Low-dose aspirin confers protection against acute cellular allograft rejection after primary liver transplantation
See editorial on Page 1825
Abstract
This study investigated the effect of low-dose aspirin in primary adult liver transplantation (LT) on acute cellular rejection (ACR) as well as arterial patency rates. The use of low-dose aspirin after LT is practiced by many transplant centers to minimize the risk of hepatic artery thrombosis (HAT), although solid recommendations do not exist. However, aspirin also possesses potent anti-inflammatory properties and might mitigate inflammatory processes after LT, such as rejection. Therefore, we hypothesized that the use of aspirin after LT has a protective effect against ACR. This is an international, multicenter cohort study of primary adult deceased donor LT. The study included 17 high-volume LT centers and covered the 3-year period from 2013 to 2015 to allow a minimum 5-year follow-up. In this cohort of 2365 patients, prophylactic antiplatelet therapy with low-dose aspirin was administered in 1436 recipients (61%). The 1-year rejection-free survival rate was 89% in the aspirin group versus 82% in the no-aspirin group (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.63–0.94; p = 0.01). The 1-year primary arterial patency rates were 99% in the aspirin group and 96% in the no-aspirin group with an HR of 0.23 (95% CI, 0.13–0.40; p < 0.001). Low-dose aspirin was associated with a lower risk of ACR and HAT after LT, especially in the first vulnerable year after transplantation. Therefore, low-dose aspirin use after primary LT should be evaluated to protect the liver graft from ACR and to maintain arterial patency.
Abbreviations
-
- ACR
-
- acute cellular rejection
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BMI
-
- body mass index
-
- CI
-
- confidence interval
-
- CIT
-
- cold ischemia time
-
- CNI
-
- calcineurin inhibitor
-
- DBD
-
- donation after brain death
-
- DCD
-
- donation after circulatory death
-
- EAD
-
- early allograft dysfunction
-
- FFP
-
- fresh frozen plasma
-
- HAT
-
- hepatic artery thrombosis
-
- HCC
-
- hepatocellular carcinoma
-
- HJ
-
- hepaticojejunostomy
-
- HR
-
- hazard ratio
-
- ICU
-
- intensive care unit
-
- IQR
-
- interquartile range
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- RAI
-
- rejection activity index
-
- RBC
-
- red blood cell
-
- RRT
-
- renal replacement therapy
-
- SRTR
-
- Scientific Registry of Transplant Recipients
-
- TACE
-
- transarterial chemoembolization
-
- TRIM
-
- transfusion-related immunomodulation
INTRODUCTION
Liver transplantation (LT) has become a standard therapy for patients with end-stage liver disease and fulminant liver failure. Postoperative morbidity still remains high,[1] especially when accounting for vascular complications.[2, 3] Hepatic artery thrombosis (HAT) occurs in 4%–9% of adult LT[4, 5] and is one of the most serious vascular complications often resulting in liver necrosis, abscess formation, ischemic cholangiopathy, and graft loss. The sequel of these adverse events has a negative impact on graft and patient survival rates and remains a life-threatening complication with high mortality and retransplantation rates. In this context, the postoperative use of low-dose aspirin after LT is practiced by many transplant centers to reduce the incidence of HAT,[6, 7] although solid recommendations do not currently exist.
Apart from the antiaggregating effect, aspirin also possesses potent anti-inflammatory properties and is, therefore, widely used as a primary and secondary preventive medication against vascular disease.[8] It inhibits pathways inherent to innate immunity, including the production of thromboxan A2,[9] and downregulates proinflammatory signaling pathways, including nuclear factor kappa B.[10, 11] This indicates that aspirin might also mitigate inflammatory processes after LT such as rejection. Although data on the antirejection effect of low-dose aspirin do not exist in LT, there are divergent findings reported for other solid organ transplantations.[12, 13]
Therefore, we conducted this cohort study to evaluate whether antiplatelet therapy with aspirin has a protective effect on the occurrence and severity of acute cellular rejection (ACR) after LT. In addition to this analysis, we also assessed the effect of aspirin on arterial patency.
