Decomposition and Transformation of Pyrene-Derivative Micelles at Intracellular Milieu and Their Influence on Cytoviability
Haisheng Wang
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorWei Yu
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorWenbo Zhang
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Changyou Gao
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorHaisheng Wang
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorWei Yu
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorWenbo Zhang
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Changyou Gao
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorAbstract
The amphiphilic pyrene-containing random copolymers with pH-sensitive hydrazone bonds are synthesized by copolymerizing hydrophobic pyrene-containing methacrylhydrazone with hydrophilic N,N-dimethylacrylamide. The hydrolysable copolymers form spherical micelles at pH 6, which are further transformed into pure 1-pyrenecarboxaldehyde nanorods due to cleavage of the hydrazone bonds. Likewise, the shape transformation is observed under pH 5.5 phosphate buffered saline. Moreover, the hydrolysable micelles being co-cultured with A549 cells cause higher cytotoxicity than the non-hydrolysable ones without shape transformation, which are synthesized by reducing the hydrazone bonds with NaHB4.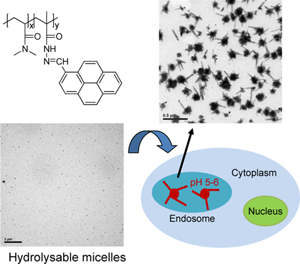
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| mabi201400338-sm-0001-SuppFig-S1.doc741 KB |
Figure S1: 1H NMR spectrum of pyrene-containing random copolymers 3 (DMSO-d6). Figure S2: Fourier transform infrared (FTIR) spectra of the nanorods and raw Py-CHO. FTIR spectroscopy was recorded on a Vector 22 spectrophotometer (Bruker optics, Switzerland). The dried samples were thoroughly mixed with KBr powders and pressed into transparent laminae. Figure S3: Fluorescence microscopy images of A549 cells incubated with (a) non-hydrolysable micelles and (b) hydrolysable micelles (1 mg/mL) for 3 h. From left to right: endosome (red), non-hydrolysable or hydrolysable micelles (green), and a merge of the two images. Scheme S1: Schematic illustration of configuration of (a) non-hydrolysable micelles and (b) hydrolysable micelles in endosomes after endocytosis. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 G. M. Whitesides, J. P. Mathias, C. T. Seto, Science 1991, 254, 1312.
- 2 H. W. Duan, D. Y. Wang, D. G. Kurth, H. Mohwald, Angew. Chem. Int. Ed. 2004, 43, 5639.
- 3 J. H. Ryu, D. J. Hong, M. Lee, Chem. Commun. 2008, 1043.
- 4 Y. F. Tu, X. H. Wan, H. L. Zhang, X. H. Fan, D. N. Lu, X. F. Chen, Q. F. Zhou, Chin. J. Polym. Sci. 2003, 21, 569.
- 5 Q. Liang, B. Guan, M. Jiang, Prog. Chem. 2010, 22, 388.
- 6 L. C. Palmer, S. I. Stupp, Acc. Chem. Res. 2008, 41, 1674.
- 7 L. Zang, Y. Che, J. S. Moore, Acc. Chem. Res. 2008, 41, 1596.
- 8 F. Reincke, W. K. Kegel, H. Zhang, M. Nolte, D. Y. Wang, D. Vanmaekelbergh, H. Mohwald, Phys. Chem. Chem. Phys. 2006, 8, 3828.
- 9 W. Li, Y. Kim, M. Lee, Nanoscale 2013, 5, 7711.
- 10 Z. Fan, Y. L. Zhao, Y. Liu, Chin. Sci. Bull. 2003, 48, 1535.
- 11 M. Y. Guo, M. Jiang, Prog. Chem. 2007, 19, 557.
- 12 E. G. Kelley, J. N. L. Albert, M. O. Sullivan, I. I. I. T. H. Epps, Chem. Soc. Rev. 2013.
- 13 Y. Wang, H. Xu, X. Zhang, Adv. Mater. 2009, 21, 2849.
- 14 M. Lee, S. J. Lee, L. H. Jiang, J. Am. Chem. Soc. 2004, 126, 12724.
- 15 P. B. Wan, H. H. Eric, X. Zhang, Prog. Chem. 2012, 24, 1.
- 16 C. Wang, Y. Guo, Y. Wang, H. Xu, R. Wang, X. Zhang, Angew. Chem. Int. Ed. 2009, 48, 8962.
- 17 A. Napoli, M. Valentini, N. Tirelli, M. Muller, J. A. Hubbell, Nat. Mater. 2004, 3, 183.
- 18 M.-P. Chien, A. M. Rush, M. P. Thompson, N. C. Gianneschi, Angew. Chem. Int. Ed. 2010, 49, 5076.
- 19 Z. Wang, H. Moehwald, C. Gao, ACS Nano 2011, 5, 3930.
- 20 T. Wang, Y. Cai, Z. Wang, E. Guan, D. Yu, A. Qin, J. Sun, B. Z. Tang, C. Gao, Macromol. Rapid Commun. 2012, 33, 1584.
- 21 Y. Bae, S. Fukushima, A. Harada, K. Kataoka, Angew. Chem. Int. Ed. 2003, 42, 4640.
- 22 L. Zhou, R. Cheng, H. Q. Tao, S. B. Ma, W. W. Guo, F. H. Meng, H. Y. Liu, Z. Liu, Z. Y. Zhong, Biomacromolecules 2011, 12, 1460.
- 23 X. J. Zhang, X. H. Zhang, W. S. Shi, X. M. Meng, C. Lee, S. Lee, Journal of Physical Chemistry B 2005, 109, 18777.
- 24 J. C. Xiao, X. Y. Xiao, Y. L. Zhao, B. Wu, Z. Y. Liu, X. M. Zhang, S. J. Wang, X. H. Zhao, L. Liu, L. Jiang, Nanoscale 2013, 5, 5420.
- 25 L. Wang, W. Li, J. Lu, Y. X. Zhao, G. Fan, J. P. Zhang, H. Wang, J. Phys. Chem. C 2013, 117, 26811.
- 26 A. Jana, B. Saha, M. Ikbal, S. K. Ghosh, N. D. P. Singh, Photochem. Photobiol. Sci. 2012, 11, 1558.
- 27 D. B. Warheit, T. R. Webb, C. M. Sayes, V. L. Colvin, K. L. Reed, Toxicol. Sci. 2006, 91, 227.
- 28 D. Bhattacharya, C. R. Santra, A. N. Ghosh, P. Karmakar, J. Biomed. Nanotechnol. 2014, 10, 707.




