovalent cross-linking. In addition, the elastomers can recover up to 3250% strain and over 94.5% of their original conductivity after self-healing at room temperature for 5 min, indicating remarkable mechanical and conductive self-healing abilities. When utilized as strain sensors to monitor real-time motion of human fingers, wrist, elbow, and knee joints, the elastomers exhibit stable and strong repetitive electrical signals, demonstrating excellent sensing performance for large-scale movements of the human body. It is anticipated that these ion-conducting elastomers will find promising applications in flexible and wearable electronics.
1 Introduction
Soft/flexible sensor, which can transduce external stimuli into measurable electrical signals, is an important component of smart devices.[1, 2] At present, soft sensors have been applied to pressure sensors, strain sensors, bioimaging, intelligent soft robots, stretchable electronics, electronic skin, photodetectors, and other fields,[3-9] Soft sensors generally require high extensibility, good mechanical strengths, excellent conductivity, good self-healing, and outstanding biocompatibility.[1, 10-12] In addition, flexible sensors are generally attached onto selected substrates for practical applications, which requires strong adhesion. So far, the material that integrates all these desired features has rarely been reported, thus greatly limiting the advancement of flexible electronics.[13] Common soft sensors include hydrogel-based soft sensors, ionogel-based soft sensors, and ion-conducting elastomer-based soft sensors.[14] Hydrogel-based soft sensors possess excellent conductivity, high sensitivity, fast responsivity, and good biocompatibility, but their properties are highly affected by the ambient environment.[15-17] Specifically, hydrogels become brittle and lose their original conductivity and sensitivity under low temperatures because of the freezing of water.[18, 19] Besides, evaporation of water in hydrogels under high temperatures is an inevitable and critical problem that limits their further applications.[20, 21] On the other hand, ionogels, which consist of ionic liquids (ILs) entrapped in 3D polymer networks, have overcome the above issues faced by the hydrogels because ILs have negligible vapor pressure.[22, 23] Although the nonvolatile nature of ILs enables ionogels to be used in extreme environment such as high or low temperatures, and even in a vacuum,[23-25] they still suffer from the leakage of solvent under stretching and compression, and difficulty in balancing mechanical properties and conductivity.[26-28] Therefore, solid-state and liquid-free ion-conducting elastomers seem to be the ideal material candidates without the abovementioned weaknesses of hydrogels and ionogels, and show promising applications in flexible and wearable electronics.[29, 30]
At present, numerous studies have been conducted on fabricating novel ion-conducting elastomers (or ionic conducting elastomers, ICEs) for healthcare applications.[31, 32] ICEs utilize ions (e.g., lithium salts and choline chloride) as current carriers and achieve good conductivity by ionic transportation in polymer chains.[33] Different from conductive hydrogels and ionogels, ICEs are solvent-free, so they can overcome the evaporation of water in hydrogels and leakage of ILs in ionogels, as well as have good stability in air without losing their original stretchability and conductivity. In 2018, Shi and co-workers introduced the concept of ionic conducting elastomers and prepared ICEs by photo-curing of precursor solution of monomer (n-butyl acrylate, BA), cross-linker (PEGDA) and lithium salts (LiTFSI). Owing to the solid-state nature of ICEs, they possess excellent thermal stability and long-term durability in open air. However, the as-fabricated ICEs have no self-healing ability.[34] To improve the self-healing ability of ICEs, Zhang et al incorporated dynamic hydrogen bonds into ICEs by co-polymerization of butyl acrylate (BA), 4-hydroxybutyl acrylate (HBA), and ethoxyethoxyethyl acrylate (EOEOEA) with the presence of lithium salts (LiTFSI). The hydrogen bonds between the ethoxy, hydroxyl and carbonyl groups endow the ICEs with great electrical self-healing ability (93%). Nevertheless, the mechanical self-healing property (< 25%) is much less than the recovery of conductivity.[35] Zhang et al proposed a dynamic cross-linking strategy to fabricate PEO ICEs. The dynamic cross-linking achieved by imine bonds can not only improve ion conductivity (2.04 × 10−4 S cm−1) by suppressing crystallinity, but also endow the elastomers with excellent stretchability (563%) and self-healing capability (96%) through a Schiff base reaction.[36] Apart from lithium salts, deep eutectic solvents (DESs) have also been used as conductive agents in ICEs.