PATIENTS AND METHODS
Study design
This is an international, multicenter, retrospective cohort study of primary adult deceased donor LT. The study includes 17 high-volume LT centers from Europe (n = 8), North America (n = 6), and Latin America (n = 3) and covers a 3-year period from 2013 to 2015 to allow a minimum 5-year follow-up (Figure S1). Each participating center required a prospective database from which data could be extracted. The project (aspirin4OLT) was implemented to investigate four specific aims regarding the effect of low-dose aspirin in patients after primary LT, including arterial patency, ACR, recurrence of hepatocellular carcinoma (HCC), and graft survival. Low-dose aspirin was defined as daily aspirin dose of 75–100 mg. We hypothesized that the use of aspirin after LT has a protective effect against ACR and lowers the incidence of HAT. All centers followed their standard of care for immunosuppression and decision making for allograft biopsies. The study has been approved by local ethic committees (2016-01889) and is registered at ClinicalTrials.gov (NCT04327427).
Data management
Aspirin4OLT.org is a clinical trial management system built on Drupal 7 that served as the backbone of our study by supporting administration, collaboration, communication, and information sharing needs among members from several participating centers worldwide. Fully anonymous patient data were stored in two separate secured sites with access given only to the relevant users. There were regular backups. Furthermore, the clinical trial management system was secured with a hypertext transfer protocol secure in combination with a secure sockets layer/transport layer security protocol and an encrypted structured query language database as previously described.[14]
Inclusion and exclusion criteria
Inclusion criteria were adult (recipient age 18 years or older) deceased donor LTs. Donor organs from donation after brain death (DBD) or donation after circulatory death (DCD; Maastricht 3 criteria) donors were included. Further inclusion criteria included primary LT and whole organs as well as arterial reconstruction with end-to-end anastomosis and arterial back-table reconstruction. Other reconstruction techniques using an aorto– or iliac–hepatic conduit were excluded. We also excluded split-liver and living donor LT as well as retransplantations.
Posttransplant outcome measures
Primary outcome measures of the study were the occurrence of ACR and arterial patency after LT. Secondary outcome measures included lengths of intensive care unit (ICU) and hospital stays and postoperative complications as well as graft and patient survival rates. Postoperative complications were ranked using the Clavien–Dindo classification.[15] Lengths of ICU and hospital stays were measured from LT to discharge or death. Rejection-free survival was measured from transplantation to last follow-up or occurrence of ACR. Occlusion-free survival was measured from transplantation to last follow-up or arterial occlusion. Graft survival was measured from transplantation to last follow-up, retransplantation, or death. Patient survival was measured from transplantation to last follow-up or death.
Definition of ACR
ACRs, which were a primary outcome measure, were classified into clinically suspected without biopsy and histologically proven rejections. ACR was defined as a clinical entity in the absence of biopsy but in the setting of elevated liver function tests and treatment of suspected rejection in the respective transplant center.
