Sulfated Alginate for Biomedical Applications
Abstract
Alginate (Alg) polymers have received much attention due to the mild conditions required for gel formation and their good bio-acceptability. However, due to limited interactions with cells, many drugs, and biomolecules, chemically modified alginates are of great interest. Sulfated alginate (S–Alg) is a promising heparin-mimetic that continues to be investigated both as a drug molecule and as a component of biomaterials. Herein, the S–Alg literature of the past five years (2017–2023) is reviewed. Several methods used to synthesize S–Alg are described, with a focus on new advances in characterization and stereoselectivity. Material fabrication is another focus and spans bulk materials, particles, scaffolds, coatings, and part of multicomponent biomaterials. The new application of S–Alg as an antitumor agent is highlighted together with studies evaluating safety and biodistribution. The high binding affinity of S–Alg for various drugs and heparin-binding proteins is exploited extensively in biomaterial design to tune the encapsulation and release of these agents and this aspect is covered in detail. Recommondations include publishing key material properties to allow reproducibility, careful selection of appropriate sulfation strategies, the use of cross–linking strategies other than ionic cross–linking for material fabrication, and more detailed toxicity and biodistribution studies to inform future work.
1 Introduction
Heparin (Scheme 1A) is a highly sulfated glycosaminoglycan with applications in medicine and biomaterials science including the treatment of thrombosis due to its anticoagulant properties,[1] and enhancement of bone and cartilage tissue engineering constructs[2] due to its high affinity for a broad class of heparin-binding proteins (HBPs), most notably growth factors (GFs).[3] There have been several decades of research developing synthetic or semi-synthetic heparin-mimetics[1] including modified polysaccharides, sulfated oligosaccharides and glycoconjugates, and non-carbohydrate-based polyanions.[1, 4-6] The advantages of using heparin-mimetics rather than heparin itself include lower cost, longer in vivo lifetime, controlled anticoagulant activity, and ability to tailor the chemistry.[7] There are several heparin-mimetics that have been approved for clinical use including; sucrose octasulfate, pentosan polysulfate, and dextran sulfate.[1]
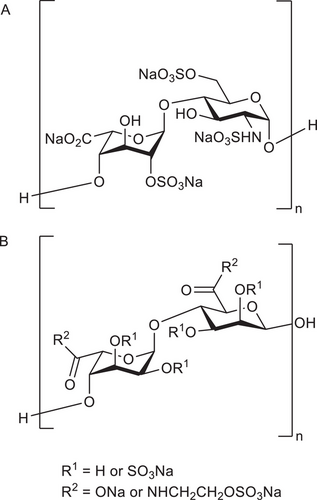
One emerging heparin-mimetic is sulfated alginate (S–Alg), produced from the naturally occurring anionic polysaccharide alginate (Alg) which is sometimes referred to as alginic acid sodium salt. Alg is a random copolymer comprised of –d-mannuronic acid (M) and -l-guluronic acid (G) residues linked via 1→4-linkages. The properties of the Alg polymer are linked to its M/G ratio and molecular weight (MW).[8-10] Alg has attracted considerable attention in biomaterials and food applications due to its mild gelling conditions using a solution of divalent cations to form a stable ionically cross–linked hydrogel in the aqueous phase. A range of sulfating agents have been used to produce S–Alg (Scheme 1B) and various applications of this heparin-mimetic have been explored. S–Alg can also be obtained commercially in different forms. This includes S–Alg products with a range of degrees of sulfation (DS), defined as the average number of sulfates per monosaccharide residue, (DS of 0.3–1.5) from ZFZ Co, and propylene glycol alginate sodium sulfate (PSS) products (DS of 1.0 or 1.3) of low MW (typically 17–18 kg mol−1) and a high M-content (M/G ratio of 2 or higher) from e.g., Haier Pharmaceutical or Lantai Pharmaceuticals Co Ltd.
Previous reviews focussing on Alg and its biomedical and biomaterials applications have included information regarding S–Alg, most notably Pawar and Edgar (2012),[8] Lee & Mooney (2012),[9] and Grøndahl et al. (2020).[10] A review about S–Alg by Arlov et al. published in 2017 covered studies reporting the synthesis of S–Alg by various methods, and the role of S–Alg in blood coagulation, immunology, and tissue engineering.[11] Arlov et al. subsequently published a review in 2021 about the sulfation of a variety of polysaccharides including Alg.[7] In this review different sulfation agents and the applications of sulfated polysaccharides as antivirals, anti-inflammatories, antioxidants, and for therapeutic delivery and regenerative medicine were detailed.
The present review gives a comprehensive overview of the synthesis and characterization of S–Alg dating back to 1972 in order to evaluate the methods that have been used to determine the DS value and to characterize this group of polymers. In addition, this review highlights the use of S–Alg as a therapeutic agent and in biomaterial science with a focus on chemical aspects including using S–Alg in the fabrication of materials, and how the presence of S–Alg can affect encapsulation efficiency (EE) and drug release rates. For these aspects, articles were identified through PubMed and Web-of-Science searches using the keywords ‘alginate sulfate or sulfated alginate’ and ‘biomedical applications’ and include studies published (e.g., available online) in the period 2017 –2023. Additionally, references included in or citing the reviews by Arlov et al. 2017 and 2021 were also checked. After careful manual verification and exclusion of articles not relevant to the topic, 22 articles were identified focusing on the use of S–Alg as a therapeutic agent, and 45 articles were identified in the field of biomaterials science. In addition, there were two recent articles focussed entirely on synthesis.
2 Synthesis of Sulfated Alginate
2.1 Methods of Sulfation
Several methods of synthesizing S–Alg from either commercial or isolated Alg polymers have been reported. The methods utilize different sulfating agents including sulfur trioxide (SO3) complexes,[12-15] chlorosulfonic acid (HClSO3),[16-23] and N,N'-dicyclohexylcarbodiimide and sulfuric acid (DCC-H2SO4).[22, 24-27] These syntheses are carried out in various solvents including water and dipolar aprotic solvents such as formamide, dimethylformamide (DMF), and dimethyl sulfoxide (DMSO). The sulfation reactions involve combining Alg with a sulfating agent for a set period of time, followed by adjusting the pH with aqueous NaOH to yield S–Alg with sulfate and carboxylate groups balanced by Na+ ions. Some approaches use Alg directly for the sulfation step (Scheme 2) while others involve the conversion of Alg from the sodium to a tetra- or tri-butylammonium salt (T-Alg) (Scheme 3) in order to improve the solubility in polar aprotic solvents. When sulfation of high MW Alg makes use of an Alg dispersion in a polar aprotic solvent in which Alg is poorly soluble,[28] heterogeneous sulfation is likely to result. However, the use of T-Alg as the precursor overcomes this issue, at least to some extent, allowing for less heterogeneous sulfation. The majority of sulfating agents will introduce sulfate groups predominantly at the C2 and/or C3 hydroxyl groups, but some studies have investigated sulfation of the carboxylate C6 (Scheme 3).

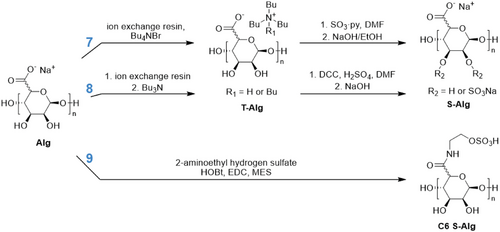
Schweiger & Andrew (1972) initially investigated a method of sulfating cellulose[12] and then Alg and pectin[12] using a sulfur trioxide-dimethylformamide complex (SO3·DMF), (Scheme 2, Reaction 1). This approach had previously been used for sulfation of chitosan (CS) as an early heparin-mimetic.[29] This was the first report of successful sulfation of Alg without excessive degradation, and while a reduction in viscosity after sulfation was attributed to some degree of degradation, it was minor compared with previous methods used for other polysaccharides.[12]
In 1979 Larm et al. described the conversion of Alg into a heparin analogue via multiple modification steps including sulfation.[30] Firstly, a fraction of the carboxylate groups were reduced to hydroxyl groups at C6, followed by partial oxidation of C2/C3 to allow for reductive amination at these sites. The final sulfation step allowed for reaction at the remaining hydroxyl groups at C2/C3 as well as C6. This sulfation process was modified from the SO3·DMF method where a sulfur trioxide-pyridine complex (SO3·py) sulfating agent was first prepared and then added to the Alg/DMF solution (Scheme 2, Reaction 2). SO3·py has been used by several other groups as a sulfating agent for Alg, sometimes with DMSO as the solvent,[13] or with a first step of converting Alg to the tetrabutylammonium or tributylammonium salt (Scheme 3, Reaction 7).[15, 31] Our recent study[31] based on the hydrodynamic radius (rH) before and after sulfation, as well as viscosity measurements, concluded that this reaction resulted in minor or no change to the chain length.
Huang et al. (2003) were the first to use a chlorosulfonic acid (HClSO3) sulfating agent for the sulfation of Alg[16] (Scheme 2, Reaction 3) as it had previously been successful at sulfating CS.[32] This is now the most widely used method of sulfation of Alg.[17-21, 33] In 2021, Mostafavi et al. used the HClSO3 reagent to produce S–Alg, but rather than converting to the Na+ form they used tributylamine (TBA) to yield a tributylammonium salt of S–Alg (hydrophobized S–Alg).[34] In 2014, Arlov et al. assessed the MW of Alg and S–Alg of different DS prepared by this reaction via size exclusion chromatography (SEC), and no depolymerization was observed as a result of the sulfation reaction.[17]
Cong et al. (2014) adapted the above method to use a chlorosulfonic acid-pyridine complex (HClSO3·py) for sulfation of Alg (Scheme 2, Reaction 4),[35] which had been used for previous work on sulfation of other polysaccharides to fabricate heparin-mimetics[36] and antitumor agents.[37] This process was patented by GH Shi in 1997 for low MW polysaccharides.[38]
Fan et al. (2011) detailed an uncommon sulfating agent trisulfamic acid, trisodium salt (N(SO3Na)3) prepared by adding a solution of sodium nitrite dropwise into sodium bisulfite (Scheme 2, Reaction 6).[39] In this study the effects of reaction pH, temperature, time, and amount of sulfating agent on the resulting DS of S–Alg were investigated. S–Alg polymers of low MW in the range of 15 to 35 kg mol−1 were obtained by oxidative degradation using hydrogen peroxide.
In 2008, Freeman et al. outlined the synthesis of S–Alg using DCC and H2SO4, with a first step of converting the Alg to the tributylammonium salt (T-Alg) (Scheme 3, Reaction 8).[24] This method was adapted from a patent by Guo et al. (2002).[40] Ma et al. (2016) conducted a study using this sulfation method directly for Alg as the starting material (Scheme 2, Reaction 5). They found that there was degradation of the Alg with this process, with more sulfating agents leading to a lower MW as determined via SEC measurements.[25] This process was patented by Cohen et al. in 2007 for bioconjugates containing sulfated polysaccharides and bioactive peptides.[41] Recently, we investigated in detail the product formed from this reaction and showed that a previously unacknowledged and unwanted adduct formed from the carbodiimide, resulting in an S–Alg product which is also functionalized with an N-acylurea.[31]
Schmidt et al. (2016) used aqueous carbodiimide coupling chemistry to achieve sulfation of the Alg carboxylate at C6 with 2-aminoethyl hydrogen sulfate (Scheme 3, Reaction 9).[42] The use of carbodiimide chemistry does not cause polymer degradation but is prone to the formation of an unwanted N-acylurea adduct from the rearrangement of the O-acylisourea intermediate.[43, 44] Sulfation at the C6 carboxylate can introduce up to one sulfate per uronic acid residue. Furthermore, it results in the same net charge for the modified polymer in contrast to the other sulfation reactions of the C2/C3 hydroxyl groups where additional charges are introduced with the sulfate groups.
Of the eight approaches described above for sulfation of Alg, some reactions provide key advantages over others. In reactions where DCC-H2SO4 is used as a sulfating agent (Reactions 5 and 8), an unwanted N-acylurea adduct is formed during the sulfation process. Particularly for biomedical applications the purity of the S–Alg product is of high importance and these methods should be avoided for such applications. Some studies have analyzed Alg and S–Alg samples via SEC, viscosity measurements, or diffusion-ordered NMR to investigate the degradation of polymers during sulfation. It should be noted that viscosity measurements alone cannot be used to evaluate degradation, as the increase in the number of charged functional groups on the polymers leads to a reduction in viscosity, regardless of chain length. Some degree of depolymerization has been reported for Reaction 1 (viscosity measurements only) and significant degradation for Reactions 5 and 8 (SEC, NMR, and viscosity measurements). Additionally, for high MW Alg polymers which are poorly solubilized in solvents such as DMF, DMSO, and formamide, it is advantageous to first convert to a tri- or tetra-butylammonium salt form to improve solubility for a subsequent sulfation step, as in Reactions 7 and 8. Based on these considerations, Reactions 3 and 7 are recommended for producing S–Alg of high purity with minimal degradation of the polymers.
2.2 Evaluation of the Degree of Sulfation
The different approaches for the synthesis of S–Alg can achieve variable DS values or the number of sulfate groups per monosaccharide. While reactions that result in sulfation at C2 and C3 hydroxyl groups have a theoretical maximum DS of 2 if sulfation selectively at the C6 carboxylate occurs then the maximum DS can be 1. The determination and reporting of DS varies widely in the literature with some reporting the number of sulfate groups per monosaccharide and some per disaccharide. In this review, all values have been normalized to represent values per monosaccharide. Many studies fail to detail the characterization of S–Alg, including the DS value, which is a key parameter to quantify in order to reproduce the work, an important aspect recently highlighted for biomedical science.[45] Figure 1A summarizes the range of DS values that have been reported for each synthetic method. Each method of sulfation yields a wide range of DS mainly depending on the ratio of sulfating agent to uronic acid monomer, while the MW of Alg and reaction conditions including temperature and reaction time may also impact the outcome. So far the highest DS reported was 1.9 using either the N(SO3Na)3 sulfating agent[39] or SO3·py.[31] When using the N(SO3Na)3 sulfating agent,[39] a reagent ratio of 2:1 (mol ratio of sulfating agent to Alg monomer) and a temperature of 40°C was found to be optimal. Furthermore, the highest DS of 1.9 was obtained at pH 9 with both lower and higher pH yielding lower DS. The method of C6 sulfation using 2-aminoethyl hydrogen sulfate was able to achieve a DS between 0.01 and 0.13 (with a theoretical maximum of 1).[42]
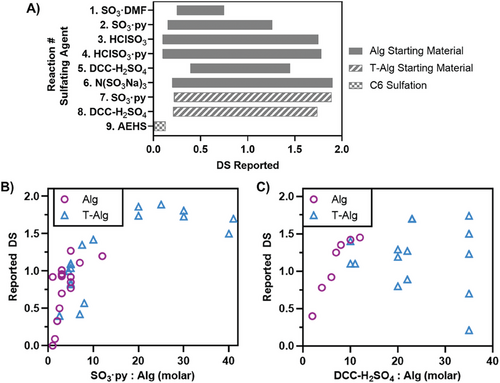
Studies using the same sulfating agent, but with either Alg or T-Alg as a starting material, were compared to assess the effect of the starting material on the resulting DS. As displayed in Figure 1B,C, Reactions 2 and 7 (SO3·py) were compared, as well as Reactions 5 and 8 (DCC-H2SO4). For S–Alg produced using the SO3·py sulfating agent there is a general trend of a higher ratio of SO3·py:Alg yielding a higher DS, regardless of whether the starting material was Alg or T-Alg. This trend is, however, not linear, and it appears that it is difficult to control DS if targeting DS values between 0.5 and 1.5. The highest DS achieved was for the T-Alg starting material which required a large excess of sulfating agent,[31] however, only relatively lower ratios of sulfating agent have been used for Alg as a starting material. When using DCC-H2SO4 for synthesis of S–Alg, there has been very high variability in the resulting DS when T-Alg was used, with no clear trend for the effect of reagent ratio on DS. While it appears that better control of DS can be achieved using Alg as starting material, all data for this reaction were from two publications from a single research group and as such reproducibility cannot be assessed.[25, 46]
To accurately determine the ratio of sulfating agent to Alg monomer, the degree of substitution and average monomer weight of T-Alg should be known and it should not simply be assumed that 100% conversion to the Bu4N+ or Bu3HN+ salt occurs. However, the characterization of T-Alg is rarely reported. Maatouk et al. (2021) reported the monomer weight of their T-Alg but did not detail how this was determined,[47] while our recent study detailed the evaluation of T-Alg monomer weight from elemental analysis.[31] The lack of this information in most reported studies means that it is presently not possible to accurately determine the effect of using T-Alg on the resulting DS from the current literature.
