Synthesis, Properties, and Doping Behavior of Optically Active Polythiophenes Bearing a Bornyl Group
Abstract
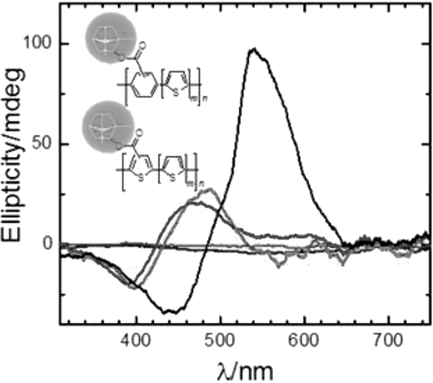
Polythiophenes bearing a bornyl group as a side chain are synthesized. The polymers, which consist of multiple thiophenes and a substituted aromatic ring in the repeat unit, demonstrate right-handed helicity in the film state. Results of energy level measurements show good agreement with the density functional theory calculation results for the model compounds. In situ electron spin resonance (ESR) studies indicate that increasing the number of unsubstituted thiophene units in the repeat unit increases susceptibility for the dopants. The chiral charge carriers are confirmed with ESR and circular dichroism spectroscopy measurements.