Novel Anti-Biofouling Soft Contact Lens: l-Cysteine Conjugated Amphiphilic Conetworks via RAFT and Thiol–Ene Click Chemistry
Chengfeng Zhang
College of Material Science and Engineering, Donghua University, Shanghai, 201620 P. R. China
Search for more papers by this authorZiyuan Liu
College of Material Science and Engineering, Donghua University, Shanghai, 201620 P. R. China
Search for more papers by this authorHaiye Wang
College of Material Science and Engineering, Donghua University, Shanghai, 201620 P. R. China
Search for more papers by this authorXiaofeng Feng
College of Material Science and Engineering, Donghua University, Shanghai, 201620 P. R. China
Search for more papers by this authorCorresponding Author
Chunju He
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai, 201620 P. R. China
E-mail: [email protected]Search for more papers by this authorChengfeng Zhang
College of Material Science and Engineering, Donghua University, Shanghai, 201620 P. R. China
Search for more papers by this authorZiyuan Liu
College of Material Science and Engineering, Donghua University, Shanghai, 201620 P. R. China
Search for more papers by this authorHaiye Wang
College of Material Science and Engineering, Donghua University, Shanghai, 201620 P. R. China
Search for more papers by this authorXiaofeng Feng
College of Material Science and Engineering, Donghua University, Shanghai, 201620 P. R. China
Search for more papers by this authorCorresponding Author
Chunju He
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai, 201620 P. R. China
E-mail: [email protected]Search for more papers by this authorAbstract
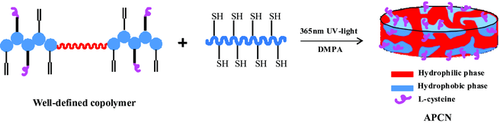
A unique l-cysteine conjugated antifouling amphiphilic conetwork (APCN) is synthesized through end-crosslinking of well-defined triblock copolymers poly(allyl methacrylate)-b-poly(ethylene glycol)-b-poly(allyl methacrylate) via a combination of reversible addition-fragmentation chain transfer (RAFT) polymerization and thiol–ene “click” chemistry. The synthesized poly(ethylene glycol) macro-RAFT agent initiates the polymerization of allyl methacrylate in a controlled manner. The vinyl pendant groups of the precursor partially conjugate with l-cysteine and the rest fully crosslink with mercaptopropyl-containing siloxane via thiol–ene click chemistry under UV irradiation into APCNs, which show distinguished properties, that is, excellent biocompatibility, more than 39.6% water content, 101 barrers oxygen permeability, optimized mechanical properties, and more than 93% visible light transmittance. What's more, the resultant APCNs exhibit eminent resistance to protein adsorption, where the bovine serum albumin and lysozyme adsorption are decreased to 12 and 21 µg cm−2, respectively. The outstanding properties of APCNs depend on the RAFT controlled method, which precisely designs the hydrophilic/hydrophobic segments and eventually greatly improves the crosslinking efficiency and homogeneity. Meantime, the l-cysteine monolayer can effectively reduce the surface hydrophobicity and prevent protein adsorption, which exhibits the viability for antifouling surface over and under ophthalmic devices, suggesting a promising soft contact lens.
References
- 1P. C. Nicolson, J. Vogt, Biomaterials 2001, 22, 3273.
- 2G. Wollensak, E. Mur, A. Mayr, G. Baier, W. Göttinger, G. Stöffler, Graefe's Arch. Clin. Exp. Ophthalmol. 1990, 228, 78.
- 3C. W. McMonnies, Clin. Exp. Optom. 2014, 97, 308.
- 4C. C. S. Karlgard, D. K. Sarkar, L. W. Jones, C. Moresoli, K. T. Leung, Appl. Surf. Sci. 2004, 230, 106.
- 5L. Szczotka-Flynn, Y. Jiang, S. Raghupathy, R. A. Bielefeld, M. T. Garvey, M. R. Jacobs, J. Kern, S. M. Debanne, Optom. Vis. Sci. 2014, 91, 3.