Elemental analysis has been the primary method used to determine DS of S–Alg. In addition, the sulfur or sulfate content of S–Alg can be determined through other techniques including ion chromatography[48, 49] or nephelometry and turbidimetry measurements of BaSO4,[14, 20, 39, 50] as well as FTIR[31] (see full description Section 2.3). Elemental analysis seems to be highly accessible these days and all recent studies (from 2017) that report DS values have used this method. It should be noted, however, that there have been different interpretations of elemental concentrations. Most research groups either use the S % only or S % and C % in formulas such as those listed in Table 1.
Equation 1 was first reported by Fan et al. (2011)[39] and is derived from the theoretical S % being equal to the mass of sulfur (DS × 32 g mol−1), divided by the monomer molar mass of Alg (C6H7O6Na, 198 g mol−1) plus the mass of SO3Na groups present (DS × 103 g mol−1) minus protons that are substituted for SO3Na (DS × 1 g mol−1), × 100%. A similar formula used by Xin et al. (2016) is Equation 2.[53] The derivation of this formula is not explained in text, other than the assumption that the carboxylate is a Na+ salt which indeed is already taken into account in Equation 1. Therefore, Equation 2 will overestimate the DS from S % obtained from elemental analysis. Equation 3 was first reported by Park et al. (2018)[26] and is derived from the of moles of sulfur (S % × 32 g mol-1) per 6 moles of carbon (6 × C % × 12 g mol−1) as there are 6 carbon atoms per uronic acid monomer. This formula had previously been applied to the sulfation of other polysaccharides with 6 carbons per monomer using a variety of sulfating agents.[56]
It is important to note that different groups make assumptions about the hydration and ionization state of S–Alg when calculating DS. Martinsen et al. conducted a study in 1991 determining MW of Alg polymers from two species of algae, including analyzing the water content by Karl Fischer titration.[57] It was found that there were approximately two water molecules bound per uronic acid monomer. Subsequent papers referring to the Martinsen et al. study follow this thinking but only assume one water molecule per uronic acid monomer.[17, 58] When determining DS from Equation 1 or 2, this does not take into account the presence of any water in the sample, so may lead to underestimating the DS. By using an element ratio such as S % : C % an accurate DS can be obtained without having to determine the amount of water present in the sample. It should be noted that for reactions that may result in the formation of an N-acylurea adduct (Reactions 5, 8, and 9), this method would underestimate DS.
It is often not explicitly stated, but most groups assume that each negative charge (sulfate and carboxylate) of S–Alg is in the sodium salt form,[16, 17] and when using Equation 1 for DS calculation this is implicit. However, few studies actually measure the sodium content of S–Alg. Almost all sulfation methods have a final step where the pH is adjusted to above 8 with NaOH (aq), and considering the pKa values of sulfate (0.5−1.5 for heparin)[59] and carboxylate (3.38−3.65 for Alg),[60] it is reasonable to assume the resulting S–Alg will have the charge on sulfate and carboxylate groups balanced by Na+ ions. However, what is often overlooked in the Fourier transform infrared (FTIR) spectra (described below) is the presence of a COOH shoulder at 1730 cm−1. If this shoulder is present in the S–Alg spectrum, then there must be some monomers that are in the carboxylic acid form.[57] This highlights the importance of using both S % and C % when calculating DS as it is not affected by the sodium content.
2.3 Spectroscopic Characterization
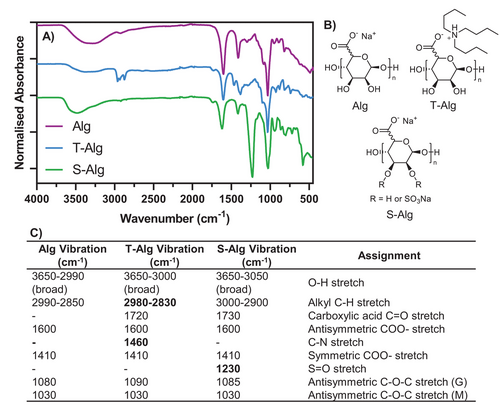
A common spectroscopic characterization technique used to verify the successful sulfation of Alg is FTIR. Figure 2A shows representative FTIR spectra for Alg, T-Alg, and S–Alg (DS = 1.8) synthesized by Reaction 7 (SO3·py method via T-Alg), with relevant chemical structures shown in Figure 2B. Figure 2C lists the assignments of the main bands in the FTIR spectra based on previous literature.[16, 25, 61, 62] Compared to the spectrum of Alg, the new bands from the tributylammonium ion in T-Alg appear between 2980 and 2830 cm−1 due to the C–H stretches, as well as a C–N stretch at 1460 cm−1 as previously reported.34, 62 Also present in the T-Alg spectrum is a small band at 1730 cm−1 which results from the C═O stretch of –COOH groups as mentioned above. The most prominent change in the FTIR spectrum for S–Alg compared to Alg or T-Alg is the band at 1230 cm−1 representing the S═O stretch. There is also a band at 1730 cm−1 suggesting some of the carboxylate groups of this polymer are in the protonated form. Some literature spectra show a similar small shoulder to the one seen in Figure 2A, however, others have more pronounced bands at 1730 cm−1 while others again have no such band present. We recently reported how FTIR can be used for estimating the DS of S–Alg.[31] Thus, a linear correlation between the intensity of the absorbance of the S═O band (1230 cm−1) relative to the Alg carboxylate band (1600 cm−1) and DS determined from elemental analysis was established for four different types of alginates (different M/G ratio and MW).
The use of 1H nuclear magnetic resonance spectroscopy (NMR) for the characterization of S–Alg is not commonly reported. This is likely due to the complex spectra where the chemical shifts for protons of the M and G residues depend on the neighboring monomers.[63, 64] This is further complicated by the presence of water in the sample, as the anomeric peaks fall at a similar chemical shift to the HOD peak and can become completely obscured. However, we recently demonstrated the usefulness of 1H NMR to evaluate if an N-acylurea adduct formed when carbodiimide chemistry is used in the sulfation reaction (Reaction 8).[31]
Low MW (up to 14 kg mol−1) sulfated poly(guluronic acid), sulfated poly(mannuronic acid), and S–Alg have been characterized using 13C NMR, allowing assignment of resonances arising from the C-atoms of each of the monomer units, and in addition for those C2 and C3 hydroxyl groups that are sulfated.[14, 17, 49, 65] To assess regioselectivity in the sulfation reaction, studies have used 1H,13C-heteronuclear single quantum coherence (HSQC) NMR for such low MW samples and observed preferential sulfation on C2 hydroxyl groups (compared to C3). This preference was observed for sulfated poly(guluronic acid), sulfated poly(mannuronic acid), and S–Alg produced using Reaction 3.[17] The use of 13C NMR to characterize S–Alg of high MW is complicated by broad peaks and significant signal overlap for the resonances for C2, C3, C4 and C5 in the 60–80 ppm range. However, using 1H,13C-HSQC NMR, we recently demonstrated successful evaluation of the regioselectivity of Reaction 7 applied to a high MW Alg polymer and found preferential C2 hydroxyl group sulfation.[31] Other work, evaluating high M-content S–Alg prepared using Reaction 4, observed a weaker signal for C2 than C3 sulfated signals for M residues, indicating a preference for substitution of the C3 hydroxyl in M monomers.[35] This was attributed to the hindrance of the axial C2 hydroxyl compared with the equatorial C3 hydroxyl in the M monomer.
Esposito et al. recently published their work utilizing multi-step strategies to achieve regioselective sulfation of Alg on the C2 or the C3 hydroxyl group as desired.[55] For this process, low MW mannuronic acid enriched alginic acid (9 kg mol−1, 83% M) was used as the starting material, and SO3·py was the sulfating agent (Reaction 2), applied after different regioselective protection strategies. 1H,13C-HSQC NMR was the primary characterization technique for confirming the site of sulfation on various S–Alg products. This is an important step toward the ability to tailor the sulfation pattern of S–Alg.
3 Application of S–Alg as a Therapeutic Agent
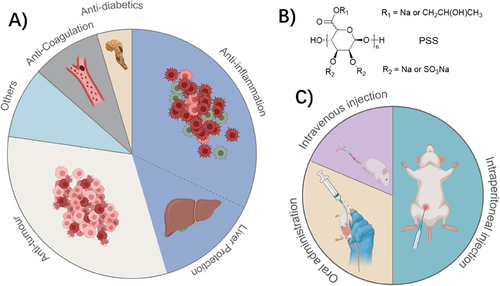
The use of S–Alg as a therapeutic agent was documented in 22 articles within the period 2017–2023. Details for the 22 recent articles can be found in Table 2 and include descriptions of the polymer used, the mode of delivery, the key studies, and major findings. S–Alg has been studied as a treatment for diabetes,[66] cancer,[67-73] and inflammation[74-83] and has been used as an anti-coagulant[46, 84] with the proportion of each application shown in Figure 3A. It is evident that there is a strong focus on exploring the anti-inflammatory properties including the use of S–Alg in liver protection. Detailed descriptions of the mechanism of action in regard to the coagulation cascade and immunological effects have been covered in-depth in previous reviews by Arlov et al. who also describe its use in reducing blood glucose levels.[7, 11]
| No | S–Alg polymer a) | Application and key studies b) | Key Findings of biological evaluation c) | Reference |
|---|---|---|---|---|
| 1 |
Alg (MW 160 kg mol−1, 1% viscosity 350–550 mPa s), Carl Roth GmbH + Co. Method of sulfation: 7. SO3·py (T-Alg) DS: 0.8–2.7 (EA) |
Antitumor therapeutics 2D in vitro antitumor model evaluation (HMT-3522 S1 and HMT-3522 T4-2 cell lines) 3D in vitro acini antitumor model evaluation (HMT-3522 S1 and HMT-3522 T4-2 cell lines) |
Growth rate of cells treated with S–Alg was consistently lower compared to untreated controls and surpassed the effect of native heparin (positive control). S–Alg preferentially hindered growth and invasion potential of tumorigenic T4–2 nodules while maintaining formation of differentiated polarized nontumorigenic HMT-3522 S1 acini. |
[67] |
| 2 |
PGM (M/G = 1:7.2, MW 10.4 kg mol−1). Method of sulfation: 3. HClSO3 DS: – (S % = 28%) |
Antitumor therapeutic In vitro cytotoxicity (293 T cells) In vivo (male C57BL/6 mice, n = 6, orally administered 400 mg kg−1 daily) Blood cell density and complement-dependent cytotoxicity assays (injected intraperitoneally with CD4 monoclonal antibody, 25 d) In vitro cell proliferation and apoptosis analysis (thymus cells isolated from C57BL/6 mice) |
PGMS had no significant inhibition on the growth of 293 T cells (concentration range 31–1000 µg mL−1). The polymer increased lymphocyte numbers more efficiently than in alkylglycerol-treated mice (day 3). No significant in vivo cytotoxicity effects on reversing hematopoietic stem cells, lymphoid progenitors, and myeloid progenitors. The polymer did not affect the thymus- derived CD4+ T cell proliferation but inhibited CD4+ T cell apoptosis. |
[69] |
| 3 |
PSS, Chia Tai Haier Pharmaceutical Co., Ltd. Method of sulfation: N/A DS: - |
Melanoma therapeutics Growth factor binding evaluation (FGF-2 and VEGF165) In vitro invasion inhibition assay (B16-F10 cells, 48 h) In vitro cell viability (B16-F10 cells) In vitro mechanism (B16-F10 cells) |
PSS inhibited serum-induced invasion of B16-F10 in a dose-dependent manner (by 34, 46, and 62% at 25, 50, and 100 µg mL−1, respectively). No inhibition of proliferation, suggesting an inhibitory effect on invasion was likely due to the inhibition of tumor cell migration or the suppression of matrix-degrading enzymes. PSS decreased the expression of MMP-2 and MMP-9 and suppressed their activity. PSS decreased the level of Vimentin in B16-F10 cells, which is known to participate in the epithelial–mesenchymal transition. |
[68] |
| 4 |
Alg (M/G = 1.9, MW 12.6 kg mol−1), PSS (M/G = 2.43, MW 17.9 kg mol−1), Chia Tai Haier Pharmaceutical Co., Ltd. Method of sulfation: N/A DS: – (S % = 11.6%) |
Antitumor therapeutics In vitro APTT assay (rat blood) Binding kinetics evaluation (P/L-selectins) In vitro selectin inhibitory activity test (HL60 and LS180 cells) In vivo antimetastatic assay (male C57BL/6 J mice, n = 7, intraperitoneally injected 30 mg kg−1 once) |
A high MW fraction (21.91 kg mol−1) exhibited optimal anticoagulant activity (APTT assay), while a low MW fraction (2.26 kg mol−1) was less effective. PSS blocked the binding of P-/L-selectin with LS180 cells and HL60, and the inhibitory effect was found higher than heparin. Compared to the untreated group, group treated with PSS exhibited a reduced number of metastatic foci in the lung induced by the B16F10 cells. In the positive control heparin-treated group, it induced death at the tested dose of 10 mg kg−1, whereas there was no death observed in the PSS-treated group. |
[70] |
| 5 |
Sodium Alg, Sigma. Method of sulfation: 7. SO3·py (T-Alg) DS: 0.4–1.35 |
Antitumor effect In vitro anti-tumor assessment (H1792 and MDA-F471 lung adenocarcinoma cell lines) Wound healing assay (H1792 and MDA-F471 cell lines, 48 h) |
The metabolic activity of H1792 cells was not affected by DS when supplemented at 10 or 100 µg mL−1. The proliferation and number of live MDA-F471 cells at 100 µg mL−1 S–Alg decreased with increasing DS. S–Alg with higher DS suppressed migration of H1792 and MDA-F471 cells by 50% and 65%, respectively. |
[71] |
| 6 |
Alg (M/G = 0.6, 2% viscosity 4500 cp), Shanghai Yuanye Co., Ltd. Oligosaccharide (MW 415–811 Da) prepared by lyase AlyB degradation. Method of sulfation: 7. SO3·py (T-Alg) DS: ≈1.3 (EA, S % = 11.77%) |
Antitumor effect In vitro antitumor assay (osteosarcoma cells) In vivo antitumor assay (female BALB/c nude mice, tail-vein injected 50 mg kg−1 daily, n = 5, 30/45 d) |
Alg and S–Alg oligos elicited antitumor effects in osteosarcoma cells with enhanced effect for S–Alg oligos. S–Alg oligo treatment was observed to trigger proapoptotic autophagy by inhibiting MEK1/ERK/mTOR signaling. The ERK activator reversed S–Alg oligo-induced autophagy. S–Alg oligos suppressed osteosarcoma cell growth and promoted autophagy through the MEK1/ERK/mTOR signaling pathway in vivo. |
