Pediatric acute liver failure: Reexamining key clinical features, current management, and research prospects
Abstract
This review aims to synthesize the most updated research, outcomes, and trends in the field of pediatric liver transplantation (LT), specifically focusing on children who have suffered from acute liver failure. Pediatric acute liver failure is a dynamic, life-threatening condition that can either self-resolve or lead to death. LT is a lifesaving intervention. With the introduction of technical variant grafts and recent immunosuppression modifications, overall patient survival, graft survival, and waitlist mortality have improved. Furthermore, recent advances in the knowledge of immunologic mediators of acute liver failure offer the possibility of more detailed understanding of the pathophysiology and new areas for research. Given the success of living donor LT for pediatric patients with acute liver failure, this option should continue to be actively considered as an alternative treatment option for patients who are listed for transplantation and are managed at a multidisciplinary tertiary care transplant center.
Abbreviations
-
- c-index
-
- concordance index
-
- CRRT
-
- continuous renal replacement therapy
-
- CT
-
- computed tomography
-
- DDLT
-
- deceased donor liver transplantation
-
- FFP
-
- fresh frozen plasma
-
- HLH
-
- hemophagocytic lymphohistiocytosis
-
- HMGB1
-
- high mobility group box 1 protein
-
- ICU
-
- intensive care unit
-
- IP10
-
- interferon gamma inducible protein 10
-
- IL2
-
- interleukin 2
-
- INR
-
- international normalized ratio
-
- LDLT
-
- living donor liver transplantation
-
- LIU
-
- Liver Injury Unit
-
- LT
-
- liver transplantation
-
- MCP1
-
- monocyte chemotactic protein 1
-
- MIG
-
- monokine induced by gamma interferon
-
- PALF
-
- pediatric acute liver failure
-
- PALFSG
-
- Pediatric Acute Liver Failure Study Group
-
- PELD
-
- Pediatric End-Stage Liver Disease
INTRODUCTION
Pediatric acute liver failure (PALF) is a rapid onset, severe disease that affects individuals of all ages, usually without any signs of preceding illness. It progresses quickly and can either self-resolve, necessitate liver transplantation (LT), or cause death. Although there are many different etiologies of PALF, the most common cause is indeterminate, accounting for up to 50% of cases.[1-3] Other etiologies include drug toxicity (especially acetaminophen), infection, genetic disorders, autoimmune hepatitis, oncologic processes or complications, perfusion-related etiologies, and metabolic diseases.[2, 3]
The current definition of PALF denotes biochemical signs of liver injury in a child without preexisting chronic liver disease, coagulopathy not correctable by vitamin K supplementation, and international normalized ratio (INR) greater than 1.5 in a child with encephalopathy or greater than 2.0 without encephalopathy.[3] Encephalopathy, one of the hallmark signs in progressive PALF, can be especially difficult to assess in children and presents a unique clinical quandary for pediatricians. Furthermore, the prognosis of PALF is very difficult to ascertain. At times, patients are treated without much understanding of the potential final outcome with regard to transplantation, death, or spontaneous recovery given the limited information available to providers to help clarify a patient's trajectory. In the United States, 10%–15% of all the pediatric LTs are indicated for acute liver failure.[2, 4] This review will summarize the contemporary diagnosis and management of PALF (Part I) and areas of ongoing research (Part II).
PART I: CLINICAL FEATURES OF PALF
Epidemiology and disease presentation
Prior to the introduction of LT for PALF, estimates of mortality were as high as 72%.[4] Dr. Starzl pioneered the field of pediatric LT in the late 1960s for biliary atresia and liver carcinoma following his first human LT in 1963 in a child.[5, 6] The Pediatric Acute Liver Failure Study Group (PALFSG), an international consortium supported by the National Institutes of Health, was formed in 1999 as a repository for patient data and outcomes, which has generated a wealth of information since its inception.[7] These milestones in LT and in the care of pediatric liver disease have been instrumental in the progress and improvement of patient outcomes.