[37, 38] DESs have received extensive attention over the past decade because of their low melting temperature, outstanding biocompatibility, green fabrication, and inexpensive biological sources.[39, 40] Usually composed of quaternary ammonium salts (acting as hydrogen bond acceptors (HBA)) and non-ionic hydrogen bond donors (HBD), DESs have low lattice energy due to the charge delocalization through hydrogen bonds and thus much lower melting points than those of individual components.[41, 42] Polymerizable deep eutectic solvents (PDESs), which contain polymerizable monomers as the non-ionic HBD, are considered as a new class of polymeric ionic liquids (PILs). They take advantage of both ILs (e.g., excellent ionic conductivity and stability) and polymers (e.g., superior mechanical properties).[43] So far, several ICEs based on PDESs have been fabricated for the development of flexible, wearable, and stretchable electronics.[44-47] Despite these achievements, the field of ICEs is still in its infancy, and many challenges remain to be explored and solved. The main issue that prevents ICEs from replacing hydrogels and ionogels in soft sensors lies in their low ionic conductivity. The strong intermolecular interactions of ICEs impede the motion of polymer chain segments and further impair the movability of ions along the polymer chains. As a result, ICEs generally have lower conductivity than hydrogels and ionongels. For example, Zhou and co-workers reported lithium salts based waterborne polyurethane elastomers with excellent ionic conductivity (8.32 × 10−4 S m−1),[48] but this value is still 2–3 orders of magnitude smaller than that of conductive hydrogels and ionogels.[24, 49] Moreover, other issues, such as weak adhesion, low transparency, and poor biocompatibility, also dramatically limit the development of ICEs in human motion detection, stretchable electronics, and electronic skins. It is highly urgent yet challenging to design liquid-free ICEs with multiple desirable properties.
In this work, we have developed a novel method to fabricate ICEs with highly stretchable, transparent, and self-healing properties. The elastomers were synthesized through a simple one-pot photo-initiated co-polymerization involving two PDESs monomers: methacrylic acid (MAA)/choline chloride (ChCl) and itaconic acid (IA)/ChCl. MAA/ChCl and IA/ChCl are two typical PDESs monomers and have been applied to hydrogels.[39, 41] Although MAA/ChCl has some applications in ICEs,[50-52] IA/ChCl has rarely been reported in this field. The difunctional carboxyl groups of IA are expected to enhance hydrogen bonds and result in better mechanical and self-healing properties because one carboxyl group can act as hydrogen bond donor for the formation of PDESs, the other one can form hydrogen bonds with hydroxyl groups and other carboxyl groups in PDESs. The dynamic hydrogen bonds between abundant hydroxyl and carboxyl moieties in the two PDESs endow as-prepared ICEs, i.e., poly(MAA/ChCl-co-IA/ChCl), with energy dissipation and network reconstruction, further contributing to the high stretchability and self-healing ability.[53] Meanwhile, the ionic liquids nature of PDESs results in excellent ionic conductivity, sensitivity, and stability of ICEs, enabling the elastomer to be employed as strain sensors to monitor the large-scale movements of the human body with stable and repeatable response. Therefore, it is anticipated that these ICEs will find promising applications in flexible and wearable electronics.
2 Results and Discussion
2.1 Synthesis of Poly(MAA/ChCl-co-IA/ChCl) ICEs
As illustrated in Figure 1a, PDESs with the molar ratio of MAA: IA: ChCl = 0.5: 0.5: 1, 1: 0.5: 1, 1.5: 0.5: 1, and 2: 0.5: 1, were prepared by co-melting the three components at 100 °C (PDESs were denoted as PDES-0.5, PDES-1.0, PDES-1.5 and PDES-2.0, respectively, with the number represents molar ratio of MAA to ChCl). The PDESs remained as transparent liquids after cooling to room temperature, indicating the successful preparation of the PDESs. After vigorous shaking, the first three PDESs kept being transparent, while PDES-2.0 became opaque due to its instability (Figure S1, Supporting Information). Then, the stable PDESs were photo-initiated to polymerize into ICEs (denoted as ICE-0.5, ICE-1, and ICE-1.5, respectively) (Figure 1a). All the ICEs have good transparency at room temperature (Figure 1b; Figure S2, Supporting Information) and their chemical structure was confirmed using the ATR-FTIR spectra (Figure S3, Supporting Information). As demonstrated in Figure 1b, all ICEs showed excellent transparency with over 85% transmittance, of which the ICE-1.5 exhibited the highest transparency of 95% transmittance.