Histologically proven ACRs were classified using the Banff rejection activity index (RAI).[16] The RAI score uses the three categories of portal inflammation, bile duct inflammation, and venous endothelial inflammation, giving one to three points according to the inflammatory extend of each category. The various possible rejection grades were accordingly categorized as follows: 0–2, no rejection; 3, borderline; 4–5, mild; 6–7, moderate; and 8–9, severe ACR.[17]
Definition of hepatic arterial patency
Primary patency, which was another primary outcome measure, was defined as time from transplantation or arterial anastomosis to occlusion or last patency follow-up. The definitions of primary patency of the hepatic artery are based on the reporting standards of the Society for Vascular Surgery and the American Association for Vascular Surgery.[18]
Statistical analysis
The primary and secondary outcome measures were compared among different patient and operation characteristics with univariate analysis. Continuous data are reported as mean and standard deviation or median and interquartile range (IQR) where appropriate. Categorical data are reported as frequencies (n) and proportions (percentages). Continuous variables were compared with the Student t, Mann–Whitney U, one-way analysis of variance, and Kruskal–Wallis tests where appropriate. Differences among proportions derived from categorical data were compared using Fisher's exact or Pearson χ2 tests where appropriate. Kaplan–Meier curves were used to estimate hepatic artery patency and rejection-free survival as well as patient and graft survival rates. Patients lost to follow-up or follow-up time ended were censored. Multivariable Cox regression analysis was performed to identify independent risk factors for cellular rejection. All p values were two-sided and considered statistically significant if p ≤ 0.05. Missing data are clearly reported in the article, and no extrapolation techniques were used to replace them. Statistical analysis was performed using R Studio Version 1.0.44 (RStudio, Inc., GNU Affero General Public License Version 3, Boston, MA) with the graphical user interface rBiostatistics.com beta version (GNU License, London, UK, 2017) and the Cloud Graphical User Interface for R Statistics and eLearning Platform (London, UK).
RESULTS
Study population
We report the effect of aspirin on ACR as well as the impact of aspirin on HAT. A total of 2366 LTs were performed in the 17 participating centers during the 3-year study period. The median follow-up time of the entire cohort was 62 months (IQR, 52–73 months); the 90-day mortality rate was 4.8%. The median recipient age of the primary LT cohort was 57 years (IQR, 49–62 years), and the majority of patients were male (n = 1587, 67%). At the time of LT, the median laboratory Model for End-Stage Liver Disease (MELD) score was 20 (IQR, 13–32), with 24% of patients having MELD scores >30. Only 15% of patients had life-support treatment before LT requiring ventilation support in 7% and/or renal replacement therapy (RRT) in 14% of cases. Most recipients (89%) received organs from DBD donors, and only 10% of organs were retrieved from DCD donors (Table 1). The piggyback technique was used in 37% of patients (n = 870), whereas 63% of recipients (n = 1496) underwent classical bicaval LT. Venovenous bypass during LT was used in 10% of patients (n = 239). Duct-to-duct anastomosis was the main biliary reconstruction technique (93%), whereas hepaticojejunostomy (HJ) was performed in 7% of patients. Further detailed characteristics of the LT population are presented in Table 2.
| Total (n = 2365) | Aspirin (n = 1436) | No aspirin (n = 915) | p-value | |
|---|---|---|---|---|
| Donor characteristics | ||||
| Demographics | ||||
| Age, years | 49 (32–62) | 48 (31–62) | 50 (34–61) | 0.55 |
| DCD | 241 (10) | 149 (10) | 92 (10) | 0.83 |
| Recipient characteristics | ||||
| Demographics | ||||
| Male sex | 1587 (67) | 966 (67) | 614 (67) | 0.96 |
| Age, years | 57 (49–62) | 52 (49–63) | 57 (49–62) | 0.5 |
| BMI, kg/m2 | 27 (23–30) | 27 (24–30) | 26 (23–30) | <0.05 |
| Liver disease | ||||
| MELD score | 20 (13–29) | 19 (13–29) | 21 (14–29) | 0.02 |
| MELD score >30 | 576 (24) | 348 (24) | 227 (25) | 0.77 |
| Pretransplant life supporta | 375 (15) | 197 (14) | 175 (19) | <0.001 |
| Ventilation | 156 (6.6) | 78 (5.4) | 77 (8.5) | <0.05 |
| RRT | 326 (14) | 172 (12) | 151 (17) | <0.05 |
| Vasopressor support | 130 (5.5) | 54 (3.8) | 74 (8.1) | <0.001 |
| HCC | 858 (36) | 514 (36) | 337 (37) | 0.63 |
| Prior TACE | 483 (29) | 322 (33) | 157 (23) | <0.001 |
| Prior radiation | 81 (5.1) | 47 (5.0) | 33 (5.0) | 1 |
| Cardiovascular risk | ||||
| Hypertension | 752 (32) | 463 (32) | 284 (31) | 0.56 |
| Dyslipidemia | 312 (13) | 206 (14) | 104 (11) | 0.04 |
| Diabetes mellitus | 638 (27) | 389 (27) | 243 (27) | 0.81 |
| Cardiovascular disease | 189 (8.0) | 141 (9.8) | 47 (5.1) | <0.001 |
| Aspirin at admission | 121 (5.1) | 105 (7.3) | 14 (1.5) | <0.001 |
- Note: Data are given as n (%) or median (IQR). A total of 14 patients were not assigned to the aspirin or no aspirin group.
