pH-Responsive Single Walled Carbon Nanotube Dispersion for Target Specific Release of Doxorubicin to Cancer Cells
Moumita Ghosh
Department of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorSayanti Brahmachari
Department of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorCorresponding Author
Prasanta Kumar Das
Department of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorMoumita Ghosh
Department of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorSayanti Brahmachari
Department of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorCorresponding Author
Prasanta Kumar Das
Department of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorAbstract
Cholesterol-based dipeptide carboxylates were synthesized and exploited for the pH responsive reversible dispersion and precipitation of single walled carbon nanotube (SWCNT) in water specifically at tumorogenic environmental pH (6.0–6.5). The nanohybrid showed excellent pH responsive drug release between pH range of 6.0 and 6.5. The exfoliation of SWCNTs was characterized by microscopic and spectroscopic studies. Importantly, drug-loaded SWCNT dispersions showed better killing efficiency compared to that of the native drug and were highly efficient in selective killing of cancer cells (Hela, HepG2) with 2.5-fold higher efficacy compared to that of normal cells (CHO, NIH3T3).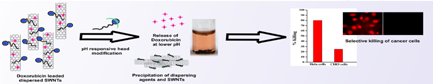
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| mabi201400290-sm-0001-SuppFig-S1.doc7.8 MB | Figure S1. a) AFM and b) TEM image of SWCNT-1 nanohybrid. Figure S2. The histogram for average bundle diameter of dispersed SWCNTs. Figure S3. The histogram for average length of the nanotubes of dispersed SWCNTs. Figure S4. % Cell viability of CHO, NIH3T3, HepG2 and Hela cells incubated with SWCNT-1 hybrid after 24 h of incubation. Figure S5. % Cell viability of CHO, NIH3T3, HepG2 and Hela cells incubated with SWCNT-2 hybrid after 24 h of incubation. Figure S6. Time dependent emission spectra of SWCNT-2-DOX. Figure S7. Stability of SWCNT-DOX at pH 8 and at lower pH. Figure S8. pH-dependent revival of intrinsic a) fluorescence b) UV absorbance peak of doxorubicin in SWCNT-1-DOX. Figure S9. pH-dependent a) fluorescence b) UV absorbance peak of doxorubicin. Figure S10. pH-dependent release profile of doxorubicin and its quantification. Figure S11. IC50 values of the doxorubicin for CHO, NIH3T3, Hela and HepG2 cells Figure S12. Bright field and fluorescence microscopic images of cells incubated with BSA-FITC loaded SWCNT-2 for 6 h in a,b) CHO cells c,d) Hela cells. Figure S13. Bright field and fluorescence microscopic images of cells incubated with BSA-FITC for 6 h in a,b) CHO cells c,d) Hela cells. Figure S14. % Cell viability of CHO and Hela cells incubated with amphiphile+DOX hybrid after 12 h of incubation. Figure S15. Bright field and fluorescence microscopic images of cells incubated with DOX loaded SWCNT-1 for 6 h in a,b) CHO cells c,d) Hela cells e,f) HepG2 cells. Scheme S1. Synthetic scheme for 1-4. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 K. D. Fowers, J. Kopecek, Macromol. Biosci. 2012, 12, 502.
- 2 A. A. Bhirde, V. Patel, J. Gavard, G. Zhang, A. A. Sousa, A. Masedunskas, R. D. Leapman, R. Weigert, J. S. Gutkind, J. F. Rusling, ACS Nano 2009, 3, 307.
- 3 R. Malhotra, V. Patel, B. V. Chikkaveeraiah, B. S. Munge, S. C. Cheong, R. B. Zain, M. T. Abraham, D. K. Dey, J. S. Gutkind, J. F. Rusling, Anal. Chem. 2012, 84, 6249.
- 4 N. Nasongkla, E. Bey, J. Ren, H. Ai, C. Khemtong, J. S. Guthi, S. F. Chin, A. D. Sherry, D. A. Boothman Gao, Nano Lett. 2006, 6, 2427.
- 5 R. I. Pakunlu, Y. Wang, M. Saad, J. J. Khandare, V. Starovoytov, T. Minko, J. Control. Release 2006, 114, 153.
- 6 H. Maeda, J. Wu, T. Sawa, Y. Matsumura, K. Hori, J. Control. Release 2000, 65, 271.
- 7 K. Raemdonck, J. Demeester, S. D. Smedt, Soft Matter 2009, 5, 707.
- 8 H. Maeda, H. Nakamura, J. Fang, Adv. Drug Deliv. Rev. 2013, 65, 71.
- 9 L. A. Doyle, W. Yang, L. V. Abruzzo, T. Krogmann, Y. Gao, A. K. Rishi, D. D. Ross, Proc. Natl. Acad. Sci. USA 1998, 95, 15665.
- 10 Z. L. Cheng, A. A. Zaki, J. Z. Hui, V. R. Muzykantov, A. Tsourkas, Science 2012, 338, 903.
- 11 Z. Liu, X. Sun, N. N. Ratchford, H. Dai, ACS Nano 2007, 1, 50.
- 12 X. Zhang, L. Meng, Q. Lu, Z. Fei, P. J. Dyson, Biomaterials 2009, 30, 6041.
- 13 J. Chen, S. Chen, X. Zhao, L. V. Kuznetsova, S. S. Wong, I. Ojima, J. Am. Chem. Soc. 2008, 130, 16778.
- 14 V. Bagalkot, O. C. Farokhzad, R. Langer, S. Jon, Angew. Chem. Int. Ed. 2006, 45, 8149.
- 15 E. S. Lee, D. Kim, Y. S. Youn, K. T. Oh, Y. H. Bae, Angew. Chem. Int. Ed. 2008, 47, 2418.