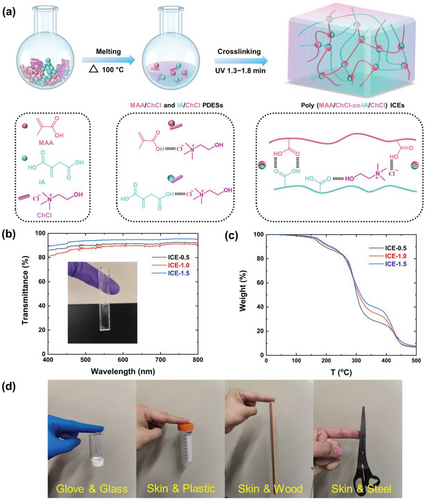
Thermal stability is a critical feature for ICEs to ensure its performance stability and enable temperature tolerance. According to TGA curves (Figure 1c; Figure S4, Supporting Information), the T5% and T10% (temperature at which a weight loss of 5% and 10% are obtained) of ICEs are 175 and 200 °C, respectively, enabling the materials to be served in a wider temperature range than common conductive hydrogels.
Adhesion is another important factor that affects the performance of soft sensors. In this study, adhesive properties were examined by simply attaching specimens to different substrates. As shown in Figure 1d, all the ICEs have strong and long-term (over 15 min) adhesion to gloves, glass, skin, plastic, wood, and steel without any additional adhesives. Therefore, poly(MAA/ChCl-co-IA/ChCl) ICEs possess desirable transparency, stability, and adhesive properties for soft sensors.
2.2 Mechanical and Mechanical Self-Healing Properties of Poly(MAA/ChCl-co-IA/ChCl) ICEs
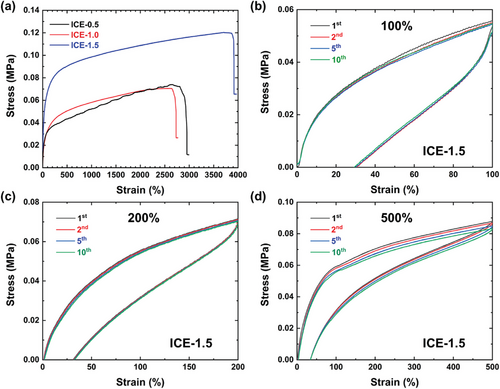
The mechanical properties of ICEs were evaluated by uniaxial tensile test and their stress-strain curves are shown in Figure 2a. Obviously, poly(MAA/ChCl-co-IA/ChCl) ICEs exhibited distinct mechanical properties by turning the molar ratio of the components. Among all three ICEs, ICE-1.5 has the highest tensile strength (0.12 MPa) and stretchability (up to 3900% elongation at break), showing excellent toughness (4.1 MPa). The high mechanical strength of ICE-1.5 can be explained by the higher polymer to small molecules ratio. On one hand, a high polymer content ensures more polymer inter-/intra-molecular interactions (i.e., chemical bonds along the chains, chain entanglement, chemical cross-linking points, hydrogen bonds, etc.). The overall number of interactions increases, and the mobility of chains decreases, leading to increased mechanical stiffness and strength. On the other hand, an increase of rigid MAA segments not only limits the movability of polymer chains, but also increases the number of carboxyl groups in the ICEs network, which are responsible for forming hydrogen bonds (as evidenced by the increased band intensity ≈1630 cm−1 in the FTIR spectra, corresponding to the carbonyl vibration in its dimeric form through hydrogen bonds[55, 56]). Upon stretching, the hydrogen bonds serve as sacrificial bonds that break before the covalent bonds, dissipating energy and protecting the polymer backbone, thus increasing the stretchability and toughness of the elastomer. In addition, hydrogen bonds not only endow ICEs with high toughness but also contribute to the good fatigue resistance of ICEs. The elasticity and anti-fatigue properties were tested by cyclic tensile tests with the maximum strain of 100%, 200%, and 500%. As presented in Figure 2b–d and Figure S5 (Supporting Information), all the tensile loading/unloading cycles have good superposition for ten cycles, despite slight decreases of mechanical strength for ICEs under 500% maximum strain. For each ICE sample, the residual strain after unloading increases with the increase of maximum strain because molecular chains require longer time to change their conformation after large deformation. However, all ICEs recovered their original shape and strength after waiting for a certain period of time (15 s for 100% and 200% strain, and 20 s for 500% strain). It is worth noting that the residual strain of ICE-1.5 for 500% maximum strain (60% after unloading) is lower than ICE-0.5 (160% after unloading) and ICE-1.0 (100% after unloading), because ICE-1.5 possesses the highest amount of inter-/intra- molecular interactions/bonds, which accelerates the recovery of strain and stress. Specifically, the noncovalent cross-linking formed by hydrogen bonds between carboxylic groups of organic acid and ChCl, combined with covalent cross-linking in the elastomer network, enhanced the elasticity and anti-fatigue properties of ICEs. It should be noted that ICE-1.5 still exhibits certain hysteresis (with a 60% residual strain after unloading from 500% maximum strain, because the polymer chains require some time to change their conformation to align with external force), owing to the dilemma in balancing conductivity and elastic resilience of ICEs: on one hand, cross-linking is a common method to improve the elasticity, but the dense cross-linking networks will reduce the movability of ions; on the other hand, introducing dynamic bonds into polymer networks can obtain high stretchability and conductivity, but it would sacrifice the elastic resilience.