[72] |
| 7 |
Alg oligosaccharide (MW 1566 g mol−1, via MS) Method of sulfation: 3. HClSO3 DS: 0.93 |
Antitumor Cytotoxicity assessment and antitumor activity (CT26.WT cell line) |
The activity of S–Alg decreased with the increasing concentration, 55% at 400 µg mL−1. Apoptosis-related proteins Bcl-2, Bax, Cox-2, and Jak-1 detected. Both Alg and S–Alg could inhibit the relative expression of Jak-1, the inhibitory effect of S–Alg stronger than that of Alg. S–Alg could inhibit the expression of Cox2 and anti-apoptotic ability of CT26.WT cells. |
[73] |
| 8 |
PSS (MW < 39 kg mol−1), Dalian Tianyu Pharmaceuticals Co. Ltd. Method of sulfation: N/A DS: - |
Acute lung injury therapeutics In vivo efficiency in LPS-induced acute lung injury mice model (C57BL/6 mice, n = 20, intraperitoneally injected 25/50 mg kg−1 once daily for 5 d) In vitro NF-κB signaling expression (murine lung epithelial 12 cells) |
Increased survival of mice that received PSS compared to control groups. PSS attenuated lung injury, as shown by lung histological detection, lung wet/dry ratio, alveolar capillary permeability, and total protein and cellular counts obtained in BALF. Inflammatory cytokine post-PSS treatment attenuated the secretion of pro-inflammatory cytokines in BALF and lung sections from LPS-induced septic mice, and it enhanced the release of anti-inflammatory cytokines. PSS had no significant effect on CXCL-1 and CXCL-2 levels of non-septic mice but down-regulated their gene transcriptions in lung tissues and decreased their secretions in BALF of LPS-induced septic mice. NF-κB signaling was inhibited by PSS in the lungs of mice subjected to LPS, as well as in the murine lung epithelial cells. |
[76] |
| 9 |
PSS, Chia Tai Haier Pharmaceutical Co., Ltd Method of sulfation: N/A DS: - PLGA NPs: unknown |
Diabetic cardiomyopathy therapeutics In vivo efficiency evaluation (male SD rats, n = 7, intraperitoneally injected 20 mg kg−1 daily) Positive controls: traditional dosage form of PSS and other drugs |
PSS-NPs had a better effect on ameliorating streptozotocin-induced cardiac dysfunction in rats and had the highest protective effect maintaining mitochondrial and microvascular structures. Increased CD31 expression in positive treatment groups, especially in PSS-NPs group. PAI-1 mRNA expression reduced by PSS-NP treatment, and fibrinolytic ability was improved. Expression of TNF-α, IL-1β, and IL-6 decreased in PSS-NP group. PSS-NPs caused the serum malondialdehyde level to decrease and serum superoxide dismutase and glutathione levels to increase. Enhanced anti-oxidative effect of PSS-NPs. |
[74] |
| 10 |
PSS, Qingdao Haier Pharmaceutical Co., Ltd. Method of sulfation: N/A DS: - PLGA particles: unknown |
Diabetic cardiomyopathy therapeutics In vivo efficiency evaluation in DCM rat model (male SD rats, n = 6, intraperitoneally injected 20 mg kg−1 daily) Control groups: prostaglandin E1, low molecular weight heparin, PSS |
Echocardiographic data showed improvement in cardiac function in PSS-NP-treated group. Abnormalities of cardiac systolic and diastolic functions were suppressed by all treatments with PSS-NP showing the strongest inhibitory effects on microvascular endothelial injuries. PSS-NPs protected the cardiac microvascular endothelium and improved endothelium dysfunction in DCM rats. PSS-NPs increased protein expression (PI3K-p85, VEGF-A, phosphorylation of protein kinase B, endothelial nitric oxide synthase). |
[75] |
| 11 |
PSS, Qingdao Zhengda haier Pharmaceutical Co., Ltd. Method of sulfation: N/A DS: - |
Hyperglycemia and hyperlipidemia In vivo characterization (C57/BL mice, 90 d, n = 12, intragastrically administered 20–100 mg kg−1 daily) |
Administration of PSS decreased the body weight of mice in the PSS 100 mg kg−1 group displaying the largest weight loss. After 90 days, the PSS administration group reduced the level of HbA1C in mice. A PSS dose of 100 mg kg−1 is comparable to Metformin administration. After 60 min of one-time injection of 1 U kg−1 insulin, the blood glucose level of PSS group was lower than model group. PSS (100 mg kg−1) reduced triglyceride, cholesterol levels, and low-density lipoprotein levels, and increased high-density lipoprotein levels. |
[77] |
| 12 |
PSS, Chiatai Haier pharmaceutical Co., Ltd. Method of sulfation: N/A DS: - |
Cardiovascular disease therapeutic In vitro release studies In vivo efficiency evaluation (beagle dogs, n = 6, orally administered 200 mg kg−1 once) ER tablet with commercial tablet as a control |
Pharmacokinetic of PSS in beagle dogs showed prolonged release within 48 h. Compared with the commercial tablets, the time to reach maximal plasma concentration, concentration-time curve, and bioavailability of PSS ER tablets increased 6-fold, 1.71-fold, and 2.17-fold, respectively. | [78] |
| 13 |
PSS, Yabao Pharmaceutical. Method of sulfation: N/A DS: - |
Acute pancreatitis therapeutics In vivo pancreatic toxicity (male Balb/C mice, n = 5) In vivo efficiency in acute pancreatitis model (male Balb/C mice, n = 12, intragastrically administered 25/50 mg kg−1 once) |
The cytokine and enzyme release as well as pathological change did not show a difference between PSS and control. Pancreatic histological scores, serum amylase and lipase activities, TNF-α, IL-1 IL-6 levels and myeloperoxidase activity were reduced by PSS via up-regulated MEK/ERK activity. Bcl-2, Bax, Lc-3, Beclin-1, P62 were reduced. |
[79] |
| 14 |
Alg (UP LVG, M/G < 0.67, low viscosity), FMC NovaMatrix Method of sulfation: 3. HClSO3 DS: 0.1–0.98 |
Osteoarthritis treatment In vitro cell assays (hCh isolated from knee cartilage) In vitro cell assay (M1-like macrophages, 24 and 72 h) |
IL-1β mediated expression of IL-6 and CXCL8 was downregulated by S–Alg (DS and concentration dependence) compared to IL-1β-treated condition. PTGS2 was not significantly reduced in any group. Among pro-inflammatory assays, the addition of Alg to M1-like cells strongly reduced the expression of the three markers in a sulfation-degree-dependent manner. Compared to control group, S–Alg reduced TNFA and CXCL10 gene expression in M1-like macrophages (DS dependence). S–Alg led to a reduction of TNF-α (DS and concentration dependence) in M1-like macrophages. |
[80] |
| 15 |
PSS (MW 20.0 kg mol−1), Dalian Tianyu Pharmaceuticals Co., Ltd. Method of sulfation: N/A DS: - |
Liver protection from ischemia-reperfusion injury In vivo hepatic IR treatment (Balb/c mice, n = 6, intraperitoneally injected 25/50 mg kg−1 once). |
No significant differences observed in ALT and AST levels in the control and PSS-treated groups. ALT and AST were higher in IR-group than in those in the sham operation, consistent with severe injury induced by hepatic IR. Apoptosis increased after IR treatment. Expression of TNF-α, IL-6, and IFN-γ mRNA in liver samples 2, 8, and 24 h after reperfusion found to be lower in the PSS-treated groups than in the IR group. LC3 and Beclin-1 upregulated and P62 downregulated in IR model, changes were inhibited by PSS pre-treatment. |
[81] |
| 16 |
PSS (MW 20.0 kg mol−1), Dalian Tianyu Pharmaceuticals Co., Ltd. Method of sulfation: N/A DS: - |
Liver injury treatment In vivo Con A-induced liver injury treatment (male Balb/c mice, n = 18, intraperitoneally injected 25/50 mg kg−1 once, 24 h). |
PSS pre-treatment reduced levels of serum liver enzymes, TNF-α, and IL-1β in a dose-dependent manner in Con A-induced liver injury in mice. Upregulation of PI3K expression in PSS pre-treated groups, whereas expression of Akt did not differ between the groups. PSS reduced Con A-induced apoptosis. |
[82] |
| 17 |
Propylene glycol Alg sodium sulfate (MW < 39 kg mol−1), Dalian Tianyu Pharmaceuticals Co., Ltd. Method of sulfation: N/A DS: - |
Liver protection from hepatic fibrosis In vivo liver protection studies (C57 mice, n = 8, intraperitoneally injected 12.5–50 mg kg−1 twice a week) In vitro cell viability and TGF-β/Smad and JAK 2/transducer and STAT3 pathway detection (LX-2 cells) |
PSS inhibited IL-6 expression, ALT, and AST activities, and reduced fibrosis. Up-regulation of Col-1 and α-SMA blocked upon PSS treatment. PSS-treated group up-regulated MMP-2 and down-regulated TIMP-1 in hepatic fibrosis. PSS suppressed TGF-β1, p-Smad2, and p-Smad3 levels. Beclin-1 suppressed by PSS. Conversely, p62, inhibited in BDL-operated and CCl4-treated groups, was promoted upon synchronous PSS injection. PSS showed no significant cytotoxicity in normal HSCs. The viability of TGF-β1–activated LX-2 cells was suppressed with PSS (dose-dependent), the IC50 = 5.8 mg mL−1. The mounting mRNA expression of Col-1 and α-SMA after activated by TGF-β1 was down-regulated in the PSS group. PSS suppressed the activation of HSCs in vitro. |
[83] |
| 18 |
PSS (MW = 16.9 kg mol−1, M/G = 2.02), Qingdao Lantai Pharmaceutical Co. Ltd. Method of sulfation: N/A DS: – (S % = 11.3%) PLGA particles (size 382 nm, ζ = −13 mV) |
Antidiabetics In vitro release study (simulated gastric medium; 24 h) In vivo bioavailability and pharmacokinetic study (Wistar rats, single oral gavage, n = 8, 48 h, oral administered 50 mg kg−1 once) In vivo efficiency (type 2 diabetic db/db mice, n = 8, 4 weeks, oral administered 100 mg kg−1 daily) |
The PSS-NPs were absorbed and metabolized faster than PSS solution; relative bioavailability was 211%. Compared with PSS solution, enteric PSS-NP had an enhanced efficiency in controlling the body weight, reducing random blood glucose, and regulating lipid metabolism. |
[66] |
| 19 |
Alg (viscosity 8 cps), Alfa Aesar Chemical Co. Ltd. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: 1.25 |
Anticoagulation In vitro anticoagulant activity (clotting time, hemolysis test, and complement activation evaluation) In vitro cell toxicity (human umbilical vein endothelial cells) In vivo anticoagulant activity (female SD rats, n = 6, tail-vein injected 0.375–1.5 mg kg−1 once) |
S–Alg exhibited excellent anticoagulant activity compared to heparin. S–Alg did not develop hemolysis, exhibited no significant blood-related complement activation, and did not trigger an inflammatory response when in contact with blood. S–Alg can enhance cell attachment and promote cell growth at low concentrations (e.g., ≤ 0.5 mg mL−1) The in vivo blood cell count indicated that the S–Alg does not affect the blood cell viability and membrane integrity in vivo. The coagulation function results demonstrated similar anticoagulant activity for S–Alg and heparin. |
[46] |
| 20 |
PG (MW 10 kg mol−1) (Shandong Provincial Key Laboratory of Glycoscience and Glycotechnology). PGS, PGS/PMS oligosaccharides from Ocean University of China (Qingdao, China). Method of sulfation: Unknown DS: 0.5–0.75 |
Target-specific anticoagulant therapeutics In vitro APTT assay (human plasma) |
Anticoagulant activity in normal plasma heparin > PMS and PGS > Fondaparinux. PGS, PGS12, PGS25, PMS6, PMS12, and PMS25 were better anticoagulants than heparin in ATIII- and HCII-double efficient human plasma, indicating that these compounds had the two-protease inhibitor-independent anticoagulant activity. | [84] |
| 21 |
PSS variants (M/G 0.42–6.70, MW 4238–19716 g mol−1) prepared from Alg (M/G 1.78, MW 7.5 kg mol−1), Chiatai Haier Pharmaceutical Co., Ltd. Method of sulfation: 3. HClSO3 DS: – (S % = 10.42–12.27%) |
Side effect determination In vivo bleeding and clotting (Kunming mice, n = 12) In vitro coagulation factor activities assessment (sheep plasma) In vivo platelet aggregation (Wistar rats, n = 8, intraperitoneally injected 45 mg kg−1 once) |
PSS with low M/G and high MW resulted in bleeding side effects in vitro by excessively inhibiting the activities of coagulation factors or promoting fibrinolysis. PSS with low M/G and high MW had higher coagulation inhibitory activity than PSS (middle M/G ratio or middle MW) in a rapid growth period between 0.01–100 µg mL−1. The in vitro and vivo results indicated PSS with a low M/G ratio reduced platelet aggregation compared with PSS (middle M/G ratio or middle MW). Heparin had a positive effect on platelet aggregation. |
[92] |
| 22 |
PSS variants (M/G 0.42–6.70, MW 4238–19,716 g mol−1) prepared from Alg (M/G 1.78, MW 7.5 kg mol−1), Chiatai Haier Pharmaceutical Co., Ltd. Method of sulfation: 3. HClSO3 DS: – (S % = 10.42–12.27%) |
Allergic response (impurities) Cytotoxicity (P815 cell line, 36 h, MTT assay) Inflammatory factors analysis (P815 cell line, 24 h) In vivo allergic response (guinea pigs, n = 8, 14 days, intraperitoneally injected at day 1, 2, and 4 for 80 mg kg−1 within Freund's adjuvant and intravenously injected at day 14 for 80 mg kg−1 in solution) |
PSS fractions (clinical grade) exhibited no significant cytotoxicity at 1000 µg mL−1. At the concentration of 100 µg mL−1, fractions (PSS-NH4+, PSS-High-MW, PSS-Low-M/G) caused histamine and β-hexosaminidase release from P815 cell. PSS group was same as control without obvious allergic symptoms. The symptom scores were enhanced by PSS-NH4+ and PSS-High-MW versus control. An improved symptom score appeared for PSS-Low-M/G only in 15–20 min. |
[91] |
- a) This column includes information about the starting Alg polymer, sulfation methods, and other Alg modifications. The abbreviations used are DCC = Dicyclohexylcarbodiimide; DS = degree of sulfation; EA = elemental analysis; MS = mass spectrometry; N/A = not applicable; PGM = polymannuroguluronate; PG = polyguluronate; PGS = sulfated PG; PM = polymannuronate; PLGA = poly(lactic-co-glycolic acid); PSS: Propylene glycol alginate sodium sulfate.