- Abbreviations: BMI, body mass index; DCD, donation after circulatory death; HCC, hepatocellular carcinoma; IQR, interquartile range; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; RRT, renal replacement therapy; TACE, transarterial chemoembolization.
- a Life support is defined as hemodialysis and/or mechanical ventilation before transplantation.
| Total (n = 2365) | Aspirin (n = 1436) | No aspirin (n = 915) | p-value | |
|---|---|---|---|---|
| Operation time, min | 366 (300–456) | 360 (295–457) | 373 (300–454) | 0.17 |
| Cold ischemia time, min | 420 (335–523) | 420 (330–510) | 430 (346–540) | <0.05 |
| Veno-venous bypass | 239 (10) | 147 (10) | 92 (10) | 0.94 |
| Simultaneous kidney transplantation | 80 (3.4) | 49 (3.4) | 31 (3.4) | 1.0 |
| Intraoperative transfusion | ||||
| RBC, units | 5 (2–10) | 5 (2–10) | 5 (3–10) | 0.14 |
| FFP, units | 8 (4–18) | 8 (4–17) | 8 (4–18) | 0.04 |
| Platelets, units | 3 (1–8) | 3 (1–9) | 4 (2–7) | 0.57 |
| Transplant technique | <0.001 | |||
| Classic | 1495 (63) | 766 (53) | 719 (79) | |
| Piggy back | 870 (37) | 670 (47) | 196 (21) | |
| Arterial anatomya | <0.001 | |||
| Type 1 | 1779 (75) | 1033 (72) | 735 (80) | |
| Type 2 | 264 (11) | 170 (12) | 92 (10) | |
| Type 3 | 198 (8.4) | 147 (10) | 50 (5.5) | |
| Type 4 | 87 (3.7) | 61 (4.2) | 26 (2.8) | |
| Type 5 | 37 (1.6) | 25 (1.2) | 12 (1.3) | |
| Additional back-table reconstruction | 299 (13) | 213 (15) | 84 (9.2) | <0.001 |
| Biliary anastomosis | 0.04 | |||
| Duct-to-duct | 2211 (93) | 1337 (93) | 860 (94) | |
| HJ | 148 (6.3) | 98 (6.8) | 50 (5.5) | |
| None | 6 (0.3) | 1 (0.1) | 5 (0.5) |
- Note: Data are given as n (%) and median (IQR). 14 patients were not assigned to the Aspirin or no aspirin group.
- Abbreviations: FFP, fresh frozen plasma; HJ, hepatico jejunostomy; LT, liver transplantation; RBC, red blood cells.
- a Classification refers to Hiatt et al.[19]: Type 1, normal; Type 2, replaced (accessory) left hepatic artery from left gastric; Type 3, replaced (accessory) right hepatic artery from superior mesenteric; Type 4, double replaced system; Type 5, common hepatic artery from superior mesenteric.