[36, 57] To address these compromises, Yin and co-works proposed a dual-bond cross-linking solid polymer electrolytes (SPEs) with chemically reversible ─C─N─ covalent bonds and dynamic hydrogen bonds to realize high elastic resilience and conductivity.[58] The combination of load-bearing covalent bonds and mechanically reversible hydrogen bonds endow the SPEs with high conductivity of 0.2 mS cm−1 and good elastic resilience without loading-unloading hysteresis. Therefore, introducing dynamic bonds into elastomers networks seems a convenient way to improve their elasticity and minimize the hysteresis because the dynamic bonds are mechanically reversible and can act as sacrificial bonds to protect loading-bearing covalent cross-linking networks. This phenomenon is also observed in this work, ICE-1.5 exhibits the lowest hysteresis for their highest hydrogen bonds content. Furthermore, the authors also point out that the polymer chain length also plays an important role in reducing hysteresis. The effects of dynamic bonds and chain length on elastic resilience will be further studied in our future work.
To endow soft materials with self-healing capability can greatly enhance their lifespan and reliability. The quasi-static mechanical self-healing performance was characterized by tensile test. Figure 3a presents the stress-strain curves of original and healed ICE-1.5. ICE-1.5 can be stretched up to 3250% strain after healing at room temperature for 5 min, giving a high self-healing efficiency of 66% as calculated by toughness. It should be noted that the tensile stress of healed ICE-1.5 declined when the strain reached 2500% due to some cross-linking points began to break and polymer chains started to slide. However, the samples did not completely fail and maintained a certain level of strength until complete failure occurred at 3250% strain, demonstrating the protective role of sacrificial hydrogen bonds on load-bearing covalent bonds and indicating good self-healing ability. Similarly, elastomers with lower MAA content., i.e., ICE-0.5 and ICE-1.0, also demonstrated high self-repairing efficiencies (Figure S6 and Table S1, Supporting Information). Moreover, the recovery of elasticity was further demonstrated by the cyclic tensile tests. As shown in Figures 3b–d, and S7 (Supporting Information), under various maximum elongations (100%, 200%, and 500% strain), the loading/unloading curves of all ICEs almost overlapped for 10 cycles, indicating good recovery of the elasticity. Among all healed specimens, healed ICE-1.5 showed the highest elasticity and strain recovery, while ICE-1.0 displayed the poorest self-healing performance, which cannot self-heal under 500% strain. The self-healing mechanism is believed to involve the breakage and reconstruction of reversible hydrogen bonds between poly(MAA/ChCl-co-IA/ChCl) molecules (Figure 3e). In the process of healing, the abundant hydroxyl, carboxyl groups and halide ions on one bisected surfaces form hydrogen bonds with corresponding functional groups on the other surface, mediating the movement of chain segments to change their conformation, together with other interactions such as van der Waals force, macromolecular entanglement and diffusion.[33, 59] finally facilitating the reformation of elastomer network. With the increase of the rigid MAA segments, the segments movability is impaired, which reduces self-healing capability. However, the more carboxyl groups available to form reversible hydrogen bonds facilitate self-healing process. Therefore, self-healing is the result of the collaboration of chain stiffness and hydrogen bonds. For ICE-1.0, the chain stiffness has more influence on its self-reparability, and thus exhibiting poor self-healing properties.