- b) This column briefly describes the key studies. The abbreviations used for the first time are APTT: activated partial thromboplastin time; BrdU = bromodeoxyuridine proliferation; CD4 = cluster of differentiation 4; DCM = diabetic cardiomyopathy; FGF = fibroblast growth factor; hCh = human chondrocytes; IR = ischemia-reperfusion; JAK = Janus kinase; LPS = lipopolysaccharides; MTT = 2,5-diphenyl-2H-tetrazolium bromide; PBS = phosphate buffer saline; qRT-PCR = quantitative real-time polymerase chain reaction; RPMI = Roswell Park Memorial Institute medium; RT-PCR = real-time polymerase chain reaction; STAT3 = activator of transcription 3; TUNEL = terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling; VEGF165 = vascular endothelial growth factor 165.
- c) This column concludes the key biological findings of each study. The abbreviations used for the first time are: ALT = alanine aminotransferase; AST = aspartate aminotransferase; ATIII = antithrombin III; BALF = bronchoalveolar lavage fluid; BDL = bile duct ligature; CXCL = C-X-C motif chemokine ligand; ER = extended-release; ERK = extracellular signal-regulated kinase; HSCs = hepatic stellate cells; KD = equilibrium dissociation constant; MEK = mitogen-activated protein kinase kinase; MMP = matrix metalloproteinase; TIMP = tissue inhibitor of metalloproteinase-1; TNF = tumor necrosis factor; PAI-1 = plasminogen activator inhibitor 1; PI3K = phosphatidylinositol 3-kinase; IL = interleukin; IFN = interferon; HCII = heparin co-factor II.
The use of S–Alg as an antitumor agent, was highlighted as a potential new application in the review by Arlov[11] where it was pointed out that non-anticoagulating heparins previously had been evaluated as anti-cancer therapeutics. Since then, seven articles have explored this aspect. The mechanisms of heparin, S–Alg, and other heparin-mimics regulating cancer cells are not completely understood, however, some researchers consider the high binding affinity between these polymers and cytokines or GFs results in suppression of tumor growth.[67, 68, 71, 85, 86] For instance, the sulfate group of polysaccharides inhibits the binding of P- and L-selectin on platelets with the cell surface mucin ligands present on the surface of cancer cells,[85, 86] thereby increasing the possibility of detection by the immune system. PSS has been identified as possessing this function based on in vitro cell work (HL60 and LS180 cells) and has demonstrated significantly suppressed lung metastasis in vivo.[70] Small fractions of S–Alg have been shown to mediate autophagy by inactivating the MEK1/ERK/mTOR signaling pathway in MNNG osteosarcoma cells.[72] Yao et al. demonstrated that these S–Alg oligomers could inhibit the expression of apoptosis and proliferation-related proteins in tumor cells (CT26.WT cell line) through molecular docking screening.[73] Binding to HBPs such as fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF) has also been shown to inhibit cancer cell growth or migration both in vitro and vivo.[67, 68, 71] Al Matari found that increasing the DS of S–Alg reduced cell viability and migration, possibly through binding to HBPs and consequentially decreasing the stemness of mouse lung adenocarcinoma cells.[71] However, this phenomenon was not significant in the human lung adenocarcinoma cell line. Ma and colleagues noted that PSS reduced the expression of metalloproteinases (MMP)-2 and -9 in B16-F10 murine melanoma, although no significant decrease in cancer cell viability was observed.[68] Sulfated low MW S–Alg has been found to boost the T cell response by inhibiting CD4+ T cell apoptosis.[69]
Many of the studies that investigate the use of S–Alg as a therapeutic agent evaluate the use of a chemically modified S–Alg polymer, specifically the commercially available PSS (Figure 3B). This drug, developed by Prof. Guan at the Ocean University of China, originated from research investigating the effects of Alg-modified barium sulfate meal on blood viscosity reduction.[87] To synthesize the heparin-mimetic PSS, Alg is hydrolyzed to reduce the chain length to ≈11 kg mol−1, followed by modification with epoxypropane and chlorosulfonic acid with the intent to mimic N-acetylglucosamine and sulfate residues of heparin, respectively. PSS, a heparinoid with anticoagulant activity mediated primarily through AT-mediated inhibition of FIIa (thrombin), was approved for the treatment of cardiovascular diseases by the Chinese Food and Drug Administration in the 1980s.
The primary routes of clinical administration for PSS are oral tablets and intravenous injection. According to Zeng et al.,[87] adverse reactions were observed in 1.03% of treated patients (235 cases out of 22 784), with only 7 cases associated with oral administration. These adverse reactions encompass allergies, bleeding, leukopenia, muscle pain, and atrioventricular block. However, due to poor absorption upon oral administration, several formulations of PSS for oral use have been developed including PSS loaded into liposomes and poly lactic-co-glycolic acid (PLGA) particles.[88-90] Three recent studies have likewise investigated the use of PLGA particles for the delivery of PSS, one using oral administration[66] whereas the other used intraperitoneal delivery.[68, 75] Only the former study included information about the particle properties (e.g. size and zeta potential, refer to Table 2) allowing for reproducibility. The routes of administration in the 16 recent articles involving animal studies are illustrated in Figure 3C. In contrast to the clinical studies, intraperitoneal administration is most used in these recent studies while one study used both intravenous and intraperitoneal injection.[91]
Two recent papers (Table 2, entries 21–22) evaluated the allergic reactions and bleeding side effects of PSS.[91, 92] This work was a continuation of an earlier study on the quality control of PSS[93] used for the prevention and treatment of hyperlipidemia and ischemic cardio-cerebrovascular diseases. In their 2018 study, Xue et al. evaluated the bleeding side effects of PSS via various in vitro and in vivo bleeding, clotting, and platelet aggregation assays. PSS fractions with low M/G ratio and high MW were found to increase the bleeding risk by excessively prolonging the activated partial thromboplastin time and thrombin time via inhibition of FIIa. Furthermore, the low M/G fraction of PSS also reduced platelet aggregation and weakened platelet function. It was concluded that the M/G ratio and MW range of PSS must be tightly controlled to guarantee its safety and effectiveness for clinical use. Their 2023 study involved the assessment of the anaphylactic potential of PSS batches of differing MW, M/G ratio, or amounts of inorganic manufacturing impurities (inorganic sulfate, ammonium salts). Qualified (clinical grade) PSS was found to be safe, however, high MW PSS and PSS with high levels (10%) of ammonium salts caused potentially serious allergic responses due to degranulation of mast cells. This study similarly concluded that good quality control of PSS (MW and levels of production impurities) is essential for safe and efficacious PSS drug products.
One aspect that is often overlooked when using S–Alg as a drug is the lack of the enzyme alginate lyase in humans which renders Alg and likely its sulfated derivatives non-degradable. So far two studies have investigated the biodistribution of Alg and one study evaluated the pharmacokinetics of PSS while one additional paper looked at the pharmacokinetics of two PSS tablet formulations for oral administration.[78]
Li and colleagues published in 2014 a pharmacokinetic evaluation in Wistar rats of FTIC-labeled PSS. The polymer had a MW of 12 kg mol−1 but the DS was not provided.[94] The study revealed that the time to peak concentration, Tmax, of a single orally administered PSS (50 mg mL−1) was 1.0 ± 1.3 h, with a T1/2 of 11.3 ± 7.9 h. In comparison, the T1/2 of a single intravenous injection (25 mg mL−1) was 1.4 ± 0.3 h. After 72 h of oral and intravenous administration, urinary excretion of PSS was 0.6% and 8.3%, respectively, and fecal excretion was 36.9% and 5.0%, respectively. It was concluded that the main metabolic pathways of PSS excretion were biliary and renal after oral and intravenous administration, respectively.
Earlier work by Al-Shamkhani and Duncan (published in 1995) evaluated the biodistribution of Alg produced from propylene glycol Alg by modification with tyrosinamide and labeled with 125I.[95] It was reported that the basic conditions used for tyrosinamide modification caused the de-esterfication of the propylene glycol ester. The final Alg polymer with an average MW of 109 kg mol−1 was used in a series of biodistribution studies. In male Wistar rats, the fate of intravenously administered Alg was greatly influenced by its MW where Alg of 48 kg mol−1 or below were cleared through renal filtration within 24 h (70% in urine samples) whereas Alg with higher MW was retained in circulation (12%) with minor accumulation in the liver (7.8%) and small intestine (2.8%) at 24 h. Similar biodistribution profiles were observed 24 h after intraperitoneal administration. In contrast, subcutaneous administration of the sample resulted in almost 70% of the sample retained at the site of injection after 24 h whereas the rest was accounted for in organs, blood, and feces. It was concluded that Alg with low MW is better suited when faster clearance is key for the end application.[95]
In a study published in 2021, we evaluated the biodistribution of ultrapure Alg (M/G ratio ≥ 0.67, MW of 530 kg mol−1) in BALB/c mice using PET and fluorescence imaging.[96] After 2 days, 64Cu-labeled Alg showed liver and spleen uptake, and this remained after 14 days as verified by in vivo and ex vivo fluorescence imaging of cyanine 5-labeled Alg. The use of MacGreen mice confirmed the uptake of Alg by macrophages in the spleen after 2 days. This extended biodistribution study confirmed the clearance of only a portion of the administered Alg biopolymer and illustrated uptake by macrophage populations in the spleen over 14 days. It was concluded that the use of high MW and high G-content Alg in biomaterial applications is only suitable when the biopolymer will not enter the bloodstream such as transdermal and oral applications.[96] It should be noted that commercial products of PSS all have MW well below that required for clearance while some S–Alg products appear to be of higher MW (see details in Table 2).
4 Material Fabrication and Properties
4.1 Cross–linking Strategies
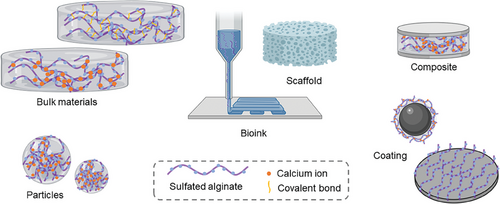
S–Alg has been used to fabricate a variety of biomaterials including bulk hydrogel materials,[23, 33, 97-104] particles,[27, 54, 102, 105-114] and scaffolds,[22, 26, 115-125] and has also been used as a component of composites and as a coating material[47, 52, 126-131] as illustrated in Figure 4. To achieve cross-linking of the S–Alg polymers to fabricate hydrogels, two main cross–linking strategies have been used: ionic and covalent. Ionic cross–linking, predominantly using calcium ions, is the most commonly used method for the fabrication of S–Alg-based materials.[8-10] It has been reported that S–Alg with a DS ≥ 0.5 cannot form a true hydrogel on the macroscale using calcium cross–linking and that the stiffness of hydrogels decreases with increasing DS.[97] Two main strategies have been used to overcome this. One is to use a mixture of Alg and S–Alg to form hydrogels with better osmotic stability and stiffness.[97, 109] The other is to use either barium or strontium salts, often in combination with a calcium salt.[23, 101, 109, 112] Both barium and strontium form stronger ionic cross–links than calcium alone and can afford cross–linking not only of the G-units but also of the M-units, hence are able to improve material properties.[8-10, 132] It should be noted, however, that barium is toxic with high doses causing paralysis or death in humans and in lower doses can be harmful in children and fetuses.[132] It is expected that all ionically cross–linked alginate hydrogels will release the cross–linking ions over time, so care needs to be taken to adjust the total amount of barium used.
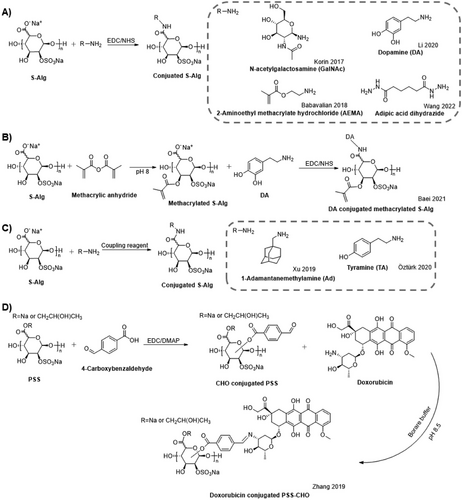
Covalent cross–linking of S–Alg polymers is often achieved by chemical modification to introduce additional chemical moieties such as vinyl groups for photo cross–linking[33, 98] or tyramine for enzymatic cross–linking using tyrosinase in the presence of oxygen.[100] Vinyl groups were introduced either using carbodiimide chemistry and reaction with 2-aminoethyl methacrylate (AEMA) (Scheme 4A) or using methacrylic anhydride (Scheme 4B), while tyramine was introduced using the coupling agent 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM) (Scheme 4C). When using carbodiimide chemistry, a reaction with the carboxylate groups of S–Alg reduces the overall charge of the polymer, while the use of an anhydride affords a reaction with the hydroxyl groups. In all cases, the synthetic strategy for producing these dual-modified Alg polymers has been to first introduce the sulfate groups (Scheme 4). In contrast, the use of boric acid as a covalent cross-linker does not require modification of S–Alg as it affords cross-linking between hydroxyl groups. Covalently cross–linked Alg and S–Alg hydrogels have higher stability and resistance to degradation under mild alkaline, low pH, or neutral conditions compared to ionically cross–linked hydrogels.[133]
4.2 Fabrication of S–Alg-based Bulk Materials
S–Alg-based bulk hydrogel materials have been used to encapsulate cells and/or proteins and the studies are summarized in Table 3 (entries 1–10). In all cases, the cargo was premixed with the polymer solution prior to cross–linking. In the cases of ionic cross–linking, various approaches were used. In one case, a calcium solution was added to the S–Alg solution with stirring until the gel formed.[104] Other studies produced a pre-gel (viscous solution of polymer and cargo) which was either cast,[97] cast and frozen,[102] or cast in silicone mold that was sandwiched between dialysis membranes.[23, 101] Cross–linking was then achieved either by immersion of the pre-gel into the cross–linking solution or by adding the cross–linking solution on top of the as-cast pre-gel. In one study by Gionet-Gonzales et al.,[23] the S–Alg polymer was mixed with Alg modified by periodate oxidation and/or modified with an RGD-peptide sequence attached via carbodiimide chemistry. It was shown that the degree of oxidation affected the rate of degradation of the hydrogel disks and as such, could be used to tune the degradation rate where a higher degree of oxidation (5% compared to 1%) caused faster degradation. Furthermore, it was confirmed that the DS value did not affect the hydrogel properties such as elastic modulus and swelling ratio.