Characteristics of the aspirin and no-aspirin groups
Prophylactic antiplatelet therapy with low-dose aspirin was administered in 1436 recipients (61%) after LT, whereas 915 recipients (39%) had no aspirin. Only two centers from the United Kingdom followed a strict policy of low-dose aspirin after LT. In all other participating centers, the decision to administer aspirin after LT was made on a rather pragmatic way, often driven by personal preference of the surgeon (Figure 1). In the aspirin group, the median postoperative day of aspirin start was Day 2 (IQR, 1–9 days). Aspirin and no-aspirin groups were comparable in terms of sex, recipient age, and MELD score (Table 1). Most of the time, aspirin was administered lifelong and after 1 and 2 years 82% and 75% of patients were under low-dose aspirin.
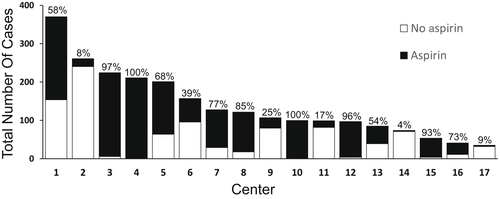
In patients requiring dialysis prior to LT, aspirin was given in 53% of cases compared with 47% of patients without any dialysis prior to transplantation (p < 0.05). The same was found with patients on life support prior to LT (53% vs. 47%; p < 0.001). Patients who underwent transarterial chemoembolization (TACE) prior to LT were more likely to receive prophylactic low-dose aspirin after LT (67% vs. 33%; p < 0.001).
Characteristics and outcome of ACR
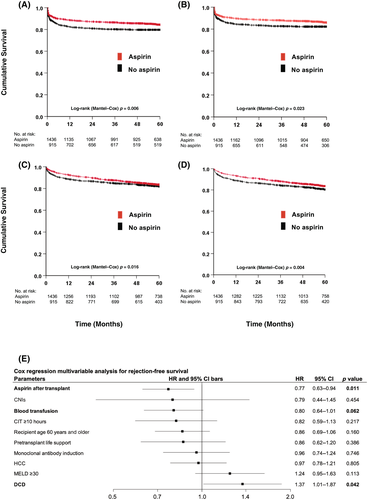
ACR was encountered in 17.7% of patients (n = 420) within the median follow-up time of 62 months (IQR, 52–73 months). Most of the rejections were biopsy proven (87%), whereas only 13% were classified as clinically suspicious without histology. The overall 1-, 3-, and 5-year rejection-free survival rates were 86%, 84%, and 83%, respectively, with 96% of the rejections occurring during the first year after LT. The 1-, 3-, and 5-year rejection-free survival rates for the aspirin versus no-aspirin groups were 89%, 87%, 84%, and 82%, 81%, 80%, respectively (p < 0.05) (Figure 2). Early ACR within 4 weeks after transplantation occurred in 6.8% (99/1436) in the aspirin versus 8.8% (81/915) in the no-aspirin group (odds ratio, 0.75; 95% confidence interval [CI], 0.55–1.03; p = 0.08). Immunosuppression between both groups was similar regarding maintenance immunosuppression with calcineurin inhibitors (CNIs), mycophenolate, and corticosteroids, but differed significantly in the use of monoclonal antibodies at the time of transplantation (aspirin 29% vs. no aspirin 15%) (Table 3). ACR was treated with steroids (37%), dose escalation of standard immunosuppressive regimen (14%), and other treatments including additional immunosuppressive medications (49%) (Table 4). Among patients with biopsy-proven ACR (n = 362), 182 (50%) were classified according to the Banff RAI by the local pathologist. Of the patients, 27% had intermediate rejection (RAI Grades 6–7), and 9.4% had severe rejection (RAI Grades 8–9).