Presentation and diagnosis
Patients meeting criteria for PALF should be evaluated with an extensive, careful history and a physical exam with an emphasis on family history, past medical history, possible ingestions or exposures, and recent travel. Physical exam findings may include ascites, jaundice, generalized edema, signs of cardiac failure, or mental status changes.[1] Some patients may have vague symptoms without evidence of end-stage liver disease or clinical instability. Hepatic encephalopathy can be difficult to assess in children, depending on their age and developmental abilities at baseline.[3] Mild features may manifest as fatigue, irritability, and confusion, and severe encephalopathy can rapidly progress to stupor, cerebral edema, seizures, and coma.[2] Diagnostic workup should include a comprehensive metabolic panel, fractionated bilirubin, gamma-glutamyltransferase, complete blood count, serum ammonia, INR, abdominal ultrasound with Doppler to assess hepatic vasculature, and investigate for possible underlying etiologies including infection, metabolic disorders, autoimmune hepatitis, and drug testing (summarized in Table 1). Further diagnostic clarity may be attained via liver biopsy, although it is important to note that biopsies are subject to sampling error and may not reliably represent the histopathology of the entire organ.[8] A transjugular approach to liver biopsy is often better tolerated than a percutaneous attempt in this population given the bleeding propensity in the setting of liver synthetic dysfunction.[8]
| Clinical manifestation | Diagnosis | Management |
|---|---|---|
| Hepatic encephalopathy and hyperammonemia | Clinical assessment, ammonia, head CT | Lactulose, rifaximin, consideration of CRRT |
| Ascites and generalized edema | Clinical assessment, quantify on abdominal ultrasound | Diuretics (furosemide and spironolactone) |
| Respiratory failure | Clinical assessment and vital signs, chest x-ray | Mechanical ventilation |
| Circulatory failure | Clinical assessment and vital signs, echocardiogram | Pressor support |
| Acute kidney injury or renal failure | Serum chemistry and creatinine, urine output | Monitor fluid status, CRRT |
| Coagulopathy or bleeding | Coagulation panel, factor levels[5, 7, 8] | Provision of FFP or cryoprecipitate if bleeding, or preprocedure; vitamin K supplementation |
| Infection | Viral and bacterial cultures | Targeted therapy as applicable with antiviral or antibiotics |
| Electrolyte derangements | Serum chemistry (particular attention to Na, glucose, phosphorus) | Maintenance of normal glucose, Na, and phosphorus levels as able; hypernatremia can improve intracranial hypertension if needed |
| Cerebral edema | Head CT, physical exam | Mechanical hyperventilation, mannitol, hypertonic saline |
- Abbreviations: CRRT, continuous renal replacement therapy; CT, computed tomography; FFP, fresh frozen plasma; PALF, pediatric acute liver failure.
Coagulopathy, part of the diagnostic criteria for PALF, is exhibited by elevated INR in the setting of a balanced loss of both pro and anti-coagulant components, which minimizes the incidence of clinical bleeding.[9] As such, elevated INR is more representative of a loss of hepatic synthetic function rather than a likelihood of bleeding, and bleeding risk has not been shown to correlate with the INR trajectory.[10] Although patients are indeed at increased risk of bleeding when compared with the general population, the risk of thrombosis may actually outweigh the risk of clinically significant bleeding.[11, 12] Patients can be treated with subcutaneous vitamin K supplementation and fresh frozen plasma (FFP) if needed, although overtransfusion can increase the risk of thrombosis and bleeding, and no standardized transfusion protocols have been established.[10] Overall, significant bleeding has been noted in 5% of patients, of which <1% are intracranial bleeds.[2, 9] Of note, the degree of coagulopathy has not been shown to correlate with hepatic encephalopathy. The PALFSG has demonstrated that 25% of patients who had Grade 3 or 4 hepatic encephalopathy and required intensive care had mild coagulopathy with INR <2.0, yet patients with this degree of encephalopathy (Grade 3–4) also demonstrated the highest rates of mortality.[7] All patients with acute liver failure require close monitoring and daily assessment of overall mental status for this reason.