| No | S–Alg polymer details a) | Material details b) | Application and key studies c) | Key biological findings d) | Reference |
|---|---|---|---|---|---|
| 1 |
Alg, Merck. Method of sulfation: 3. HClSO3 DS: 1.3 (13% S, EA) Chemical modification: EDC coupling AEMA, DM: - |
Photo-cross–linked S–Alg discs (d = 6 mm) encapsulating PDGF |
Wound healing In vitro release (PBS, 21 d) In vivo wound healing (Albino-N-Mari male rats, n = 5, 21 d) |
S–Alg disk sustained the release in vitro of PDGF compared to Alg control. In vivo, the PDGF loaded S–Alg disk showed enhanced vascularization, and better hair follicle formation compared to no treatment and Alg/S–Alg disk. |
[98] |
| 2 |
Alg (M/G: 0.8, MW: 120–200 kg mol−1), Sigma. Method of sulfation: 3. HClSO3 DS: 0.45 and 0.67 (EA S %) 6. N(SO3Na)3 DS: 0.31 (EA S %) Chemical modification: Methacrylic anhydride, DM: 3%. EDC coupling Cat, DM: 16–17%. |
Material 1: Calcium cross–linked S–Alg/ CS, and S–Alg-Cat/CS disks (d = 10 mm, h = 10 mm) encapsulating TGF and KGN. Materials 2: Photo and calcium cross–linked DN S–Alg/CS disk (d = 10 mm, h = 10 mm) Materials 3: DN S–Alg/CS disk (d = 10 mm, h = 10 mm) encapsulating MSCs and CHs |
Cartilage tissue engineering In vitro release (PBS, 21 d, material 1) In vitro degradation (tissue culture medium, 21 d, material 2) In vitro cellular characterization (14 d) and in vivo (New Zealand white male rabbits, 6 and 12 w, material 3) |
Sustained release of TGF-β1 and KGN. DN/S–Alg-Cat hydrogel slower degradation (7%) and higher mechanical stability (81% retention of stiffness) in the presence of lysozyme. Greater metabolism of the MSC and CH encapsulated in S–Alg/CS hydrogels compared to Alg/CS. S–Alg-Cat/CS and DN/SAlg-Cat better regeneration of full-thickness articular cartilage defects in vivo. |
[33] |
| 3 |
Alg, Merck. Method of sulfation: 3. HClSO3 DS: - |
Calcium cross–linked S–Alg discs (d = 18 mm) encapsulating TGF-β1-expressing Sp2/0-Ag14 cells or encapsulating Ad-MSCs |
Cartilage tissue engineering In vitro cell distribution and viability in hydrogels (Ad-MSCs, 3 d) In vitro cell migration (Ad-MSCs, 4 d) In vitro RNA expression (co-culture, 21 d) |
Homogeneous distribution of encapsulated Ad-MSCs (> 90% viability) compared to non-encapsulated. Ad-MSCs co-cultured with discs encapsulating Ag14 cells were found to migrate significantly, compared to the control. Encapsulated Ad-MSCs expressed higher levels of chondrogenic genes (Col II, Ag, and Sox-9) when co-cultured with Ag14 cells. |
[99] |
| 4 |
Alg (M/G: <0.67, MW: <75 kg mol−1) Pronova. Method of sulfation: 3. HClSO3 DS: 0.05–0.38 (DMMB assay) Chemical modification: Oxidation, DO: 1 and 5%, EDC coupling RGD-peptide, DM: – |
Barium or calcium cross–linked S–Alg/RGD-O-Alg/RGD-Alg discs (d = 8 mm) encapsulating MSCs and HGF |
Fundamental tissue engineering In vitro HGF retention (serum-free media, 7 d) In vitro endothelial tubulogenesis (HDMECs, 6 h) In vitro macrophage migration and subsequent polarization (IC-21 macrophages, 24 h) |
HGF retained within S–Alg longer, slower release compared to Alg hydrogels. HDMECs tubule formation using conditioned media from Alg > 1% S–Alg > 2% Alg hydrogels. No significant difference in macrophage response. |
[23] |
| 5 |
Alg (MW: VLVG < 75 kDa, LVG 100 kg mol−1), Pronova. Method of sulfation: 3. HClSO3 DS: - Chemical modification: see No. 4 |
Barium or calcium cross–linked S–Alg/RGD-Alg/O-RGD-Alg hydrogel discs (d = 8 mm) loaded with MSC spheroids |
Muscle tissue engineering In vivo crush injury recovery study (Sprague Dawley rats, 6 w, n = 4) |
S–Alg hydrogel decreased collagen deposition, improved myogenic marker expression, and increased neuromuscular junctions, 2 weeks after injury. The quantity of fibrotic tissue decreased at 6 weeks for the S–Alg group, while the Alg group maintained a similar fibrotic area. |
[101] |
| 6 |
Alg (low viscosity), Sigma. Method of sulfation: 3. HClSO3 DS: - Chemical modification: EDC coupling Ad APS/Fe2+ polymerization EDOT |
PEDOT:S–Alg-Ad assembled with Pβ-CD through host-guest interaction, discs (d = 6 mm), encapsulating C2C12 cells. |
Muscle tissue engineering In vitro cell proliferation, cell differentiation, and gene expression (7 d) |
Electroconductive S–Alg hydrogel induced formation of large spheroid colonies. Compared to control (S–Alg-Ad /Pβ-CD hydrogels), the cells in PEDOT:S–Alg-Ad /Pβ-CD hydrogels up-regulating myoblast differentiation genes (myogenin, troponin T, and MyHC-1). |
[103] |
| 7 |
Alg (M/G: <0.67, MW: >200 kg mol−1), Pronova. Method of sulfation: 3. HClSO3 DS: 0.44 (EA S %) Chemical modification: EDC coupling TA, DM: 2% |
Covalently cross–linked S–Alg-TA hydrogel rings (d = 4 mm, h < 2 mm), encapsulating bovine or human CHs |
Cartilage tissue engineering In vitro cell viability (21 d) In vitro chondrocyte adhesive properties to bovine articular cartilage In vivo stability (female NU/NU nude mice, 4 w) |
75% viability of chondrocytes in S–Alg-TA and Alg-TA hydrogels. S–Alg-TA displayed strong adhesion to cartilage rings with in situ tyrosinase cross–linking. In vivo toxicity was not observed. Deposition of collagen-2 matrix by human chondrocytes in hydrogels. |
[100] |
| 8 |
Alg (M/G = 0.47, MW = 270 kg mol−1), FMC Biopolymer. Method of sulfation: 3. HClSO3 DS: 0.32, 0.90 (ICP-MS) |
Calcium cross–linked Alg/S–Alg hydrogel disc (V = 30 µL) encapsulating human chondrocytes or IL-1β. |
Cartilage tissue engineering In vitro cell viability and inflammatory stimulation of chondrocytes with IL-1β. In vitro IL-1β release (24, 48 h). |
Good viability of encapsulated chondrocytes in all hydrogels. S–Alg promoted a homogeneous matrix deposition in hydrogels. S–Alg displayed anti-inflammatory and anti-catabolic effects on encapsulated chondrocytes stimulated by IL-1β. |
[97] |
| 9 |
Alg, Sigma. Method of sulfation: 3. HClSO3 DS: 0.02 and 0.21 (ICP-OES) |
Calcium cross–linked S–Alg/Alg disk (d = 8 mm) encapsulating PB nanozymes |
Wound healing In vitro cytotoxicity (HUVEC, NIH 3T3, and Raw264.7, 24 h) In vitro macrophage phenotype modulation (BMDMs) In vivo effects on deep second-degree burn wounds (C57 mice, 21 days) |
Hydrogels showed no cytotoxic effects on all cell lines. The macrophage phenotype was regulated by the S–Alg disks. Compared to the control (saline) group having 26%, 55%, and 62% of wound closure rates, S–Alg hydrogel with PB NPs has 65%, 90%, and 98% wound closure rates on days 7, 14, and 21 respectively. |
[104] |
| 10 |
Alg (1% solution viscosity 15 – 25 cps), Sigma. Method of sulfation: 4. HClSO3 DS: – |
Calcium cross–linked Alg/S–Alg/HEP-Alg disks (d = 13 mm, h = 3 mm, 0.5 mL) and microspheres (d = 37 µm) encapsulating VEGF. |
Vascular tissue engineering In vitro release of GF (PBS, 37 °C, 14 d) Matrigel tube formation assay (hPCS-derived endothelial cells (37 °C, 24 h) Ex vivo pro-angiogenic activity (Sprague dawley rats, 37 °C, 7 d). |
Alg/S–Alg/HEP-Alg disks and microspheres showed sustained release VEGF and better control of release compared with control (Alg disk and microsphere) Alg/S–Alg/HEP-Alg microspheres are more efficacious at inducing vascular morphogenesis in hPSC-endothelial cells compared to control (without microspheres). |
[102] |
| 11 |
Alg, Haihong Chemicals. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: 1.34 (EA S %, C %) |
Calcium cross–linked Alg/S–Alg particles (size 323–771 nm, ζ = −7.2 mV) encapsulating sCT, |
Bone tissue engineering In vitro sCT release (PBS, 15 d) In vitro degradation (PBS, lysozyme, 15 d) In vitro cytotoxicity and differentiation (MC3T3 cells, 7 and 10 d, respectively) |
S–Alg prolonged release of sCT. Degradation ranges from 9% (Alg/S–Alg) to 14% (S–Alg). Enhanced cell proliferation and osteogenic ability (ALP levels, intra- and extra-cellular calcium) of MC3T3 in Alg/S–Alg group. |
[27] |
| 12 |
Alg, Sigma. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: 1.7 (EA S %) |
Calcium cross–linked S–Alg particles (size 3.8 µm) encapsulating BMP-4 |
Fertility enhancement In vitro protein release (PBS, 5 d) In vitro cell differentiation (embryonic stem cells) |
Reduced initial burst of BMP-4 from S–Alg particles. BMP-4 loaded S–Alg particles increased germ cell differentiation from embryonic stem cells at least 2-fold compared to conventional solution delivery. Meiosis-related genes are significantly upregulated. |
[105] |
| 13 |
Alg (UP LVG, M/G: <0.67, MW: 75–200 kg mol−1), Pronova. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: – |
Calcium cross–linked S–Alg particles (size 270 nm, ζ = −15 mV) encapsulating pDNA |
Cancer therapy In vitro cytocompatibility (CT26-EGFP, CFs, HepG2, and MDA-MB-231, 72 h) In vitro cellular transfection (CFs, HepG2, MDA-MB-231, 72 h) In vitro effects on human peripheral blood mononuclear cells (PBMCs, RPMI 3 h treatment, measured after 72 h) |
Cell viability depended on calcium content for CF and MDA-MB-231. All cell types showed uptake of particles (≥ 2.5 mM Ca2+) greater than free pDNA. The mechanism via an active endocytosis, is mainly clathrin-mediated. Particles displayed no significant lymphocyte activation on native PBMCs compared to pos controls. |
[106] |
| 14 |
Alg (UP VLVG, M/G: <0.67, MW: < 75 kg mol−1), Pronova. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS not reported Chemical modification: EDC coupling GalNAc, DM: – |
Calcium cross–linked S–Alg-GalNAc particles (size 130–150 nm, ζ < −10 mV), encapsulating siRNA |
Cancer therapy In vitro cell viability, cellular uptake, and gene silencing (HepG2, 24 h) In vivo biodistribution (female Balb/c mice, n = 3, 5 h) |
High cell viability of all particles. Cellular uptake of particles (S–Alg:siRNA = 1:1) in ≈86% of the cells. Significant reduction in STAT3 mRNA levels reaching ≈82–91% silencing. 3-fold stronger fluorescence in the liver with liver-targeted particles compared with non-targeted particles and lower accumulation in the kidney. |
[107] |
| 15 |
PSS (MW 20 kg mol−1), Ocean University of China Method of sulfation: Unknown DS: – (S % = 12%) Chemical modification: EDC coupling 4-CB, DM: 12, 28, 50% |
Borate cross–linked particles encapsulating DOX (DOX covalently attached to PSS) and CXB (size < 150 nm) |
Cancer therapeutic In vitro release (PBS, pH 7.4, 6.5 and 6.0) In vitro cellular uptake (4T1 cells, 12 h) In vitro cytotoxicity and metastasis inhibitory (4T1 cells) In vivo biodistribution and antitumor efficiency (female BALB/c mice, n = 10, 24 h) |
DOX, but not CXB, showed pH-sensitive release where the release rate increased with decreasing pH. Particles with DOX and free DOX showed similar DOX uptake by cells. Based on IC50 values, encapsulated DOX is less efficient than free DOX. Encapsulated CXB displayed stronger metastasis inhibitory ability. Particles decreased DOX distribution in the lungs and increased tumor accumulation after 6 h compared to free DOX. DOX encapsulation increased retention at the tumor site. Tumor growth was inhibitory by 73% for particle formulation. |
[114] |
| 16 |
Hydrolyzed Alg (MW: 30–50 kg mol−1) Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: - |
TCH encapsulated and cross–linked S–Alg particles. |
Fundamental drug delivery In vitro release (PBS, RT, 5 d) |
Rapid (5 h) release of 85% TCH and complete release over 5 days. | [108] |
| 17 |
Alg LVG (M/G: 0.47, MW: 237 kg mol−1), MVG (M/G: 0.52, MW: 235 kg mol−1), LVM (M/G: 1.13, MW: 235 kg mol−1), and VLVG (M/G: 0.45, MW: 32 kg mol−1), Pronova. Method of sulfation: 3. HClSO3 DS: 0.76-0.83 |
Barium or calcium or strontium cross–linked Alg/S–Alg particles (size 350–550 µm) |
Fundamental biological study In vitro stability (0.9% (w/v) NaCl) In vitro inflammatory property evaluation (whole blood, n = 5, 14 d) In vivo (female C57BL/6JRj mice, 118 d) In vivo fibrinogen deposition (female C57BL/6JRj mice, 14 d) |
Particle stability in vitro affected by Alg/S–Alg ratio and cross–linker ion. Lower recovery in vivo for S–Alg particles, the minimal effect of gelling ion. Low cytokine responses in human blood compared to controls Lower fibrinogen deposition for Alg/S–Alg (80/20). |
[109] |
| 18 |
Alg (MVG, M/G = 0.49, MW: 235 kg mol−1), Pronova. Method of sulfation: 3. HClSO3 DS: 0.65 |
Barium or calcium cross–linked S–Alg particles (size 625 µm) encapsulating hPSC-Heps |
Therapeutic for acute liver failure In vitro functionality assays (24 h) In vivo intraperitoneal implantation (immunocompetent C57BL/6J male mice, n = 16, 10 d) |
Enhanced in vitro albumin secretion in S–Alg-encapsulated hPSC-Heps compared to Alg control. Higher fibrotic overgrowth on Alg than S–Alg particles. |
[112] |
| 19 |
Alg (medium viscosity, MW 280 kg mol−1), Sigma. Method of sulfation: 7. SO3·py (T-Alg) 8. DCC-H2SO4 (T-Alg) DS: 0.8, 1.1, 1.7 (EA S %, C %) |
Calcium and CS cross–linked S–Alg particles (size 200–600 nm) encapsulating Lf |
Fundamental study Effect of DS and fabrication method on particle size. |
[54] | |
| 20 |
Alg (MW 30–50 kg mol−1), Pronova. Method of sulfation: 3. HClSO3 DS: - |
HGF or IGF-1 encapsulated and cross–linked S–Alg particles (size 50–100 nm) |
Heart tissue engineering In vivo (intramyocardial-injection, Sus scrofa pigs, n = 12, 7 weeks) |
Infarct size is significantly smaller for particles than PBS control. Particle-treated pigs show increased LVEF accompanied by improved myocardial remodeling. | [113] |
| 21 |
Alg (M/G = 0.66, MW: 200 kg mol−1), Pronova. Method of sulfation: 3. HClSO3 (M1) 6. N(SO3Na)3 (M3) 8. DCC-H2SO4 (T-Alg) (M3) DS: 0.68 (M1), 0.58 (M2), 0.65 (M3) (EA) |
S–Alg coated calcium cross–linked Alg particles (size 600–700 µm) encapsulating pancreatic islets |
Anti-diabetes application In vitro/vivo toxicity (7 d) In vivo transplantation (alloxan-induced diabetic NMRI male mice, n = 6, 42 d) |
Cell viability > 90% for S–Alg particle supernatants. M1- and M3-coated particles had less severe immune cell infiltration in vivo. Secreted collagen highest for M2 coated sample. FBG levels for Alg particles returned to hyperglycemia (d 17). M1- and M3-coated particles showed control of FBG (healthy 42 d). M1-coated particles had lower collagen deposition than M3-coated particles. |
[111] |
| 22 |
Alg (LVG, >65% G content, MW: 128 kg mol−1), Pronova Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: - |
HBPep cross–linked S–Alg particles (size 5 µm) |
Attenuates ventricular remodeling In vivo release (Balb/C mice, n = 3 or 4, 6 days) |
The particles were injected into the hip muscles of mice. After 47 h, the proteins remained in the injected tissue, while no protein was found in the internal organs. | [110] |
| 23 |
Alg (M/G: <0.67, MW: 75–200 kg mol−1), Pronova. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: - |
