Development of a Redox-Responsive Polymeric Profluorescent Probe
Kai-Anders Hansen
School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, Queensland, 4001 Australia
Search for more papers by this authorKathryn E. Fairfull-Smith
School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, Queensland, 4001 Australia
Search for more papers by this authorSteven E. Bottle
School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, Queensland, 4001 Australia
Search for more papers by this authorCorresponding Author
James P. Blinco
School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, Queensland, 4001 Australia
E-mail: [email protected]Search for more papers by this authorKai-Anders Hansen
School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, Queensland, 4001 Australia
Search for more papers by this authorKathryn E. Fairfull-Smith
School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, Queensland, 4001 Australia
Search for more papers by this authorSteven E. Bottle
School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, Queensland, 4001 Australia
Search for more papers by this authorCorresponding Author
James P. Blinco
School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, Queensland, 4001 Australia
E-mail: [email protected]Search for more papers by this authorAbstract
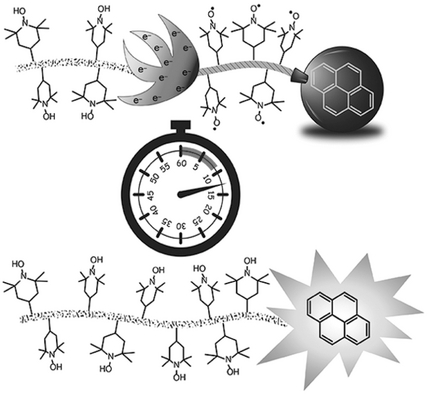
Profluorescent nitroxides (PFNs) have emerged as an important class of imaging agents for monitoring intracellular redox status and levels of oxidative stress. However, the fast reduction of nitroxides upon incubation within cells limits the window of opportunity for detection. By increasing the concentration of nitroxides per fluorophore, their reduction to the corresponding hydroxylamines and the subsequent switch-on of fluorescence can be delayed. Herein the preparation of nitroxide-containing polymers of different chain length coupled to a fluorophore is reported and their reduction with pentafluorophenylhydrazine is examined. The fluorescence switch-on kinetics and radical concentrations are monitored by fluorescence and electron paramagnetic resonance spectroscopy and compared to a conventional PFN bearing a single nitroxide moiety. The polymeric PFNs display significant delays in reduction and fluorescence switch-on and higher turn-on ratios than their single-nitroxide counterparts. The results of this study indicate that polymeric PFNs are a promising architecture for future imaging agents.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| macp201600147-sup-0001-S1.pdf1.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. Ueno, T. Nagano, Nat. Methods 2011, 8, 642.
- 2E. A. Lemke, C. Schultz, Nat. Chem. Biol. 2011, 7, 480.
- 3F. H. Kasten, in Fluorescent and Luminescent Probes for Biological Activity, 2nd ed. (Ed: W. T. Mason), Academic, London 1999, p. 17.
10.1016/B978-012447836-7/50004-X Google Scholar
- 4H. Neuweiler, M. Sauer, Curr. Pharm. Biotechnol. 2004, 5, 285.
- 5R. Roy, S. Hohng, T. Ha, Nat. Methods 2008, 5, 507.
- 6L. Ming, W. Nianqiang, in Biosensors Based on Nanomaterials and Nanodevices (Eds: L. Jun, W. Nianqiang), CRC Press, Boca Raton, FL 2013, p. 71.
- 7Q. Li, C. Wang, H. Tan, G. Tang, J. Gao, C.-H. Chen, RSC Adv. 2016, 6, 17811.
- 8Q. Mei, R. Tian, Y. Shi, Q. Hua, C. Chen, B. Tong, New J. Chem. 2016, 40, 2333.
- 9F. Hu, B. Zheng, D. Wang, M. Liu, J. Du, D. Xiao, Analyst 2014, 139, 3607.
- 10D. Aigner, S. M. Borisov, P. Petritsch, I. Klimant, Chem. Commun. 2013, 49, 2139.
- 11E. W. Miller, S. X. Bian, C. J. Chang, J. Am. Chem. Soc. 2007, 129, 3458.
- 12J. Matko, K. Ohki, M. Edidin, Biochemistry 1992, 31, 703.
- 13P. Lederhose, N. L. Haworth, K. Thomas, S. E. Bottle, M. L. Coote, C. Barner-Kowollik, J. P. Blinco, J. Org. Chem. 2015, 80, 8009.
- 14V. C. Lussini, J. P. Blinco, K. E. Fairfull-Smith, S. E. Bottle, Chem. - Eur. J. 2015, 21, 18258.
- 15J. P. Blinco, K. E. Fairfull-Smith, B. J. Morrow, S. E. Bottle, Aust. J. Chem. 2011, 64, 373.
- 16J. P. Blinco, D. J. Keddie, T. Wade, P. J. Barker, G. A. George, S. E. Bottle, Polym. Degrad. Stab. 2008, 93, 1613.