| Total (n = 2365) | Aspirin (n = 1436) | No aspirin (n = 915) | p-value | |
|---|---|---|---|---|
| Peak transaminases | ||||
| AST, U/L | 989 (452–1985) | 946 (388–1873) | 1091 (552–2167) | 0.84 |
| ALT, U/L | 685 (346–1350) | 703 (358–1423) | 661 (331–1252) | 0.12 |
| Primary graft function | 0.51 | |||
| Normal allograft function | 2114 (89) | 1310 (91) | 791 (86) | |
| Early allograft dysfunctiona | 229 (9.7) | 114 (7.9) | 114 (12.5) | |
| Primary non-functionb | 22 (0.1) | 12 (8.1) | 10 (6.2) | |
| Clavien-Classificationc | <0.05 | |||
| None or Minor (0-IIIa) | 1496 (63) | 945 (66) | 542 (59) | |
| Major (IIIb-V) | 869 (37) | 491 (34) | 373 (41) | |
| Bleeding complicationd | 353 (15) | 182 (12.6) | 171 (18.6) | <0.05 |
| Hepatic arterial occlusion | 59 (2.5) | 15 (1.0) | 44 (4.8) | <0.001 |
| Hospitalization | ||||
| ICU stay, days | 4 (2–8) | 4 (2–8) | 4 (2–8) | 0.04 |
| Hospital stay, days | 16 (10–27) | 15 (10–25) | 18 (11–29) | <0.001 |
| Readmission within 90 days | 713 (30) | 447 (31) | 261 (29) | 0.18 |
| Immunosuppression | ||||
| Corticosteroids | 2188 (92) | 1331 (93) | 848 (93) | 1.0 |
| Mycophenolate | 1646 (70) | 996 (71) | 647 (69) | 0.49 |
| Tacrolimus | 2139 (93) | 1325 (92) | 801 (88) | <0.05 |
| Cyclosporine | 293 (12) | 159 (11) | 134 (15) | 0.01 |
| Everolimus | 105 (4.4) | 84 (5.8) | 20 (2.2) | <0.001 |
| Monoclonal antibody | 476 (20) | 211 (29) | 265 (15) | <0.001 |
| Acute cellular rejection | ||||
| Total episodes | 420 (18) | 239 (17) | 181 (20) | 0.05 |
| Rejection therapy | ||||
| Steroids | 161 (73) | 91 (70) | 70 (81) | 0.08 |
| Dose escalation IS | 72 (33) | 44 (34) | 28 832) | 0.88 |
| Additional IS | 53 (24) | 35 (27) | 18 (21) | 0.34 |
| Watchful waiting | 24 (11) | 7 (8.0) | 17 (13) | 0.28 |
- Note: Data are given as n (%) and median (IQR). 14 patients were not assigned to the Aspirin or no aspirin group. Normal allograft function is defined as function not meeting the criteria of early allograft dysfunction and primary non-function.
- Abbreviations: ALT, alanin aminotransferase; AST, aspartat aminotransferase; ICU, intensive care unit; LT, liver transplantation.
- a Refers to Olthoff et al.[20]
- b Refers to Hartog et al.[21]
- c Refers to Dindo et al.[15]
- d Overall, including all Clavien-Dindo-Complications.
| No rejection (N = 1945) | Biopsy-proven rejection (N = 364) | Clinically suspected rejection (N = 56) | p | |
|---|---|---|---|---|
| Age, years | 57 (49–62) | 55 (47–61) | 60 (50–66) | <0.001 |
| Male sex | 1312 (68) | 236 (65) | 39 (70) | 0.57 |
| MELD | 20 (13–29) | 20 (13–31) | 19 (14–22) | 0.68 |
| DCD | 192 (10) | 42 (12) | 7 (13) | 0.53 |
| Cold ischemia time, hours | 7 (6–9) | 7 (5–8) | 8 (6–9) | <0.001 |
| Aspirin at discharge | 1197 (62) | 210 (57) | 50 (80) | 0.07 |
| RBC | 1457 (77) | 257 (72) | 40 (80) | 0.07 |
| HCC | 706 (36) | 133 (37) | 19 (34) | 0.93 |
| Immunosuppression | ||||
| CNI-Inhibitor maintenance | 1777 (91) | 314 (86) | 48 (86) | <0.05 |
| Monoclonal antibodies | 394 (20) | 68 (19) | 14 (25) | 0.52 |
| Rejection treatment | <0.001 | |||
| Corticosteroids | – | 133 (37) | 27 (48) | |
| Dose escalation | – | 52 (14) | 19 (34) | |
| Others | – | 179 (49) | 10 (18) |
- Note: Data are given as n (%) and median (IQR).