Calcium cross–linked Alg/S–Alg hydrogel scaffolds in a microfluidic device (size 144 µm) encapsulating MCF-7, CCD-1129SK, and MCF10a cells. |
Cancer model On-chip cell viability and toxicity assays (0, 5, or 10 µM Doxorubicin, 96 h) |
Cell viability was observed to decrease within 48 h, then remained stable until 96 h. Compared to the control (Alg scaffolds), Alg/S–Alg scaffolds displayed significantly higher viability. |
[117] |
| 24 |
Alg (UP LVG, M/G: <0.67, MW: 75–200 kg mol−1) Pronova. Method of sulfation: 7. SO3·py (T-Alg) DS: 0.4, 1.0, 1.3 (EA S %) |
Calcium cross–linked S–Alg printed patterns (square, d = 10 µm, h = 70 µm, nozzle d = 50 ± 10 µm) encapsulating primary rat hippocampal neurons |
Neural tissue engineering In vitro characterization 3D patterned neural network (21 d) |
Improved neuron viability, neurite numbers, and mean branch length in S–Alg material compared to Alg control. |
[120] |
| 25 |
Alg (MW: 2% viscosity 100–300 cP) Sigma. Method of sulfation: 3. HClSO3 DS: 0.016, 0.068, 0.248 (ICP-MS) |
Thermally cross–linked PVA/S–Alg nanofibers (fiber d = 273 nm, 21G needle) scaffolds encapsulating TGF-β1 |
Fundamental GF delivery In vitro mass loss (PBS, 2 months) In vitro cytotoxicity (hMSCs, 7 d) In vitro TGF-β1 release (ELISA, 128 h) |
The degradation rate is similar for S–Alg and Alg-based nanofibrous scaffolds. PVA/S–Alg nanofibrous scaffold non-cytotoxic. Improved retention of TGF-β1 in PVA/S–Alg nanofibrous scaffold compared to Alg-based control. |
[121] |
| 26 |
S–Alg (MW: 76 kg mol−1), ZFZCo. Method of sulfation: Unknown–various DS: 0.65 |
Electrospun and GTA vapor cross–linked PVA/S–Alg nanofiber scaffolds (fiber d = 169–488 nm) |
Neural tissue engineering In vitro cell viability (C6 and Schwann cells, 7 d and hBMSCs, 14 d) In vitro neural differentiation evaluated from cell morphology and biomarker expression (hBMSCs, 21 d) |
Cell viability (C6, Schwann cells, and hBMSCs) improved in scaffold groups compared to PVA control. The presence of S–Alg in scaffolds improved cellular proliferation and differentiation (hBMSCs). Expression of neural marker significantly up-regulated in hMSCs on PVA/S–Alg scaffolds. |
[118] |
| 27 |
S–Alg (MW: “medium”) ZFZCo. Method of sulfation: Unknown–various DS: 0.5 |
Electrospun and GTA vapor cross–linked PVA/S–Alg nanofiber scaffolds (fiber d = 185 nm) |
Cartilage tissue engineering In vitro viability study (hBMSCs, 72 h) In vitro chondrogenic differentiation (hBMSCs, 21 d) |
Cells on PVA/S–Alg (7:3) scaffold showed a higher survival rate compared to PVA control. The presence of S–Alg improved chondrogenic differentiation. |
[119] |
| 28 |
Alg (M/G: 1.56, MW: 758.4 kg mol−1) Sigma. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: 0.21 (ICP S %, C %) |
Material 1: calcium cross–linked Alg/S–Alg LbL scaffold (square, d = 2 cm, h = 3.5 mm, nozzle d = 300 µm) encapsulating BMP-2 Material 2: calcium cross–linked Alg/S–Alg LbL scaffold (square, d = 2 cm, h = 3.5 mm, nozzle d = 300 µm) encapsulating MC3T3-E1 and BMP-2 |
Bone tissue engineering In vitro protein release (PBS, 10 d, material 1) Cell printing and in vitro cell culture (7 d, material 2) |
Printed Alg/S–Alg scaffold released 73% BMP-2 within 2 d, while the Alg group released 90%. Osteoblastic proliferation and differentiation in vitro were significantly promoted by Alg/S–Alg 3D-printed scaffolds with an optimal composition of 3% Alg and 2% S–Alg. |
[26] |
| 29 |
Alg (M/G: >0.65, MW: VLVG 32 kg mol−1, LVG 100 kg mol−1), FMC Biopolymers. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: - |
Calcium cross–linked S–Alg/EV coating onto the gelatin/RGD conjugated Alg scaffolds (d = 10 mm, h = 2 mm) encapsulating human THP-1 cells |
Extracellular vesicles delivery In vitro EVs release (11 d) In vitro cell metabolic activity (7 d) |
Incorporation of S–Alg-EV complexes on shell bioink sustained EV release (88% compared with 92% without S–Alg, 4 d). S–Alg prolonged release lasting 11 d. Cellular scaffold maintained metabolic rate and cell viability. |
[116] |
| 30 |
Alg (M/G: 0.8, MW: 120–200 kg mol−1) Sigma. Method of sulfation: 3. HClSO3 DS: 0.9 (EA S%) |
Calcium cross–linked S–Alg/PVA electrospun fiber mat (fiber d 75 -283 nm, 19 G needle) |
Fundamental study Morphology of electrospun scaffolds. |
[22] | |
| 31 |
Alg (LVG, M/G: 0.49, MW: 140 kg mol−1), Pronova. Method of sulfation: 3. HClSO3 DS: 0.32, 0.9 (ICP-MS) |
Calcium cross–linked cellulose/S–Alg hydrogel discs and 3D printed grid-like constructs (l = 20 mm, w = 15 mm, 22–30 G needle) encapsulating chondrocytes |
Cartilage tissue engineering In vitro cell viability and morphology (14 and 28 d) In vitro immunohistochemistry (28 and 42 d) |
Cell viability is similar for 3D printed construct and hydrogel discs. The geometry of the 3D-printed construct affected cell spreading and morphology. Increased production of proteoglycan and collagen I at d 42 compared to d 28. Collagen I and II are lower in printed samples compared to hydrogel disc control. |
[115] |
| 32 |
Alg (UP LVG, M/G: <0.67, MW: 75 kg mol−1), Pronova. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: 1.5 |
Covalently cross–linked GelMA scaffold with calcium cross–linked Alg/S–Alg interpenetrating network encapsulating TGF-β3 (pore size 20 µm; d = 5 mm, h = 3 mm, 25 G needle). |
Cartilage tissue engineering In vitro protein release (21 d) In vitro MSCs chondrogenesis (42 d) In vivo MSCs chondrogenesis (BALB/c OlaHsd-Foxn1nu nude mice, n = 2, 28 d) |
TGF-β3 release is slower from S–Alg-containing scaffold compared to Alg control (42 vs 66% at 7 d). TGF-β3 loaded S–Alg scaffold enhanced GAG accumulation and collagen type X deposition compared to Alg control in vitro (42 d). TGF-β3 loaded S–Alg scaffold enhanced matrix development (GAG and collagen type II) in vivo. |
[125] |
| 33 |
Alg (UP VLVG, M/G: <0.67, MW: 30–50 kg mol−1), Pronova. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: – |
Calcium cross–linked Alg/S–Alg scaffold (d = 6 mm, V = 100 µL) encapsulating TGF-β1 and BMP-4 in distinct layers |
Technical Paper In vitro release (PBS, 7 d) In vitro growth factor bioactivity (mouse and rat primary cardio-fibroblasts, 3 d) In vitro cell culture (hMSCs, 14 d) |
TGF-β1 showed slower release from S–Alg-based scaffolds compared to Alg control. Differentiation of hMSCs to chondrocytes and osteoblasts in distinct layers depending on growth factor present |
[123] |
| 34 |
S–Alg (medium molecular weight), ZFZ Co. Method of sulfation: Unknown DS: 0.9 |
EDC cross–linked S–Alg/gelatin scaffold (d = 10 mm, h = 3 mm) |
Wound healing In vitro degradation and S–Alg release (PBS, 21 d) In vitro cytotoxicity, adhesion, and proliferation (HDFs, 14 d) In vivo analysis of scaffolds as dermal substitute, diabetic wounds (NMRI male mice, n = 4, 20 d) |
The presence of S–Alg in the scaffold (50/50) increased the degradation rate. Gelatin/S–Alg (90/10) scaffold showed slower initial (24 h) release of S–Alg compared to the (70/30) scaffold. Scaffolds were non-cytotoxic. Fibroblasts showed enhanced adhesion and proliferation on gelatin/S–Alg(90/10) scaffold compared to 70/30, 50/50, and gelatin control. Gelatin/S–Alg (70/30) scaffold induced angiogenesis and lower foreign body reaction compared to controls. |
[124] |
| 35 |
S–Alg (DS = 0.9, MW 140 kg mol−1), ZFZCo. Method of sulfation: Unknown DS: 0.9 |
GTA cross–linked PVA/S–Alg electrospun scaffold (fiber d = 169–488 nm) incorporating d-ECM |
Tympanic membrane tissue engineering In vitro degradation (PBS, n = 3) In vitro cytotoxicity (NIH 3T3, 7 d) In vitro cell migration (NIH 3T3, 24 h) In vivo (Sprague Dawley rats, n = 33, 14 d) |
The presence of S–Alg increased the degradation rate. The presence of S–Alg enhanced cellular response and viability. Cell migration improved in d-ECM-containing scaffolds. Enhanced cell migration for the S–Alg scaffolds. TMP regeneration (d-ECM group) complete with control group (natural healing) incomplete (80%). |
[122] |
| 36 |
S–Alg (MW: 140 kg mol−1), ZFZCo. Method of sulfation: Unknown DS: 0.8 |
Ionically cross–linked Alg/S–Alg hydrogels disc (d = 8 mm, h = 1 mm) encapsulating KGN-loaded PLGA particles, laminated composite with PCL mat |
General tissue engineering In vitro degradation (PBS, 37 °C, 30 d) In vitro release (PBS, 37 °C, 30 d) In vitro cell proliferation (Ad-MSCs, 37 °C, 7 d). |
The Alg/S–Alg hydrogel degraded faster than the electrospun mat and PLGA particles. Linear release profile of KGN from the composite, 2-fold less than particles only. Optimized composite scaffold displayed no cytotoxic effect and could improve cell growth. |
[130] |
| 37 |
S–Alg (MW: 140 kg mol−1), ZFZCo. Method of sulfation: Unknown DS: 0.8 |
Calcium cross–linked Alg/S–Alg/d-ECM hydrogels (fiber d = 400 µm, pore size 600 µm, d = 10 mm, h = 2 mm) encapsulating adipose-derived MSCs, filled within fused PCL scaffold |
Cartilage tissue engineering In vitro cytotoxicity (adipose-derived MSCs, 14 d) In vitro chondrogenic differentiation (Ad-MSCs, 14 d) |
Enhanced cell viability, cell proliferation, and chondrogenic differentiation (expression of SOX9, ACAN, and COL II significantly increased) in optimized scaffolds. |
[131] |
| 38 |
Alg (LVG, MW: 75 kg mol−1), Pronova. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: - |
Covalently cross–linked S–Alg/Alg scaffold (square, fiber d = 100 µm, pore size 25–125 µm, d = 8 mm, h = 3 mm) within PLCL frame, encapsulating TGF-β3 and MSCs |
Cartilage tissue engineering In vitro TGF-β3 release study (ELISA, 21 d) In vitro MSC co-culture (Porcine bone marrow-derived MSCs, 3 weeks) |
A sustained initial release (≈35%) in the first 7 days was observed from the Alg/S–Alg scaffolds compared to the Alg scaffold (55%) and followed the release in a plateau at day 21. Live/dead staining revealed high cell viability for both culture conditions (92% and 95% cell viability for static and dynamic culture respectively). | [129] |
| 39 |
Alg (MW: low viscosity 75–200 kg mol−1), Pronova. Method of sulfation: 7. SO3·py (T-Alg) DS: 1 (EA S %) |
Calcium cross–linked S–Alg core within PCL particle shell (size 236 nm), encapsulating BSA |
Wound healing In vitro BSA release (PBS, 37 °C, 28 d) In vitro cytotoxicity (HaCaT cells, 72h) In vitro cellular uptake (HaCaT cells, 24h) |
The presence of S–Alg rather than Alg increased the release of BSA. HaCaT cells with treatment of up to 50 µg mL−1 of particles showed no cytotoxic effects after 72 h. S–Alg particles showed lower cellular uptake efficiency, than Alg particles. |
[47] |
| 40 |
Alg (MW 200–300 kg mol−1) and S–Alg, Sigma. Method of sulfation: Unknown DS: - |
Calcium cross–linked S–Alg/Alg scaffold (d = 9 mm, V = 140 µL) encapsulating ECM and hASCs, sandwiched between PCL and GT electrospun mat |
Cartilage tissue engineering In vitro biodegradation (PBS, 21 d) In vitro cytotoxicity (hASCs, 14 days) In vitro chondrogenic differentiation (hASCs, 21 d) |
The degradation rate decreased with an increasing number of hydrogel layers. Optimal metabolic activity of hASCs observed for 3-layer composite. Enhanced GAG synthesis by hASCs within scaffold containing ECM compared to scaffold without ECM. |
[128] |
| 41 |
Alg (M/G = 0.82, MW: 32 kg mol−1), Sigma. Method of sulfation: 6. N(SO3Na)3 DS: 0.65 |
S–Alg coated on plasma-treated PCL nanofibrous scaffold |
Cartilage tissue engineering In vitro cytotoxicity (MSCs, 72 h) In vitro differentiation (MSCs, 21 d) |
MSC viability higher on S–Alg coated scaffold than PCL control. S–Alg coating capable of supporting cell growth, proliferation, and differentiation better than PCL control. |
[52] |
| 42 |
Alg (M/G: 0.8, MW 12–40 kg mol−1), Sigma. Method of sulfation: 3. HClSO3 (T-Alg) DS: - |
Heat-casted elastomers of polyurethane modified with S–Alg |
Vascular tissue engineering In vitro anticoagulation (37 °C, 1 min) In vitro biocompatibility (HUVECs, 24 h) In vivo biodegradation (male Wistar rats, n = 3, 8 weeks) |
S–Alg material had increased anticoagulant activity compared to Alg material. Cell attachment and growth were enhanced in the S–Alg group, compared to the control. Elastomers with S–Alg caused smaller amounts of mast cells and foreign body giant cells in vivo compared to control. |
[34] |
| 43 |
Alg, Alfa-Aesar Corp. Method of sulfation: 8. DCC-H2SO4 (T-Alg) DS: - Chemical modification: EDC coupling with dopamine, DM = 26% (NMR) |
Covalently cross–linked dopamine-S–Alg coating on Fe3O4 particles (particle size 955 nm, ζ = −48 mV) |
Hemodialysis In vitro clotting (whole blood) In vitro hemolysis (whole blood, 3 h) In vitro platelet activation (whole blood) |
The clotting time was prolonged for S–Alg coated particles compared to uncoated particles and increased with increasing the S–Alg content. S–Alg coated particles enhanced suppression of hemolysis compared to uncoated control. The S–Alg coated particles displayed lower complement activation, platelet activation, and contact activation compared to the uncoated control. |
[126] |
| 44 |
Alg (UP LVG, M/G: <0.67, MW: 75–200 kg mol−1), Pronova. Method of sulfation: 7. SO3·py (T-Alg) DS: 0.4, 1.0 (EA S %, C %) Chemical modification: EDC coupling with biotin, DM: 0.02 |
Biotin-S–Alg LbL film coating on streptavidin-gold substrate, encapsulating FGF-2 |
Neural tissue engineering In vitro cell viability (A172, SH-SY5Y and PC-12, 72 h) Neurite outgrowth (PC-12, 72 h) |
Cell viability increased with FGF-2 binding and higher DS. S–Alg substrates supported the attachment and growth of neurite-positive PC-12 cells with increased performance for FGF-2-loaded high DS coatings. |
[127] |
| 45 |
Alg (LVG, M/G: 0.49, MW: 140 kg mol−1), Pronova. Method of sulfation: 3. HClSO3 DS: 0.4, 1.0, 1.3 (EA) |
Collagen/S–Alg LbL assembly (2-bilayers) |
General tissue engineering In vitro cell morphology (ADSCs, 24 h) |
ADSCs on S–Alg terminated surfaces exhibited very long filopodia. Increase in filopodia length with increasing DS. | [136] |
- a) This column includes information about the starting Alg polymer, sulfation methods, and other Alg modifications. The abbreviations used are 4-CB = 4-carboxybenzaldehyde; Ad = 1-adamantanemethylamine; AEMA = 2-aminoethyl methacrylate hydrochloride; APS = ammonium persulfate; Cat = catechol; DMMB = 1,9-dimethylmethylene blue zinc chloride double salt; DCC = Dicyclohexylcarbodiimide; DM = degree of modification (%), DO = degree of oxidation (%); EA = elemental analysis; EDC = 1-ethyl-3-(3-dimethylaminopropyl)carbodiim-ide hydrochloride; EDOT = 3,4-ethylenedioxythiophene; GalNAc = N-acetyl galactosamine; ICP-MS = inductively coupled plasma – mass spectrometry; ICP-OES = inductively coupled plasma – optical emission spectrometry; RGD = Arg-Gly-Asp peptide; TA = tyrosinase.