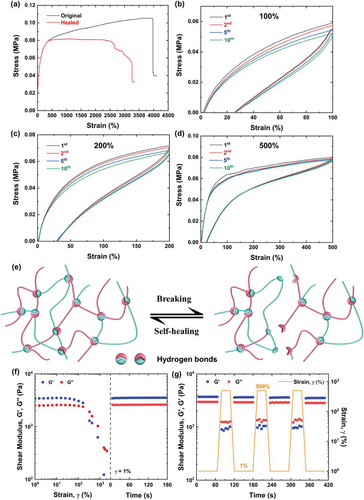
The dynamic mechanical self-healing ability was evaluated by performing oscillatory strain sweep measurement. As depicted in Figure 3f, the storage modulus (G′) and the loss modulus (G′′) of ICE-1.5 remained constant with the shear strain lower than 100%, showing good shear resistance. When shear strain reached 100%, G′ and G′′ began to decrease, and the cross-over of G′ and G′′ appeared at 200% strain, indicating large deformation of the elastomer structure. After that, both G′ and G′′ experienced dramatical decrease until the strain reached 2000%, implying severe damage in the elastomer network. However, after the strain changed to 1%, the network structure reconstructed immediately as proved by the recovery of G′ and G′′ to their initial strength within 5 s. Instant regeneration of G′ and G′′ also occurred in ICE-0.5 and ICE-1.0 (Figure S8a,b, Supporting Information). Then cyclic strain step tests were applied to demonstrate the repeating self-healing ability for ICE-1.5 (Figure 3g). Upon continuous 1% strain, the values of G′ (3.7 kPa) and G′′ (2.9 kPa) kept constant, whereas their values suddenly decreased to 0.9 kPa and 1.2 kPa, respectively, and became unstable upon continuous 500% strain owing to the rupture of network. Nevertheless, when strain switched to 1%, the values of G′ and G′′ instantly recovered to their original values and kept almost 100% self-healing efficiency in at least 3 cycles. The other two ICEs also exhibit such repeatable and outstanding dynamic self-healing capabilities (Figure S8c,d, Supporting Information) because of the fast reconstruction of reversible hydrogen bonds.
2.3 Conductive and Electrical Self-Healing Properties of Poly(MAA/ChCl-co-IA/ChCl) ICEs
The electrical properties were measured using an electrochemical workstation and the conductivities (σ) were calculated from Nyquist plots of ICEs (Figure S9 and Table S2, Supporting Information). All ICEs possessed excellent conductivities with the value for ICE-1.5 up to 1.35 × 10−2 S m−1, which stands out of recently reported ion-conducting elastomers and some hydrogels.[60-62] The excellent conductivity of ICEs might be attributed to the combination of flexibility and ion concentration: although ICE-1.5 has the lowest ion concentration, the increased number of carboxyl groups facilitates ion transportation along the polymer chains through hydrogen bonds, ultimately improving the conductivity.
The strain-sensitive electrical properties of ICEs were further measured, as shown in Figures 4a,b, and S10 (Supporting Information). The gauge factor (GF) is an important parameter to assess the strain sensitivity of strain sensors,[63] which can be obtained from the slop of the relative resistance change (ΔR/R0, calculated by ΔR/R0 = (R-R0)/R0, where R and R0 represent the real-time and initial resistances) versus strain curve. From Figure 4a,b, ICE-1.5 exhibits good GF of 0.5 at 100% strain and 1.89 at 500% strain, as calculated from the 1st differentiation of the fitting curves, displaying promising potential to be applied as strain sensors with high sensitivity and stability in a wide strain range.