- Abbreviations: CNI, calcineurin inhibitor; DCD, donation after cardiac death; HCC, hepatocellular carcinoma; MELD, Model for End Stage Liver Disease; RBC, red blood cells.
On multivariate Cox regression analysis, aspirin was the strongest independent predictor for rejection-free survival with an HR of 0.77 (95% CI, 0.63–0.94; p = 0.01) followed by blood transfusions (Figure 2E). DCD organs showed the opposite effect, significantly triggering ACR with an HR of 1.37 (95% CI, 1.01–1.87; p < 0.05).
Hepatic artery patency rates
Overall hepatic arterial occlusion or stenosis occurred in 5.7%of patients (n = 135). Nearly all occlusions happened during the first year after LT (95%). The 1-year primary arterial patency rates were 99% in the aspirin group and 96% in the no-aspirin group with an HR of 0.23 (95% CI, 0.13–0.40; p < 0.001) (Figure S2).
Patient and graft survival
The overall patient survival rate of primary LT after 1 year was 92% and 82% after 5 years. The 1-year graft survival rates in the aspirin group versus no-aspirin group were 93% and 88%, respectively, and 84% and 80% after 5 years, respectively (HR, 0.79; 95% CI, 0.65–0.96; p < 0.05) (Figure 2C). The overall graft survival rates after 1 and 5 years were 91% and 83%, respectively. The 1- and 5-year patient survival rates in the aspirin group versus no-aspirin group were 93% versus 89% and 83% versus 82%, respectively (Figure 2D).
DISCUSSION
The central finding that low-dose aspirin was associated with a lower risk of developing ACR is a somehow new and interesting aspect reflecting the anti-inflammatory properties of aspirin. Furthermore, the study findings support the concept of a prophylactic low-dose aspirin policy to prevent HAT. Because most of the rejection and arterial occlusion events occurred during the first 12 months after LT, this implies a low-dose aspirin policy at least for this early posttransplant period.
ACR is an acute T cell–mediated rejection that occurs frequently during the first year after LT. Data from a systematic review of randomized controlled trials showed that 15%–25% of recipients who took tacrolimus-based immunosuppression medications developed ACR.[22] The most recent Scientific Registry of Transplant Recipients (SRTR) report from 2019 showed that 12.3% of all adult LT recipients develop at least one rejection episode during the first posttransplant year.[23] Although the 1-year rate of ACR might be underreported, this figure mainly applies to a large contemporary cohort with more than 80%–90% of patients on tacrolimus-based immunosuppression. These figures compare with the finding of the present study, where the 1-year rate of biopsy-proven ACR was 17%, with 96% of rejection episodes occurring during the first posttransplant year.
In the present study, most rejections were based on histological evaluation, which is still the gold standard to diagnose and grade ACR.[24, 25] Liver biopsies for allograft rejection are usually performed in the clinical scenario of abnormal liver function tests in the absence of vascular or biliary complications.[26] In our study, only 13% of all rejections were not biopsy proven and were categorized as clinical suspicious rejection. Some clinicians have treated such rejections empirically and reserve liver biopsies for unresponsive treatment.[26]
The histological diagnosis of ACR is based on the three categories of portal, bile duct, and venous endothelial inflammation and is usually graded using the Banff RAI.[16] Aspirin, which is frequently used either after LT to prevent HAT or for medical vascular conditions, has anti-inflammatory properties[8] and might mitigate rejection-associated inflammation. This assumption triggered our hypothesis that aspirin might confer anti-inflammatory protection against ACR. We have shown that the use low-dose aspirin after LT decreased the rate of ACR and was associated with superior rejection-free survival, especially during the first posttransplant year along with a small but significant protective effect against HAT. To exclude any confounding effect of immunosuppression with the use of aspirin, we included both the use of CNI and induction therapy with monoclonal antibodies in our Cox regression analysis and independently identified aspirin as a protective factor against ACR. This central and novel finding implies that the prophylactic application of low-dose aspirin after LT can be used as dual protection against ACR as well as HAT, especially during the vulnerable period of the first posttransplant year.