- b) This column summarizes how the materials were fabricated. The abbreviations used for the first time are: Ad-MSCs = adipose-derived mesenchymal stem cells; ADSCs = adipose-derived stem cells; BMP = bone morphogenetic protein; CHs: chondrocytes; CS = chitosan; d-ECM = decellularized extracellular matrix; CXB = celecoxib; DN = double-network; DOX = doxorubicin; EV = extracellular vesicles; GelMA = gelatin methacrylate; GT = gelatin; GTA = glutaraldehyde; hASC = human adipose-derived stem cells; HBPep = heparin-binding peptide; HEP = heparin; HGF = hepatocyte growth factor; HDMECs = human dermal microvascular endothelial cells; hPSC = human pluripotent stem cell; HUVECs = human umbilical vein endothelial cells; IGF = insulin-like growth factor; IL-1β = interleukin-1β; KGN = kartogenin; LbL = layer-by-layer; MSCs: mesenchymal stem cells; Pβ-CD = poly-β-cyclodextrin; PB = Prussian blue; PCL = polycaprolactone; PDGF = platelet-derived growth factor; PLCL = poly(l-lactide-co-ε-caprolactone);); PLGA = poly(lactic-co-glycolic acid); PEDOT = poly EDOT; PVA = poly(vinyl alcohol); VEGF = vascular endothelial growth factor; sCT = salmon calcitonin; TCH = tetracycline hydrochloride; TGF = transforming growth factor.
- c) This column briefly describes the key studies. The abbreviations used for the first time are BMDMs = bone-marrow-derived macrophages; CFs = cardiac fibroblasts; ELISA = enzyme-linked immunosorbent assay; hBMSC = human bone marrow stromal cells; HDFs = human dermal fibroblasts; PBMCs = aperipheral blood mononuclear cell, PBS = phosphate-buffered saline; RPMI = Roswell Park Memorial Institute medium; RT = room temperature.
- d) This column concludes the key biological findings of each study. The abbreviations used for the first time are ALP = alkaline phosphatase; FBG = fasting blood glucose; GAG = glycosaminoglycans; LVEF = left ventricular ejection fraction; NPs = nanoparticles.
Two studies fabricated hydrogels by mixing two polymers with complementary functional groups. In one study, an S–Alg polymer modified with adamantane groups (Scheme 4C) was mixed with a poly-β-cyclodextrin (CD) to form a cross–linked hydrogel via host-guest interactions.[103] It was demonstrated that disassembly of the hydrogel could be controlled by adding β-CD monomer which can compete with poly-β-CD for the adamantane groups. In another study, polyelectrolyte complexes formed between S–Alg and CS upon mixing, and these were further cross–linked with calcium ions.[33] This study found that the degree of sulfation affected the compressive modulus of the gels, both as prepared and after partial degradation.
Formation of covalently cross–linked S–Alg-based hydrogel materials has been achieved using various synthetic strategies. Tyramine modified S–Alg (Scheme 4D) was cross–linked using tyrosinase and activation by oxygen.[100] It was found that there was no difference in the compressive modulus compared to control hydrogels made from tyramine-modified Alg, nor was the swelling behavior different, which was not observed for control hydrogels prepared using ionic cross–linking where the swelling behavior was dependent on the DS. UV-cross–linking of methacrylated S–Alg was utilized in two studies, one which used S–Alg modified with AEMA through carbodiimide chemistry (Scheme 4A)[98] and one which used S–Alg modified using methacrylic anhydride (Scheme 4B),[33] in both cases for cell encapsulation.
In the study by Baei et al., two different approaches to the formation of S–Alg/CS hydrogels were explored.[33] One approach used S–Alg modified with catechol groups through carbodiimide chemistry (Scheme 4B). This polymer was mixed with CS and cross–linking facilitated through oxidation with periodate with the intent to oxidize the catechol to o-quinone which would then react with the amine group of CS. It was acknowledged that periodate can also cause oxidation of the polysaccharides, potentially leading to Schiff base formation with the amine groups of CS. The second approach involved methacrylation of S–Alg followed by attachment of catechol groups (Scheme 4B) as well as methacrylation of CS. This allowed the formation of a dual-covalently cross–linked hydrogel with superior mechanical properties compared to the singly-covalently cross–linked hydrogel. In both cases, the hydrogels were additionally ionically cross–linked by calcium ions which affected their stiffness. The catechol-containing hydrogels were black in color, attributed to polymerized catechol.
4.3 Fabrication of S–Alg Particles
Various methods have been used for the fabrication of S–Alg-based particles for cell and drug delivery. To encapsulate cells or cell spheroids in S–Alg-based particles, premixing of the components was done prior to cross–linking in all cases.[109, 111, 112] When encapsulating drugs, however, both a pre-mixing and a post-loading approach have been reported. Particle fabrication was achieved using nanoprecipitation,[54, 106-108, 110, 113, 114] as well as spraying or injection into a cross–linker-containing bath.[27, 102, 105, 109, 111, 112, 134] These studies are summarized in Table 3 (entries 10–22). In this review, the term nanoparticles will refer to particles up to 300 nm in diameter, while particles ranging from 301 to 999 nm will be termed sub-micron particles. Any particles larger than 1 µm will be classified as micron-sized particles. This follows the definition of polymer particle sizes set down by the National Science and Technology Council, USA.[135]
Fabrication of submicron-sized particles has been achieved using a mixture of Alg and S–Alg to encapsulate the peptide calcitonin where the peptide–polysaccharide mixture was injected into a calcium bath.[27] While pure peptide self-assembled into particles (680 nm), the presence of the Alg–S–Alg mixture greatly reduced the size to 320–380 nm at optimized reagent ratios.[27] VEGF was loaded into particles prepared from a mixture of Alg and S–Alg by premixing and spraying the mixture into a calcium bath, achieving micron-sized particles.[102] An alternative approach of post-loading was used to encapsulate bone morphogenetic protein 4 (BMP-4) into micron-sized particles produced by injecting S–Alg or mixtures of Alg and S–Alg into a calcium bath.[105]
Nanoparticles have been produced using nanoprecipitation which involves the mixing of precursor solutions with agitation. Work by the group of Cohen, encapsulated oligonucleotides using nanoprecipitation with vortexing and stirring.[106, 107] In one study, the effect of the order of mixing S–Alg, calcium, and small interfering ribonucleic acid (siRNA) solutions demonstrated no difference in particle size which was 130–140 nm.[107] Encapsulation of plasmid deoxyribonucleic acid (pDNA) in calcium cross–linked S–Alg resulted in a particle size of 270 nm which was significantly larger than those encapsulating siRNA related to the different sizes of the oligonucleotide.[106] Our recent work evaluated the encapsulation of lactoferrin in S–Alg particles using sonication in combination with nanoprecipitation.[54] A reduction in size was found for particles prepared using S–Alg (175 nm) compared to those made from Alg (245 nm), and while similar particle sizes were observed using S–Alg with DS values of 0.8 and 1.1, a significantly larger particle size was found using S–Alg with a DS of 1.7. Nanoprecipitation was also used to form core–shell nanoparticles from PSS with the anticancer drug doxorubicin (DOX) covalently attached through an imide bond (Scheme 4D). Particle fabrication was achieved by dropwise addition of an organic solution of the PSS-DOX conjugate mixed with a second anticancer drug CXB into an aqueous solution of borax acting as the cross-linking agent. This allowed the formation of particles <150 nm in size.[114]
The requirement for ionic cross–linking using calcium ions was explored in our study where it was demonstrated that the HBP lactoferrin could form S–Alg-based nanoparticles in the absence of calcium ions.[54] Direct assembly of nanoparticles without the use of a cross–linker was likewise achieved using proteins with high binding affinity for S–Alg.[110] Specifically, a small matrix MMP-7 inhibitor protein, C9, modified with heparin-binding protein domains was mixed with S–Alg, vortexed, and incubated overnight to form spherical nano-sized (≈10 nm) complexes. Another example encapsulating the HBPs hepatocyte growth factor (HGF) and insulin-like growth factor 1 (IGF-1) in S–Alg particles using nanoprecipitation with vortex and no calcium cross–linker formed particles of 50–100 nm in size.[113] These studies demonstrate that polyelectrolyte complex formation between high pI proteins and S–Alg can facilitate particle formation.
4.4 Fabrication of S–Alg Particles
This section includes hydrogel scaffolds that incorporate S–Alg. In some cases, S–Alg is a major component while S–Alg has also been used to prepare hydrogel scaffolds where the major component is for example Alg, gelatin methacrylate (GelMA) or polyvinyl alcohol (PVA). These studies are summarized in Table 3 (entries 23–35). Scaffolds have been made using bioprinting,[26, 115, 116, 120, 125] electrospinning,[22, 118, 119, 121, 122] particle assembly,[117] or freeze drying.[123, 124] Cross–linking of the S–Alg component in the scaffolds was done mainly using a calcium source, although other strategies have been used with examples included below.
Bioprinting of hydrogel scaffolds involves the fabrication of a bioink that is printed onto a platform and subsequently or simultaneously, the bioink is cross–linked. An important factor for the printability of a bioink is its rheological properties. While some studies have reported that the addition of S–Alg into Alg bioinks causes a decrease in the viscosity of the bioink[115] or a decrease in scaffold stiffness,[120] other studies have not observed such effects.[26] The reduction in viscosity of S–Alg compared to Alg has been demonstrated to depend both on the MW and DS of S–Alg.[31] Therefore, it is important to consider the DS and the amount of S–Alg included in bioinks.
The use of bioprinting allows for the incorporation of cells within the scaffold as demonstrated in some studies.[26, 116, 120, 125] An interesting approach to bioprinting was demonstrated using S–Alg (DS = 0.8) combined with Alg, where selective printing of primary neurons in the S–Alg gel within an Alg carrier restricted the mobility of the neurons.[120] Another example of compartmentalizing different components using bioprinting is the encapsulation of cardiac cells in an RGD-modified Alg matrix which was surrounded by an outer shell of S–Alg encapsulating extracellular vesicles (EVs).[116] A study making use of Alg and S–Alg in GelMA bioinks and creating scaffolds through dual ionic and UV-cross–linking demonstrated that the presence of S–Alg improved the compressive properties of cell and protein-laden scaffolds.[125]
Studies that have prepared fibrous scaffolds using electrospinning have in all cases used a combination of S–Alg and PVA. Different cross–linking strategies were used including ionic,[22] thermal,[121] and covalent using glutaraldehyde as the cross–linker.[118, 119, 122] When using high weight ratios of S–Alg (DS = 0.9) (PVA:S–Alg from 7:3 to 5:5), nanofibers with diameters ranging from 75 to 280 nm could be produced.[22] Furthermore, it was shown that increasing the S–Alg content caused less bead formation on the nanofibers. Another study found that using S–Alg (30 wt.%) rather than pure PVA enhanced nanofiber uniformity and reduced the fiber diameter significantly from 450 to 185 nm.[119] Similar observations of lower average fiber diameter and higher uniformity when fibers are produced with S–Alg have likewise been made in other studies.[118, 121, 122] It was demonstrated that increasing the S–Alg content caused an increase in swelling and degradation rate and furthermore, a reduction in mechanical properties.[122]
4.5 Fabrication of S–Alg Particles
For applications of S–Alg in composites and as coatings, S–Alg does not form the main component and is combined with materials such as thermoplastic polymers,[47, 52, 128-131] polyurethane elastomers[34] or metal oxide particles,[126] while some studies used a model gold substrate for fundamental studies.[127, 136] These studies are summarized in Table 3 (entries 36–45).