Identifying the underlying etiology of PALF is of the utmost importance, as determining contributing factors or underlying disease processes will impact the medical team's decision to list for LT. For instance, PALF attributed to acetaminophen ingestion can be treated using protocols including N-acetylcysteine and thus has been shown to be less likely to require LT as these patients recover more often than not.[3, 11] Conversely, herpes simplex virus, Wilson disease, and gestational alloimmune liver disease have been associated with more severe illness and multiorgan system involvement, which may sway the medical teams to list these patients earlier or even exclude them from listing altogether given the critical nature of illness.[12] Similarly, some patients may not be eligible for transplant because of their underlying cause of PALF, such as patients with hemophagocytic lymphohistiocytosis (HLH) or chemotherapy-induced PALF with active malignancy.
Given the nuances of determining etiology, the need for early aggressive management, and the potential need for emergent LT, patients should be cared for in pediatric LT centers as early in the disease course as possible given the rapidly progressive and unpredictable nature of disease.[13] Patients who are transferred earlier to pediatric transplant centers, before developing hepatic encephalopathy, have better outcomes than those who are transferred later.[1]
Physiology of multiorgan failure in PALF
The natural course of PALF results in either recovery or death. For patients with decompensating and progressive disease, intensive care is imperative. Patients may develop cardiac dysfunction, including peripheral vasodilation with decreased systemic vascular resistance and low mean arterial blood pressure in the setting of hyperdynamic cardiac failure.[9] Respiratory compromise can lead to acute respiratory distress syndrome as a result of fluid overload, and patients may require endotracheal intubation, especially to protect the airway in the setting of hepatic encephalopathy. Acute kidney injury or even renal failure is also a commonly observed complication among the PALF population and is associated with increased mortality.[2] Kidney injury can be attributed to intrarenal vasoconstriction and decreased renal perfusion as well as drug toxicity, hypovolemia, and sepsis.[9] Renal function typically recovers as hepatic function improves. Patients with PALF require careful monitoring of electrolytes as they are at risk of hypoglycemia, hypokalemia, hypophosphatemia, hypocalcemia, and hypomagnesemia.[9] Persistent hypoglycemia can represent an ominous sign of complete hepatic necrosis, with an inability to maintain euglycemia in the absence of gluconeogenesis.[14] For the most part, maintenance of normal electrolyte ranges is optimal. In the event of increased intracranial pressure, hypernatremia may be indicated, but outside of cerebral complications, normal serum Na levels ought to be maintained.
Although hyperammonemia is involved in the development of hepatic encephalopathy because of its neurotoxic nature, the degree of elevation does not always correlate with the extent of encephalopathy that a patient may exhibit.[9, 15] Careful and frequent neurological assessments are important as the high ammonia level can cause increased intracellular osmolarity in astrocytes, leading to enhanced water diffusion into the cells and ultimately cerebral edema.[9] According to the PALFSG, 55% of children present with hepatic encephalopathy, usually Grade 1 or 2.[9] Patients with Grade 3 or 4 encephalopathy usually require brain imaging to evaluate for any signs of intracranial hemorrhage.[2] It is important to ensure the ammonia level is collected from a free-flowing blood sample that is quickly placed on ice to reduce the chance of falsely elevated results.
Intensive care unit management of PALF
LT represents the definitive cure for PALF. Between 2010 and 2013 in the United States, 11.2%–12.5% of all pediatric LTs were performed for PALF.[9, 12] If applicable, treatment may be directed toward the underlying etiology of PALF, such as the administration of N-acetylcysteine for acetaminophen toxicity. However, the majority of etiologies lack a targeted therapeutic option. Stabilizing the patient in the intensive care unit (ICU) and managing multisystem organ failure remains the mainstay of therapy both while awaiting LT and to avoid LT as outlined in Table 1. Determining which patients might benefit from LT versus exhibit potential for recovery has been difficult to ascertain. Therefore, patients are monitored and treated supportively, with their clinical and mental statuses continuously reassessed to determine the likelihood of self-resolution versus progressive decompensation (Figure 1).