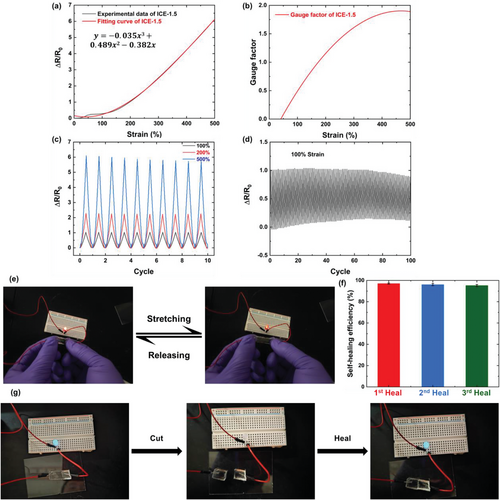
By cyclic tests with the maximum strain of 100%, 200%, and 500%, as demonstrated in Figure 4c, the ICE-1.5 showed excellent electrical signal repeatability and stability, owing to good elasticity of the elastomer. Similar repeatable sensing behaviors were observed in ICE-1.5 with 100% maximum strain for 100 cycles (Figure 4d) and in other materials compositions (Figure S11, Supporting Information). The repeatable changes of relative resistance in the stretching/releasing cycles were also proved by the luminance variation of an LED bulb during the process (Figure 4d; Video S1, Supporting Information).
The electrical self-healing properties of ICEs were also evaluated. The electrical self-healing efficiency, defined as the ratio of conductivity of healed specimen to that of the original ICEs, was calculated from Nyquist plot (Figure S12, Supporting Information). The self-healing efficiency of ICEs exceeded 94.5% for the first healing and 80.8% after 3 cutting/healing cycles at the same position of tested specimens, manifesting outstanding repeatable recovery of the conductivity. The self-healing efficiency of all ICEs in each cutting/healing cycle were presented in Figure 4e, and Figure S13 and Table S3 (Supporting Information), ICE-1.5 maintained an extraordinary self-healing efficiency of 95.18% after three cutting/healing cycles due to its outstanding flexibility, which could promote the movability of molecular chains and ions. The self-healing capability was further studied by the changes of luminance of a LED bulb during several cutting/healing cycles. As shown in Figure 4f and Video S2 (Supporting Information), a piece of ICE-0.5 was employed as a conductor of the circuit, the LED bulb kept illuminating, while the light extinguished immediately after ICE-0.5 was cut into two halves because of the open circuit. However, the bulb was turned on instantly once the two bisected pieces contacted, showing fast recovery of electrical properties. Although ICE-0.5 is the least self-healable elastomer, there is no obvious luminance decrease observed in at least 3 cutting/healing cycles, indicating excellent and repeatable electrical self-repairability due to the rapid and efficient reconstruction of conductive path fulfilled by dynamic hydrogen bonds. Owing to their outstanding stretchability, excellent conductivity, remarkable sensitivity and repeatable self-healing capability, poly(MAA/ChCl-co-IA/ChCl) ICEs exhibit great potential for a wide range of applications such as wearable sensors, electronic skins, soft robotics, bioelectronics, and so on.
2.4 Sensing Performance and Recovery of Sensing Performance of Poly(MAA/ChCl-co-IA/ChCl) ICEs
The ICEs were further applied as strain sensors to detect diverse human activities in real-time by directly attaching ICEs to different parts of human body. As displayed in Figure 5a and Video S3 (Supporting Information), ICE-1.5 was capable of monitoring the changes of relative resistance when the index finger was bending with different angles and speeds. When the bending angle switched from a small one to a large one, or the bending speed chanced from a low speed to a fast speed, the changes of relative resistance can be easily observed and distinguished from the plots, illustrating outstanding sensitivity of sensing properties. Benefiting from its excellent stretchability and electrical sensitivity, the bending activities of other joints, such as wrist, elbow, and knee (Figure 5b–d), could be detected with repeatable and reliable electrical signals. ICE-0.5 and ICE-1.0 also exhibited excellent reversibility as well as fast response to the bending of these joints (Figure S14, Supporting Information). In addition to human joint bending, a robot was employed to mimic human walking, and an ICE-1.5 specimen was attached upon one knee of the robot to record walking data (Figure 5e). The robot first walked 4 steps forward and then 4 steps backward to its original position. As presented in Figure 5f, and Figure S15 (Supporting Information) for ICE-0.5 and ICE-1.0, each forward and backward process contains 4 peaks which represent 4 steps in the process. There is no patent signal discrepancy between all forward processes, or backward processes, but the peaks for forward and backward processes look different (Figure 5g) owing to different knee bending. The backpack makes the weight point of the robot slightly backward, when the robot walks backward, its body and knees shake more vigorously than walking forward, resulting peaks for backward more fluctuating than those of forward processes. The different shapes of signal can be applied to distinguish different walking modes of the robot and further prove the excellent sensing performance and sensitivity of ICEs.