In some composites, the different components of the composite are fabricated separately before final assembly. One example is ketogenic-loaded PLGA nanoparticles inside a calcium cross–linked Alg–S–Alg hydrogel which was placed between poly(ε-caprolactone) (PCL)–gelatin electrospun mats to enhance the mechanical performance.[130] A similar material was made from a calcium Alg–S–Alg hydrogel sandwiched between PCL–gelatin electrospun mats for cell delivery.[128] This study found that increasing the number of electrospun mat layers reduced the degradation rate and this was attributed to decreasing the ion exchange inside the composite. Another approach is to add a mixture of Alg, S–Alg, and decellularized extracellular matrix inside a 3D printed PCL scaffold prior to freeze-drying and cross–linking the hydrogel with calcium solutions.[131] Alternatively, a single-step fabrication approach was used to make core-shell particles with PCL forming the shell and S–Alg loaded with GFs forming the core via a water-in-oil-in-water double emulsion method.[47] Covalent attachment of S–Alg to the end-groups of NCO-terminated cationic polyurethane allowed fabrication of supramolecular polyurethanes that were ionically cross–linked, facilitated by the sulfate and carboxylate groups of S–Alg, with the amines of the polyurethane.[34]
There are a few studies that have coated S–Alg or a mixture of Alg and S–Alg onto scaffolds. One approach was to covalently cross–link Alg and S–Alg with the cross–linker adipic acid dihydrazide using carbodiimide chemistry (Scheme 4A) as a coating on a poly(lactide-co-ε-caprolactone) (PLCL) scaffold.[129] This study found that the presence of Alg was required to increase the solution viscosity. A different approach to coating was applied to PCL nanofiber scaffolds that were surface-activated by cold atmospheric plasma to facilitate direct coating with S–Alg.[52] S–Alg coatings have also been applied to Fe3O4 particles.[126] The use of dopamine-conjugated S–Alg facilitated not only electrostatic attraction between S–Alg and the Fe3O4 particles but also afforded covalent attachment of the mussel-inspired adhesive macromolecule. The coated particles had a negative zeta potential (−48 mV) while the parent particles were positively charged (+ 52 mV). Based on a reduction in mean particle size after applying the coating (from 930 to 350 nm) it was concluded that the S–Alg coating suppressed particle aggregation. Unfortunately, these studies do not evaluate the longevity of the coating which is a parameter that is difficult to predict and even some covalently attached coatings have shown poor adhesion stability after immersion in simple solutions.[137]
5 Applications of S–Alg-based Biomaterials
S–Alg-based materials have been fabricated for a wide range of biomedical applications as detailed in Table 3 and illustrated in Figure 5. As described in Section 3, one aspect that is often overlooked is the lack of the enzyme alginate lyase in humans which makes Alg and its derivatives non-biodegradable. In relation to using S–Alg in the material design, this means that after the biomaterial has degraded the Alg polymer will remain. As detailed in Section 4, some studies have used dual-modified Alg where oxidation of the polymer renders it biodegradable in vivo. In some applications, the incorporation of S–Alg into a material is due to the inherent biological properties of S–Alg such as anti-diabetes[111] or anti-coagulation.[114, 126] However, the most common property of S–Alg that is being utilized is the ability to bind HBPs which is discussed in Section 5.1. The main area of research included in 24 articles, involves encapsulation of GF or other therapeutic agents and this aspect is described in Section 5.2. Cell encapsulation is a common approach in tissue engineering, particularly for cartilage regeneration. While Alg hydrogels have been observed to lack cell adhesion for encapsulated cells, S–Alg hydrogels demonstrated optimal cell adhesion, spreading, and proliferation.[115] This function has been attributed to the high binding affinity of S–Alg to β-1 integrins, which mediate cell adhesion and proliferation.[15] S–Alg hydrogels therefore excel in mimicking the structure of the native ECM, providing an optimal microenvironment for encapsulated cells to adhere, proliferate, and differentiate effectively.[138] Moreover, as detailed in Section 5.1, hydrogels incorporating S–Alg exhibit high binding affinity to various HBPs which has the potential to assist tissue regeneration by facilitating the retention and gradual release of essential HBPs. Previous research primarily focussed on encapsulating chondrocytes within S–Alg bulk hydrogels,[33, 97, 100, 115] with some studies exploring the feasibility of co-encapsulating HBP and MSCs.[99, 129, 131] To encapsulate cells, most of these studies employed a post-encapsulation method,[33, 52, 97, 99, 100, 119, 129, 131] and only a few studies cross–linked the hydrogel scaffolds in the presence of cells to facilitate cell encapsulation.[115, 128, 139]
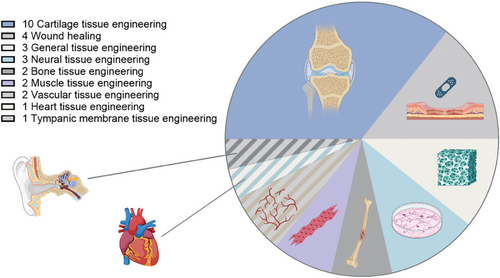
5.1 Binding affinity of S–Alg to Proteins and Other Therapeutics
One important property of S–Alg, is the ability to bind HBPs with high binding affinity similar to heparin and heparan sulfate. Earlier work evaluated a range of GFs including EGF and IGF and the cytokine interleukin 6 (IL-6).[24, 54, 140] In comparison, Alg does not show specific binding affinity to HBPs and this has been verified in recent works. Evaluation of the binding affinity is achieved using either surface plasmon resonance (SPR) or a quartz crystal microbalance with dissipation (QCM-d). In both techniques, the S–Alg polymer is often attached to a surface using various conjugation chemistries and as such, the biomolecule may be attached in different conformations.
In recent years a number of additional HBPs have been evaluated. Itzhar et al. produced heparin-binding peptides and used SPR to evaluate the affinity of three such peptides to S–Alg. All demonstrated high binding affinity (KA = 2.6 × 106–5× 107 M−1) to S–Alg polymer, as well as heparin.[110] Anitha et al., determined the binding affinity of lactoferrin to S–Alg (DS = 0.6) using SPR to be KA = 2.0 × 107 M−1 which was similar to that reported for heparin.[54] SPR has also been used to evaluate binding of EVs to S–Alg where very high binding affinity (KA = 3 × 1014 M−1) was similar to that of heparin (KA = 1 × 1014 M−1).[116] A study by Ma et al., investigated the binding affinity between PSS and FGF-2 as well as VEGF165.[68] It was observed that FGF-2 had a similar binding affinity to PSS (KA = 3.6 × 107 M−1) as to heparin (KA = 3.6 × 107 M−1), while VEGF165 showed specific binding to heparin (KA = 1.2 × 106 M−1) and only weak binding to PSS (KA = 5.6 × 103 M−1).
The Group led by Mhanna has used QCM-d to study the binding of the HBP FGF-2 to S–Alg in different ways. In their 2017 study, layer-by-layer assemblies of collagen and S–Alg were created on the gold-coated QCM crystal, and it was shown that higher binding affinity was achieved when using S–Alg with higher DS (DS = 0.4, 1.0, 1.3).[136] In their 2019 study, the S–Alg polymers were attached to the QCM crystal via end-group functionalization. A similar finding was made in that the DS (0.4, 1.0, 1.3) of S–Alg was found to affect the binding of FGF-2 with an increased binding affinity for the polymers with higher DS.[127] Their 2020 study evaluated the effect of the concentration of S–Alg used for attachment to the QCM crystal and found higher FGF-2 and β-NGF binding affinity (both HBPs) when a higher concentration of S–Alg had been used.[120]
5.2 Application of S–Alg to Sustain the Release of Therapeutic Agents
Encapsulation of a range of pharmaceutical compounds in a hydrogel-based material fabricated from or incorporating S–Alg has been demonstrated in numerous studies. This includes small molecule drugs, HBPs, other proteins, oligonucleotides, EVs, as well as nanoparticles encapsulating drugs. In the area of wound healing (a total of four articles identified) various approaches have been taken including encapsulation of platelet-derived growth factor (PDGF) for enhanced vascularization[98] and encapsulation of Prussian blue nanozymes for enhanced would closure and macrophage phenotype regulation.[104] In bone tissue engineering, strategies include delivery of the single-chain 32 amino acid polypeptide hormone salmon calcitonin (sCT)[27] and co-encapsulating BMP-2 with MC3T3-E1 cells to promote osteogenesis.[26]
The presence of S–Alg can effectively regulate both the EE and release profile of many therapeutic compounds. Enhanced EE has been demonstrated for transforming growth factor-beta 1 (TGF-β1) in a Ca2+-cross–linked S–Alg scaffold where a higher amount was encapsulated in the S–Alg materials compared with the Alg control (151 ng and 128 ng, respectively),[141] and for the positively charged small molecule drug tetracycline hydrochloride with EE of 35% in S–Alg particles, compared to 1% in Alg particles.[108] The EE of the low pI protein BSA can also be increased when using S–Alg compared to Alg in double emulsion PCL particles (94 versus 88%, respectively). However, the presence of S–Alg rather than Alg increased the rate of release of BSA in PBS in accordance with higher electrostatic repulsion for the S–Alg containing particles.[47] In contrast, Munarin and colleagues found no to a minor decrease in EE upon encapsulating VEGF in S–Alg compared to Alg control in Ca2+ cross–linked materials (EE of 66 and 70%, respectively). The S–Alg containing materials were, however, able to attain a better controlled and sustained release of VEGF in PBS compared to the Alg control.[102]
Many studies have observed a similar ability of S–Alg to control and prolong the release of HBP which has been attributed to the higher binding affinity of S–Alg compared to Alg for HBPs as discussed in Section 5.1. Studies include PDGF-BB encapsulated in photo-cross–linked S–Alg based materials,[98] BMP-4 in Ca2+-cross–linked particles,[105] TGF-β1 in thermally cross–linked PVA/S–Alg nanofibrous scaffolds,[121] BMP-2 in Ca2+-cross–linked 3D printed scaffolds.[26] In the study by Baei et al., there was a direct correlation between the rate of release of TGF-β1 encapsulated in an interpenetrating network (IPN) scaffold and DS (0–0.67) with the total amount released over 14 days, reducing from 83% to 42% with increasing DS.[33] In addition to its high binding affinity to HBPs, the negative charges of S–Alg also contribute to slowing down the release of the positively charged polypeptide hormone sCT when encapsulated in Ca2+-cross–linked particles where the presence of S–Alg reduced the burst release compared to the Alg control and furthermore, extended the overall release.[27]
It should be noted that in the studies described above, PBS was used as the release medium. The name PBS is used for buffers of different compositions (with or without added Ca2+ and Mg2+, different ionic strength) which is not always explicitly reported. The importance of this is that PBS has a significantly higher phosphate content than serum and that PBS (without added calcium ions) will superficially enhance the release rate of cargo from Ca2+-cross–linked Alg-based materials due to the high affinity between Ca2+ and phosphate ions.[142]
Many studies have used tissue culture medium to study the release of HBP for S–Alg-based materials and in these studies, the presence of S–Alg again controlled and prolonged the rate of release. The cumulative release over 7 days of TGF-β3 from IPN constructs was 66% for control Alg materials compared to 42% for the S–Alg containing materials, and sustained release of TGF-β3 over 7 days was observed for the S–Alg containing IPN.[139] The same lab reported an initial burst release of TGF-β3 from Alg-containing scaffolds (55% in 3 days) while the sustained release was observed from S–Alg-containing scaffolds.[129] The release of TGF-β1 from a Ca2+-cross–linked S–Alg scaffold and Alg control displayed a burst release of 20 and 56%, respectively, and while the HBP was released (≈100%) from the Alg control in 3 days, the S–Alg containing scaffold afforded release (≈92%) over 7 days.[123]
An interesting observation was made in the study by Gionet-Gonzales et al. who investigated the binding and release of IGF-1, a non-HBP high pI protein (pI = 8.5[143]), and the HBP HGF from ionically cross–linked disks in serum-free media.[23] They observed higher burst release for the disks produced using S–Alg with a high DS compared to Alg and S–Alg with a low DS for IGF-1, whereas a direct correlation between DS and EE and release rate was observed for HGF, with faster release from the Alg control. This demonstrates the specific affinity of S–Alg for HBPs.
6 Conclusion
Much research published on the use of S–Alg in biomedical applications is lacking detailed characterization of the S–Alg polymer and often the DS or S % are not reported. This prohibits reproducibility of the work, a major obstacle to progress in biomedical research. Recent advances in the synthesis of S–Alg have demonstrated i) the need for careful and detailed characterization to fully elucidate the structure and the presence of any unwanted adducts, and ii) strategies for regioselective sulfation. Future work should make use of these advances to carefully select appropriate sulfation strategies thereby ensuring well-defined and well-characterized materials are used.
Many studies have used interesting chemistry to fabricate S–Alg containing materials, yet, there is still a high reliance on using ionic cross–linking. Considering the well-reported uncontrolled degradation of such materials, more sophisticated strategies such as those detailed in this review should be implemented more broadly. It is also noted that when using nano-precipitation and encapsulation of HBPs, the protein itself can act as the cross–linker. Many studies incorporating S–Alg into biomaterials have highlighted the benefits of enhanced EE and sustained release of HBP and other positively charged therapeutic agents. The heparin-mimicking structure and high negative charge of S–Alg thus make it a promising material for fabricating controlled drug delivery systems. S–Alg, and often specifically PSS, continues to be evaluated as a drug. Interesting new discoveries of S–Alg as an anticancer drug could potentially be exploited in the preparation of nanoparticle carriers for anticancer drugs gaining dual effects.
Before substantial progress and translation can be achieved, however, it is recommended that more detailed toxicity and biodistribution studies be performed for the general class of S–Alg polymers. Knowledge of the effects of MW, M/G ratio, or DS is currently missing. This makes it difficult to make informed decisions as to the choice of S–Alg polymer for specific applications.
Acknowledgements
A.L.M. and J.Y. contributed equally to this work. Author A.L.M. is thankful to the Australian Government RTP scholarship funding; author J.Y. acknowledges scholarship funding from The University of Queensland and China Scholarship Council (CSC); author A.A. is thankful to the National Health and Medical Research Council, Australia for the Early Career Research Fellowship (APP1123340, 2017–2021) as well as the UQ Amplify Fellowship (2021 Dec-2023 Nov) for providing the financial support.
Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Alexandra Mutch completed her PhD in polymer chemistry at The University of Queensland in 2022 under the supervision of Prof Lisbeth Grøndahl. She was a Lecturer at Australian Catholic University before commencing her role as a Postdoctoral Fellow at the University of Tasmania in 2023. Her research interests include the fabrication of polymeric biomaterials and surface modification and characterization. She is currently exploring the use of polymerizable eutectics for new methods of materials synthesis.

Jiankun Yang completed his bachelor's in pharmacy in 2017 at Jiangsu University and his Master's degree in Biotechnology in 2019 at The University of Queensland. He worked as an Assistant Editor and Special Issue Editor for the International Journal of Molecular Science and Biomedicines from Sep 2020 to Jan 2021. He was awarded the Chinese Scholarship Council – The University of Queensland joint PhD scholarship and commenced his PhD study in July 2021, at The University of Queensland under the supervision of Prof Lisbeth Grøndahl. His research focuses on the chemical modification of alginate and nanogel fabrication for protein delivery applications.

Vito Ferro obtained his PhD from the University of Western Australia in 1992. Following postdoctoral studies at the Carlsberg Laboratory, Denmark, and the University of British Columbia, Canada, he joined Progen Pharmaceuticals Ltd (Brisbane, Australia) where he spent 12 years in various positions, including Director of Drug Discovery. Following a brief period at Queensland University of Technology, he moved to the University of Queensland in 2010 where he is a Professor in the School of Chemistry and Molecular Biosciences and is also a member of the Australian Infectious Diseases Research Centre. His research interests are in carbohydrate and medicinal chemistry with a particular focus on glycosaminoglycans (especially heparan sulfate and heparin) and their mimetics as potential drugs for a range of diseases.

Anitha A. obtained her PhD in Nanobiotechnology from Amrita Vishwa Vidyapeetham University, India, in 2014. She has held several Fellowships including a UQ postdoctoral Fellowship and an NHMRC early career research Fellow at the School of Chemistry and Molecular Biosciences, The University of Queensland, Australia working in the research group of Prof Lisbeth Grøndahl. Dr. Anitha's research interests are in the development of natural and synthetic polymer-based therapeutic formulations toward drug delivery especially for cancer as well as bone fracture healing applications.

Lisbeth Grøndahl obtained a PhD in chemistry from the University of Copenhagen, Denmark, in 1995. After a number of teaching and research positions including two Fellowships, she joined the School of Chemistry and Molecular Biosciences at the University of Queensland, Australia, in 2002 as Lecturer and now holds a position as Professor. She is a fellow of Biomaterials Science and Engineering and works in the interdisciplinary field of biomaterials science with a focus on the development of approaches to and characterization of functional polymeric biomaterials in the form of membranes, scaffolds, hydrogels, and nanoparticles.