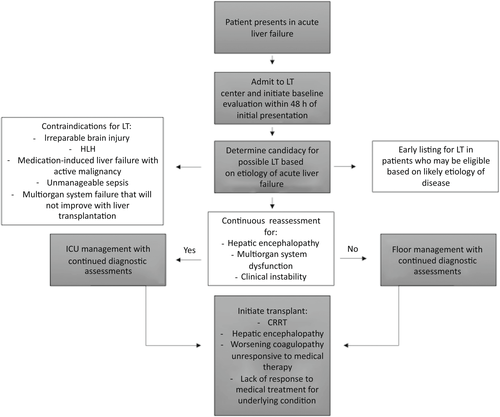
During PALF, patients are susceptible to transudative fluid accumulation in extravascular compartments, which may lead to cardiovascular compromise. In the setting of resuscitation, norepinephrine tends to be an appropriate choice of vasoconstrictor because of its ability to maintain central perfusion pressure despite fluid overload.[16] According to the PALFSG, almost 40% of patients require ventilator support for respiratory failure, with low tidal volumes and higher positive end expiratory pressure.[11] Continuous renal replacement therapy (CRRT) may be used to manage patients with acute kidney injury and fluid or significant acidosis and has been proven to successfully reduce serum ammonia and lactate levels while improving transplant-free survival.[11, 17] FFP infusions should only be given preprocedurally or in the setting of significant bleeding given the risk of volume overload and loss of INR trajectory as a prognostic indicator, and patients should be treated with proton pump inhibitors to decrease the incidence of stress ulcers. Sedative medications should be used sparingly as they may limit the accurate assessment of encephalopathy. Hepatic encephalopathy can be treated with medical management, such as lactulose, rifaximin, or neomycin, in attempts to decrease the buildup of ammonia, yet supportive data for these interventions are minimal.[18] Cerebral edema may require the head of the bed to be elevated, hyperventilation, hyperosmolar therapy, electroencephalogram monitoring, and imaging with a computed tomography (CT) scan.[19, 20] Ascites can be managed with fluid restriction or diuretics such as furosemide or spironolactone, yet it is important to avoid overdiuresis, which can lead to hepatorenal syndrome. Patients with evidence of sepsis or infection may require empiric treatment with broad-spectrum antibiotics.[1]
While managing patients' disease sequelae, the ever-present decision of listing for LT must be reassessed. Many centers favor listing patients while workup and intensive management is ongoing within the first 24–48 h.[12] If possible, obtaining a tissue sample can provide diagnostic clarity and may even invite medical therapy to potentially save the patient from LT, such as activated CD8+ T cell hepatitis, which may respond to corticosteroids.[21] Some patients, such as those with underlying mitochondrial conditions, may not be eligible for LT. Furthermore, patients with life-threatening cerebral complications also may not be candidates for LT.[11] Even so, the possibility remains to remove a child from the waiting list once signs of recovery are demonstrated.