- 6A. Saini, C. J. Rapuano, P. R. Laibson, E. J. Cohen, K. M. Hammersmith, Eye Contact Lens 2013, 39, 324.
- 7G. Guzman, S. S. Es-haghi, T. Nugay, M. Cakmak, Adv. Healthcare Mater. 2016, 6, 1600775.
- 8E. J. Kepola, E. Loizou, C. S. Patrickios, E. Leontidis, C. Voutouri, T. Stylianopoulos, R. Schweins, M. Gradzielski, C. Krumm, J. C. Tiller, M. Kushnir, C. Wesdemiotis, ACS Macro Lett. 2015, 4, 1163.
- 9G. Kali, B. Iván, Eur. Polym. J. 2016, 84, 668.
- 10C. Fodor, G. Kali, B. Iván, Macromolecules 2011, 44, 4496.
- 11G. Kali, S. Vavra, K. László, B. Iván, Macromolecules 2013, 46, 5337.
- 12A. Domján, C. Fodor, S. Kovács, T. Marek, B. Iván, K. Süvegh, Macromolecules 2012, 45, 7557.
- 13B. Iván, M. Haraszti, G. Erdődi, J. Scherble, R. Thomann, R. Mülhaupt, Macromol. Symp. 2005, 227, 265.
- 14N. Bruns, J. Scherble, L. Hartmann, R. Thomann, B. Iván, R. Mülhaupt, J. C. Tiller, Macromolecules 2005, 38, 2431.
- 15J. Scherble, R. Thomann, B. Iván, R. Mülhaupt, J. Polym. Sci., Part B: Polym. Phys. 2001, 39, 1429.
- 16J. Scherble, B. Iván, R. Mülhaupt, Macromol. Chem. Phys. 2002, 203, 1871.
- 17G. Erdodi, J. P. Kennedy, Prog. Polym. Sci. 2006, 31, 1.
- 18T. K. Georgiou, C. S. Patrickios, P. W. Groh, B. Iván, Macromolecules 2007, 40, 2335.
- 19M. D. Rikkou, M. Kolokasi, K. Matyjaszewski, C. S. Patrickios, J. Polym. Sci., Part A: Polym. Chem. 2010, 48, 1878.
- 20G. Moad, E. Rizzardo, S. H. Thang, Aust. J. Chem. 2012, 65, 985.
- 21J. T. Lai, D. Filla, R. Shea, Macromolecules 2002, 35, 6754.
- 22C. Boyer, A. Granville, T. P. Davis, V. Bulmus, J. Polym. Sci., Part A: Polym. Chem. 2009, 47, 3773.
- 23J. W. Chan, B. Yu, C. E. Hoyle, A. B. Lowe, Chem. Commun. 2008, 40, 4959.
- 24C. Boyer, T. P. Davis, Chem. Commun. 2009, 40, 6029.
- 25G. Erdödi, B. Iván, Chem. Mater. 2004, 16, 959.
- 26G. Kali, T. K. Georgiou, B. Iván, C. S. Patrickios, E. Loizou, Y. Thomann, J. C. Tiller, Macromolecules 2007, 40, 2192.
- 27A. P. Chapman, Adv. Drug Delivery Rev. 2002, 54, 531.
- 28S. Lee, J. Vörös, Langmuir 2005, 21, 11957.
- 29S. Demming, C. Lesche, H. Schmolke, C. P. Klages, S. Büttgenbach, Phys. Status Solidi A 2011, 208, 1301.
- 30R. K. Dey, A. R. Ray, Biomaterials 2003, 24, 2985.
- 31R. E. Holmlin, X. Chen, R. G. Chapman, S. Takayama, G. M. Whitesides, Langmuir 2001, 17, 2841.
- 32G. B. Sigal, M. Mrksich, G. M. Whitesides, J. Am. Chem. Soc. 1998, 120, 3464.
- 33E. Ostuni, R. G. Chapman, M. N. Liang, G. Meluleni, G. Pier, D. E. Ingber, G. M. Whitesides, Langmuir 2001, 17, 6336.