- 17J. M. Colwell, J. R. Walker, J. P. Blinco, A. S. Micallef, G. A. George, S. E. Bottle, Polym. Degrad. Stab. 2010, 95, 2101.
- 18S. Stevanovic, B. Miljevic, G. K. Eaglesham, S. E. Bottle, Z. D. Ristovski, K. E. Fairfull-Smith, Eur. J. Org. Chem. 2012, 2012, 5908.
- 19B. J. Morrow, D. J. Keddie, N. Gueven, M. F. Lavin, S. E. Bottle, Free Radical Biol. Med. 2010, 49, 67.
- 20H.-Y. Ahn, K. E. Fairfull-Smith, B. J. Morrow, V. Lussini, B. Kim, M. V. Bondar, S. E. Bottle, K. D. Belfield, J. Am. Chem. Soc. 2012, 134, 4721.
- 21N. B. Yapici, S. Jockusch, A. Moscatelli, S. R. Mandalapu, Y. Itagaki, D. K. Bates, S. Wiseman, K. M. Gibson, N. J. Turro, L. Bi, Org. Lett. 2011, 14, 50.
- 22C. L. Rayner, S. E. Bottle, G. A. Gole, M. S. Ward, N. L. Barnett, Neurochem. Int. 2016, 92, 1.
- 23H. Nam, J. E. Kwon, M.-W. Choi, J. Seo, S. Shin, S. Kim, S. Y. Park, ACS Sens. 2016.
- 24Y. Matsuoka, M. Yamato, T. Yamasaki, F. Mito, K.-I. Yamada, Free Radical Biol. Med. 2012, 53, 2112.
- 25K. E. Fairfull-Smith, S. E. Bottle, Eur. J. Org. Chem. 2008, 2008, 5391.
- 26J. P. Blinco, J. C. McMurtrie, S. E. Bottle, Eur. J. Org. Chem. 2007, 2007, 4638.
- 27S. M. Hahn, C. M. Krishna, A. Samuni, W. DeGraff, D. O. Cuscela, P. Johnstone, J. B. Mitchell, Cancer Res. 1994, 54, 2006s.
- 28B. K. Hughes, W. A. Braunecker, A. J. Ferguson, T. W. Kemper, R. E. Larsen, T. Gennett, J. Phys. Chem. B 2014, 118, 12541.
- 29M. A. Sowers, J. R. McCombs, Y. Wang, J. T. Paletta, S. W. Morton, E. C. Dreaden, M. D. Boska, M. F. Ottaviani, P. T. Hammond, A. Rajca, J. A. Johnson, Nat. Commun. 2014, 5, 5460.
- 30K. Gallas, A.-C. Knall, S. R. Scheicher, D. E. Fast, R. Saf, C. Slugovc, Macromol. Chem. Phys. 2014, 215, 76.
- 31E. M. Simpson, Z. D. Ristovski, S. E. Bottle, K. E. Fairfull-Smith, J. P. Blinco, Polym. Chem. 2015, 6, 2962.
- 32L. Rostro, S. H. Wong, B. W. Boudouris, Macromolecules 2014, 47, 3713.
- 33W. Jakubowski, K. Matyjaszewski, Angew. Chem. 2006, 118, 4594.
10.1002/ange.200600272 Google Scholar
- 34S. Liu, X. Chu, H. Wang, F. Zhao, E. Tang, Ind. Eng. Chem. Res. 2015, 54, 5475.
- 35T. Janoschka, A. Teichler, A. Krieg, M. D. Hager, U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem. 2012, 50, 1394.
- 36G. Hauffman, J. Rolland, J.-P. Bourgeois, A. Vlad, J.-F. Gohy, J. Polym. Sci., Part A: Polym. Chem. 2013, 51, 101.
- 37K. Matyjaszewski, J.-L. Wang, T. Grimaud, D. A. Shipp, Macromolecules 1998, 31, 1527.
- 38A. K. Nanda, K. Matyjaszewski, Macromolecules 2003, 36, 8222.
- 39Y. Guillaneuf, D. Gigmes, S. R. A. Marque, P. Tordo, D. Bertin, Macromol. Chem. Phys. 2006, 207, 1278.
- 40J. M. Otón, A. U. Acuña, J. Photochem. 1980, 14, 341.
- 41S. K. Lower, M. A. El-Sayed, Chem. Rev. 1966, 66, 199.
- 42J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd ed., Springer, Baltimore, MD 2006, p. 278.
- 43O. Lagrille, N. R. Cameron, P. A. Lovell, R. Blanchard, A. E. Goeta, R. Koch, J. Polym. Sci., Part A: Polym. Chem. 2006, 44, 1926.
- 44H. Kautsky, Trans. Faraday Soc. 1939, 35, 216.