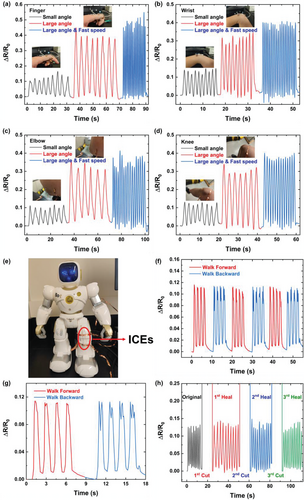
The recovery of sensing performance was further evaluated by directly mounting original and healed ICEs on the index finger to monitor bending of the finger, and the changes of relative resistance with time were recorded. As shown in Figure 5h and Figure S16 (Supporting Information), the original ICEs specimens were able to detect the movement of finger through changes of relative resistance, which has been proved previously. After cutting into two fragments, the relative resistance became infinite due to open circuit. Upon bringing the two fragments together, the relative resistance promptly recovered. The healed ICEs demonstrated sensitive monitoring of the bending and straightening of fingers through at least three cutting/healing cycles, indicating rapid restoration of sensing performance and long-term reliable detection of human motion. Leveraging their high sensitivity, stability, and self-healing capability, poly(MAA/ChCl-co-IA/ChCl) ICEs hold promise for application as wearable strain sensors for detecting human motions.
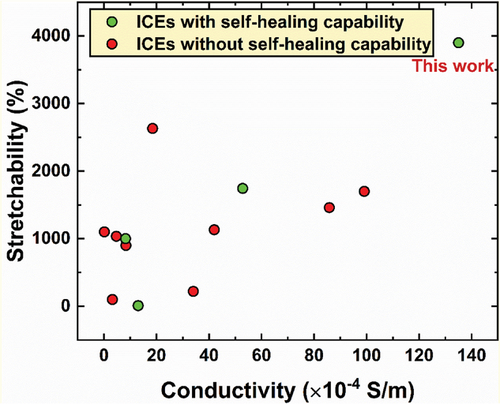
As shown in Figure 6, the combination of ultra-stretchability, desirable elasticity, brilliant conductivity, and repeatable self-healing properties enable the poly(MAA/ChCl-co-IA/ChCl) ICEs to stand out of currently existing conductive elastomers,[33-35, 38, 48, 60, 64-69] and the detailed data were shown in Table S4 (Supporting Information). Together with their good transparency, reliable environmental stability, and outstanding sensing performance, the prepared ICEs can serve as promising wearable sensors for long-term, multiscale human motion detection.
3 Conclusion
In this study, poly(MAA/ChCl-co-IA/ChCl) ICEs have been successfully fabricated through a facile one-pot photo-initiated co-polymerization of two monomers, MAA/ChCl and IA/ChCl PDESs. The synthesized ICEs possess high transparency and thermal stability which enables the ICEs to serve at a wide temperature range. The abundant hydrogen bonds formed between carboxylic groups of organic acid and ChCl in the elastomer network endow ICEs with ultra-stretchability and good mechanical self-healing ability due to the breakage and reconstruction of reversible hydrogen bonds. In addition, hydrogen bonds not only endow ICEs with high toughness but also contribute to the good elasticity of ICEs. ICEs exhibit high elasticity and anti-fatigue properties with good superposition of stress-strain cycles in the cyclic tensile loading/unloading tests because of the collaboration of noncovalent cross-linking formed by hydrogen bonds and covalent cross-linking formed by cross-linker. The ICEs also display excellent self-healing ability, reaching a high stretchability up to 3250% and fully recovering from a 500% strain after self-healing. Additionally, ICEs show outstanding conductivity since the movement of ions of ChCl was facilitated by the flexible polymer chains, and the results of cyclic tests for electrical properties display stable and repeatable electrical signals with high sensitivity. Moreover, ICEs can maintain high conductivity in cutting/healing cycles, in which ICE-1.5 can recover 95.18% conductivity after 3 cycles. Notably, ICEs are integrated with desirable and self-healable sensing performance, which can distinguish different bending activities of human joints with fast response and high sensitivity. Owing to all the excellent properties, poly(MAA/ChCl-co-IA/ChCl) ICEs hold great potential as wearable sensors for long-term reliable human motion detection. This work provides valuable insights into the development of advanced flexible electronics.