- 16 Y. Obata, S. Tajima, S. Takeoka, J. Control. Release 2010, 142, 267.
- 17 M. Talelli, M. Iman, A. K. Varkouhi, C. J. F. Rijcken, R. M. Schiffelers, T. Etrych, K. Ulbrich, C. F. van Nostrum, T. Lammers, G. Storm, W. E. Hennink, Biomaterials 2010, 31, 7797.
- 18 C. Fabbro, H. Ali-Boucetta, T. D. Ros, K. Kostarelos, A. Bianco, M. Prato, Chem. Commun. 2012, 48, 3911.
- 19 E. Yan, Y. Ding, C. Chen, R. Li, Y. Hu, X. Jiang, Chem. Commun. 2009, 2718.
- 20 J.-W. Kim, C. V. Dang, Cancer Res. 2006, 66, 8927.
- 21 C. Sinthuvanich, A. Salome Veiga, K. Gupta, D. Gaspar, R. Blumenthal, J. P. Schneider, J. Am. Chem. Soc. 2012, 134, 6210.
- 22 Z. Zhao, H. Meng, N. Wang, M. J. Donovan, T. Fu, M. You, Z. Chen, X. Zhang, W. Tan, Angew. Chem. Int. Ed. 2013, 52, 7487.
- 23 J.-Z. Du, T.-M. Sun, W.-J. Song, J. Wu, J. Wang, Chem. Int. Ed. 2010, 49, 3621.
- 24 L. Niu, L. Meng, Q. Lu, Macromol. Biosci. 2013, 13, 735.
- 25 B. G. Cousins, A. K. Das, R. Sharma, Y. Li, J. P. McNamara, I. H. Hillier, I. A. Kinloch, R. V. Ulijn, Small 2009, 5, 587.
- 26 M. Benincasa, S. Pacor, W. Wu, M. Prato, A. Bianco, R. Gennaro, ACS Nano 2011, 5, 199.
- 27 A. B. Dalton, A. Ortiz-Acevedo, V. Zorbas, E. Brunner, W. M. Sampson, L. Collins, J. M. Razal, M. M. Yoshida, R. H. Baughman, R. K. Draper, I. H. Musselman, M. Jose-Yacaman, G. R. Dieckmann, Adv. Funct. Mater. 2004, 14, 1147.
- 28 G. K. C. Lee, C. Sach, M. L. H. Green, L.-L. Wong, C. G. Salzmann, Chem. Commun. 2010, 46, 7013.
- 29 J. T. Robinson, G. Hong, Y. Liang, B. Zhang, O. K. Yaghi, H. Dai, J. Am. Chem. Soc. 2012, 134, 10664.
- 30 C. Backes, C. D. Schmidt, K. Rosenlehner, F. Hauke, J. N. Coleman, A. Hirsch, Adv. Mater. 2010, 22, 788.
- 31 V. Georgakilas, K. Kordatos, M. Prato, D. M. Guldi, M. Holzinger, A. Hirsch, J. Am. Chem. Soc. 2002, 124, 760.
- 32 Y. Li, B. G. Cousins, R. V. Ulijn, I. A. Kinloch, Langmuir 2009, 25, 11760.
- 33 S. Brahmachari, D. Das, P. K. Das, Chem. Commun. 2010, 46, 8386.
- 34 C. G. Salzmann, M. A. H. Ward, R. M. J. Jacobs, G. Tobias, M. L. H. Green, J. Phys. Chem. C 2007, 111, 18520.
- 35 M. G. Hahm, A. L. M. Reddy, D. P. Cole, M. Rivera, J. A. Vento, J. Nam, H. Y. Jung, Y. L. Kim, N. T. Narayanan, D. P. Hashim, C. Galande, Y. J. Jung, M. Bundy, S. Karna, P. M. Ajayan, R. Vajtai, Nano Lett. 2012, 12, 5616.
- 36 Z. Guo, Y. Feng, D. Zhu, S. He, H. Liu, X. Shi, J. Sun, M. Qu, Adv. Funct. Mater. 2013, 23, 5010.
- 37 Z. Guo, Y. Feng, S. He, M. Qu, H. Chen, H. Liu, Y. Wu, Y. Wang, Adv. Mater. 2012, 25, 584.
- 38 S. Brahmachari, D. Das, A. Shome, P. K. Das, Angew. Chem. Int. Ed. 2011, 50, 11243.
- 39 S. Brahmachari, M. Ghosh, S. Dutta, P. K. Das, J. Mater. Chem. B 2014, 2, 1160.
- 40 S. Dutta, T. Kar, S. Brahmachari, P. K. Das, J. Mater. Chem. 2012, 22, 6623.
- 41 T. Kar, S. Debnath, D. Das, A. Shome, P. K. Das, Langmuir 2009, 25, 8639.
- 42 M. B. Hansen, S. E. Nielsen, K. Berg, J. Immunol. Methods 1989, 119, 203.
- 43 H. A. Boucetta, K. T. Al-Jamal, D. McCarthy, M. Prato, A. Bianco, K. Kostarelos, Chem. Commun. 2008, 459.
- 44 Y. Tan, D. E. Resasco, J. Phys. Chem. B 2005, 109, 14454.
- 45 A. Ishibashi, N. Nakashima, Chem. Eur. J. 2006, 12, 7595.
- 46 W. Wenseleers, I. I. Vlasov, E. Goovaerts, E. D. Obraztsova, A. S. Lobach, A. Bouwen, Adv. Funct. Mater. 2001, 14, 1105.
- 47 X. Yang, X. Zhang, Z. Liu, Y. Ma, Y. Huang, Y. Chen, J. Phys. Chem. C 2008, 112, 1755.