Poor prognostic indicators for PALF include age younger than 1 year at the time of presentation, presence of Grade 4 encephalopathy, and need for pretransplant dialysis.[2] Although predictive models have been proposed, no scoring system has been successfully applied to reliable clinical outcome modeling. The Liver Injury Unit (LIU) score, which uses peak serum total bilirubin, prothrombin time/INR and ammonia, is able to predict death or LT by 4 weeks of patient presentation with an area under the curve of 88.5%–90.5%.[22] In a multicenter validation study, the LIU was able to predict LT status better than death with a concordance index (c-index) of 0.84 and a c-index of 0.81 for the prediction of survival without LT.[23] However, its use in the clinical setting is limited by a reliance on peak laboratory values, which is of minimal use in the day-to-day practical setting and does not account for patient's age. The King's College model incorporates the presence of hepatic encephalopathy, which is an unreliable feature in pediatrics, impeding its applicability. When validated in a large cohort of patients with PALF, the sensitivity and specificity of death prediction were low at 61% and 70%, respectively.[4, 24] The Pediatric End-Stage Liver Disease (PELD) score was developed in an attempt to predict mortality pretransplant in children with liver disease and is calculated using albumin, bilirubin, INR, presence of growth failure, and age. Although the PELD score is widely used for chronic disease, it is unreliable in PALF and is not an appropriate grading score. As such, predicting patient outcomes remains challenging.[9, 12] Without a corroborated scoring system, some patients will be listed for and ultimately receive LT who would have otherwise spontaneously recovered.[12]
Evolving use of LT in PALF
LT remains the standard of care and can be lifesaving for PALF. The PALFSG demonstrated a reduced frequency of listing patients for transplant and a reduced number of transplants without an increase in mortality after describing the trends from 1999 to 2014.[12, 25] Patients most likely to be listed had higher INR (median, 3.0), total bilirubin (median, 15 mg/dl), lactate (median, 2.8 mmol/L), ammonia (63 μmol/L), and lower liver enzymes (alanine aminotransferase, 1635 IU/L) than patients who were not listed because of a categorization of “not sick enough.”[12] Listed patients were also more likely to be boys and require mechanical ventilation or vasopressor support.[12] Indeterminate PALF was the most common diagnosis for listing.[12] Patients who were not listed because they were “medically unsuitable” were more likely to have viral causes of PALF, shock, or HLH as well as a higher incidence of mechanical ventilation and vasopressor requirement.[12] Contrary to adult data, irreversible brain damage was not a predominant exclusionary reason for listing.[12] Overall, approximately one-third of patients with PALF were listed for transplant during the course of the study period.[12]
As the rate of transplant listing and pursuit decreased during the 15-year study period, it can be presumed that identification of more treatable causes of PALF have been determined. However, the time to listing remained stable throughout, usually within 1 day of study enrollment, which contradicts previous studies documenting the decision to list patients for transplant as a process that unfolds over time. More likely, patients are listed quickly to optimize the potential for organ receipt given the continued organ shortages and unpredictable waitlist times.[12]
Both patient survival and waitlist mortality improve for patients listed for transplant at centers that perform more than 50 technical variant graft transplants (split, reduced, and living donor) when compared with lower volume centers. This is likely due to the enhanced wealth of experience performing these technically demanding procedures in small children in the busier centers.[25] Waitlist mortality on the whole has improved for pediatric patients since the incorporation of technical graft variants.[13] Younger patients tend to have the longest waitlist times, especially when younger than 1 year of age, in whom waitlist mortality is >20%, and these patients are more likely to receive split and living donor grafts compared with whole livers.[25] Age may affect both the severity of illness as well as the likelihood of transplant listing. Because of limited organ availability, difficult clinical assessments of encephalopathy, and the increased incidence of multisystem organ failure, younger children (aged younger than 3 years) tend to have worse prognoses without LT.[3]
Increased death and graft loss are observed in patients who have public insurance, and health-related quality of life, fatigue, and school performance are all worse in patients who have received LT and in patients who recovered from PALF when compared with the general population.[26-28] Sexual disparity among LT recipients exists on a national level, with only 35.7% female recipients in 2017.[12] Reasons for this remain unknown.