4 Experimental Section
Materials
Methacrylic acid (MAA, 98%), choline chloride (ChCl, 98%), itaconic acid (IA, 99%), poly(ethylene glycol) diacrylate (PEGDA, average Mn = 250), 2-hydroxy-4′-(2-hydroxyethoxy)−2-methylpropiophenone (photo-initiator, Irgacure 2959, 98%) were purchased from Sigma–Aldrich and used as received.
Synthesis of MAA/ChCl and IA/ChCl PDESs
PDESs with different molar ratios of MAA, IA, and ChCl were carefully synthesized. Briefly, desired amount of MAA, IA, and ChCl were added to a flask under stirring at 100 °C in an oil bath until transparent homogeneous liquids were obtained.[54] In this process, ChCl acted as hydrogen bond acceptor, while MAA and IA served as hydrogen bond donors.
Preparation of Poly(MAA/ChCl-co-IA/ChCl) ICEs
Copolymer poly(MAA/ChCl-co-IA/ChCl) was synthesized by photopolymeri-zation process. In general, photo initiator 2959 (1 mol% to comonomers) and cross-linker PEGDA (2 mol.% to comonomers) were uniformly dispersed into the prepared PDESs. After curing under UV light for 1.3–1.8 min, transparent ICEs were obtained.
Characterizations of Poly(MAA/ChCl-co-IA/ChCl) ICEs
The chemical structure of ICEs was characterized by a FTIR spectrometer (Nicolet iS50, Thermo Fisher Scientific, USA) over a range of 500–4000 cm−1 with a resolution of 4 cm−1. To measure the transparency of the ICEs synthesized, UV–vis spectra were employed on a UV–vis spectrophotometer (Evolution 300, Thermo Fisher Scientific, USA) with the length wavelength ranging from 400 to 800 nm (visible light). Thermal stability of ICEs was tested using TGA (Q500, TA Instruments, USA) with the temperature rising from 25 to 500 °C.
Mechanical properties of Poly(MAA/ChCl-co-IA/ChCl) ICEs
The stretchability of the ICEs was tested at a crosshead rate of 10 mm min−1 using an AGS-X universal tensile testing machine (Shimadzu, Japan) equipped with a load cell of 50 N at room temperature. Specimens to be tested were cut into rectangular strips of 20 mm × 9.5 mm × 1.5 mm and at least 3 duplicates were tested for each composition.
To evaluate the elasticity and anti-fatigue properties of the elastomers, cyclic tensile tests were performed at a constant crosshead rate of 50 mm/min and multiple predetermined maximum strains of 100, 200, and 500% at room temperature. The rest time between cycles was set to 15 s for 100 and 200% maximum strains and 20 s for 500% maximum strain because the polymer chains of the specimens under 500% maximum strain require more time to change their conformation to align with applied stress.
Self-Healing Properties of Poly(MAA/ChCl-co-IA/ChCl) ICEs
The mechanical self-healing abilities were also measured through tensile and cyclic tensile tests. For tensile tests, the specimen was cut into halves using a blade, and the two pieces were brought together to heal at room for 5 min. Then the healed specimen was tested at the same parameters to determine mechanical self-healing efficiency. Mechanical self-healing efficiency was defined as the ratio of the toughness of the healed specimen to that of the original specimen, as toughness accounts for the recovery of both strain and stress, which provides a comprehensive measure of the material's ability to restore its mechanical properties after damage,[38] and the toughness was defined as the area surrounded by the stress-strain curves. For cyclic tensile test of self-healed samples, specimens were healed at room temperature for 30 min before testing.
The dynamic self-healing properties were characterized through oscillatory rheological tests using an AR-G2 rheometer (TA Instruments, USA) with a 20 mm, 2° cone geometry. Oscillatory strain amplitude sweep was carried out at a constant frequency of 10 rad s−1 with strain ranging from 1 to 2000% to cause a failure of the elastomer network, then strain immediately returned to 1% at 10 rad s−1 to induce the reconstruction of elastomer network. Furthermore, the dynamic self-healing capability was captured by cyclic experiment with strain shifting steps between 1% and 500% at 10 rad s−1.
The sensing self-healing ability was obtained by recording the real-time I-t (current versus time) curves during finger bending in three cutting-healing cycles.
Electrical and Sensing Properties of Poly(MAA/ChCl-co-IA/ChCl) ICEs
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Foundation for Innovation (CFI), and the Canada Research Chairs Program.
Conflict of Interest
The authors declare no conflicts.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