- 34M. Mrksich, G. B. Sigal, G. M. Whitesides, Langmuir 1995, 11, 4383.
- 35O. R. Bolduc, J. F. Masson, Langmuir 2008, 24, 12085.
- 36B. S. Sumerlin, A. P. Vogt, Macromolecules 2009, 43, 1.
- 37J. D. Flores, N. J. Treat, A. W. York, C. L. Mccormick, Polym. Chem. 2011, 2, 1976.
- 38J. E. Rosen, F. X. Gu, Langmuir 2011, 27, 10507.
- 39G. Erdodi, J. P. Kennedy, J. Polym. Sci., Part A: Polym. Chem. 2005, 43, 3491.
- 40C. H. Lin, W. C. Lin, M. C. Yang, Colloids Surf., B 2009, 71, 36.
- 41C. H. Lin, W. C. Jao, Y. H. Yeh, W. C. Lin, M. C. Yang, Colloids Surf., B 2009, 70, 132.
- 42Y. K. Chong, J. Krstina, T. P. T. Le, G. Moad, A. Postma, E. Rizzardo, S. H. Thang, Macromolecules 2003, 36, 2256.
- 43C. Schilli, M. G. Lanzendörfer, A. H. E. Müller, Macromolecules 2002, 35, 6819.
- 44G. Levesque, P. Arsène, V. Fanneau-Bellenger, T. N. Pham, Biomacromolecules 2000, 1, 387.
- 45C. Barner-Kowollik, Handbook of RAFT Polymerization, John Wiley & Sons, Wiley, 2008, p. 1.
10.1002/9783527622757.ch1 Google Scholar
- 46S. Dech, V. Wruk, C. P. Fik, J. C. Tiller, Polymer 2012, 53, 701.
- 47J. Xu, M. Qiu, B. Ma, C. He, ACS Appl. Mater. Interfaces 2014, 6, 15283.
- 48C. H. Lin, Y. H. Yeh, W. C. Lin, M. C. Yang, Colloids Surf., B 2014, 123, 986.
- 49J. Pozuelo, V. Compañ, J. M. González-Méijome, M. González, S. Mollá, J. Membr. Sci. 2014, 452, 62.
- 50N. Efron, P. B. Morgan, I. D. Cameron, N. A. Brennan, M. Goodwin, Optom. Vis. Sci. 2007, 84, 328.
- 51M. Guillon, C. Maissa, Contact Lens Anterior Eye 2007, 30, 5.
- 52T. S. Bhamra, B. J. Tighe, Contact Lens Anterior Eye 2011, 34, S22.
- 53A. López-Alemany, V. Compañ, M. F. Refojo, J. Biomed. Mater. Res. 2002, 63, 319.
- 54J. Scherble, R. Thomann, B. Iván, R. Mülhaupt, J. Polym. Sci., Part B: Polym. Phys. 2001, 39, 1429.
- 55P. C. Nicolson, Eye Contact Lens 2003, 29, 192.
- 56R. I. Myers, D. W. Larsen, M. Tsao, C. Castellano, L. D. Becherer, F. Fontana, N. R. Ghormley, G. Meier, Optom. Vis. Sci. 1991, 68, 776.
- 57C. E. Soltys-Robitaille, D. M. Ammon Jr., P. L. Valint Jr., G. L. Grobe III, Biomaterials 2001, 22, 3257.
- 58K. W. Gellatly, N. A. Brennan, N. Efron, Optom. Vis. Sci. 1988, 65, 937.
- 59F. P. Carney, C. A. Morris, M. D. P. Willcox, Aust. N.Z. J. Ophthalmol. 1997, 25, 36.
- 60S. L. Willis, J. L. Court, R. P. Redman, J.-H. Wang, S. W. Leppard, V. J. O'Byrne, S. A. Small, A. L. Lewis, S. A. Jones, P. W. Stratford, Biomaterials 2001, 22, 3261.