The observation that the nonaspirin group was sicker might imply that adverse outcomes were more likely to occur compared with the aspirin group. This finding might assume that the beneficial effects of aspirin were rather related to the higher acuity of the recipient than the anti-inflammatory and antithrombotic properties of aspirin. To address this potential confounding effect, we included parameters of medical acuity such as pretransplant life support (dialysis and/or ventilation) and MELD scores <30 in our multivariate analysis.
Our multivariate analysis also identified two other important risk factors for ACR that we would like to highlight. First, perioperative red blood cell (RBC) transfusions improved rejection-free survival after primary LT. This effect, also known as transfusion-related immunomodulation (TRIM), has been initially reported in the renal transplant setting, when significantly improved renal allograft survival was observed in transplant recipients who received RBC transfusions.[27] Although the immunosuppressive mechanisms of TRIM are not yet fully elucidated, impaired natural killer cell function and macrophage phagocytosis, defective antigen presentation, and suppression of lymphocytic proliferation are described as immunosuppressive alterations.[28-32] These immunosuppressive effects of RBC TRIM might play an important role in why the factor RBC transfusion was an almost independent protective factor against ACR in our multivariate analysis. Second, our multivariate analysis identified allografts from DCD as an independent risk factor for ACR. However, this finding appears divergent from many studies reporting similar rejection rates for DBD and DCD organs.[33-35]
Aspirin was also associated with significantly superior graft and overall survival rates in the present study, although the magnitude of this benefit was smaller compared with rejection-free survival. Although past studies suggested no association between ACR and graft survival,[36-38] a recent study analyzing the Adult-to-Adult Living Donor Liver Transplantation Cohort Study and SRTR cohort found that ACR was associated with an increased risk of developing graft failure and graft failure–related death.[39] How much the effect of aspirin on ACR exactly contributed to the superior graft and patient survival is unclear, but other beneficial effects on arterial patency and medical conditions might have contributed as well.
The strength of this study relates to the large multicenter study design with a well-defined contemporary study population of more than 2300 primary LT recipients. The contemporary nature of the study population is not only reflected by the recent 3-year study period but also by the fact that more than 90% of the recipients were on tacrolimus-based immunosuppression. In addition, the median follow-up time of more than 5 years provided a long observational basis for the primary outcome measures. The study was also associated with certain limitations that relate to the heterogeneity of posttransplant management among centers, including aspirin use, immunosuppression, and liver-biopsy decision making. In addition, the lack of biopsy-proven diagnosis in the scenario of clinically suspicious rejection might misallocate those patients to the rejection versus nonrejection group.
In conclusion, low-dose aspirin protects against both rejection and HAT, which translates to improved graft and patient survival rates. The findings of this contemporary cohort study should encourage evaluating low-dose aspirin use after primary LT to protect the liver graft from ACR and maintain arterial patency.
ACKNOWLEDGMENT
The authors thank Patricia Martinez, user-experience designer (ch.linkedin.com/in/pmartinezch/en), for the map she created. Open access funding provided by Universitat Zurich.
CONFLICT OF INTEREST
Parissa Tabrizian received honoraria from Bayer AG. Varvara Kirchner consults for Natera.