PALF transplant outcomes
Prior to the introduction of LT, the primary outcome of PALF was death, with a mortality rate of 70%–95%.[12] Since the implementation of LT for PALF, mortality rates have decreased to 11% for the 21-day outcome.[12] However, long-term outcomes after transplant for PALF may be inferior when compared with those patients who received transplants for chronic liver disease.[25]
A single-center study comparing outcomes of living donor LT (LDLT) and deceased donor LT (DDLT) for PALF in Poland demonstrated shorter cold ischemia times for LDLT with median times of 4 and 9.2 h, respectively.[29] Decreasing the cold ischemia time can improve organ preservation complications, including primary nonfunction of the graft. This study also demonstrated improved graft quality from the LDLT compared with the DDLT, which also had higher rates of graft primary nonfunction.[29] Similarly, a 2016 Brazilian retrospective, single-center study of 115 patients with PALF showed that LDLT was associated with a higher long-term survival rate (72.4%) compared with DDLT (40.0%) as well as lower incidences of primary nonfunction.[30] The study also demonstrated improved waitlist mortality after the initiation of LDLT.[30] In parts of the world with limited access to deceased donor organs, urgent LDLT should be considered in PALF.
High-risk populations in PALF
PALF can affect children of any age, and managing the youngest patients, younger than 90 days, can be particularly challenging. The PALFSG has studied this subset of patients, revealing the median age of enrollment was 18 days of life, with most commonly presenting with lethargy.[31] Of these patients, 38% were identified as indeterminate PALF, and 64% lacked encephalopathy at the time of study enrollment.[31] Resolution of disease without transplant was more likely in patients without encephalopathy.[31] Overall, 60% of the patients recovered, usually with indeterminate PALF, followed by metabolic disease, which accounted for 20.5%.[31] Of these patients, 41% were listed for LT, 40% of whom were successfully transplanted within 21 days.[31] The mortality rate of this younger patient cohort is worse than their older counterparts, with 24% of the infants enrolled in the study succumbing to sepsis, multiorgan failure, pulmonary hemorrhage, or cerebral edema.[31] This clearly demonstrates the negative impact of young age on PALF outcomes.
Pediatric LT remains a more complicated task given the prevalence of donor–recipient size mismatch and the general lack of pediatric donors.[25] The rapid progression of PALF can drastically reduce the time available for transplant, necessitating emergency evaluations both of living and deceased donors. In some parts of the world, transplant relies more heavily on living donation because of a lack of deceased donor availability. In a 2016 study from Turkey, 16 patients were emergently listed for LT, and 12 received living donations after no deceased donor became available: nine received a left lateral segment donation, two received a right lobe donation, and one patient received a left lobe organ donation.[32] The remaining four patients died while awaiting organ donation.[32] Published statistics between 2005 and 2014 in Turkey are 3–4.8 deceased donors per 1 million people.[32] Such scenarios amplify the importance of expedited living donor evaluation in PALF.[33]
PART II: RESEARCH TOPICS IN PALF
Immunologic mechanisms of PALF
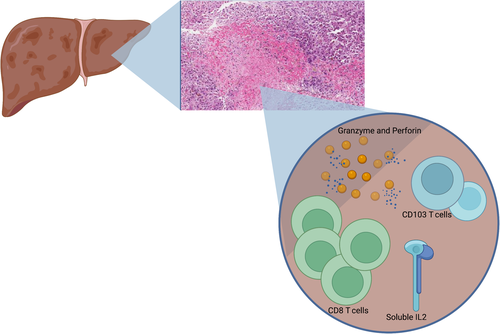
Patients with indeterminate PALF generally have worse outcomes than patients with identifiable etiologies of acute liver failure and are more likely to require LT.[34] New data suggest that some patients with indeterminate PALF can be characterized by CD8+ T cell infiltrates that are immune mediated, driving hepatocyte injury in a new classification of liver injury known as “activated CD8 T cell hepatitis.”[21,34] These patients may be responsive to steroid therapy, which could potentially save them from LT. Liver biopsy specimens from this newly identified cohort show dense CD8 positivity along with perforin and CD103 T cell staining patterns when compared with biopsy specimens of patients with other non-PALF diagnoses.[21] Although this can be helpful in making diagnoses in patients with otherwise indeterminate disease, other etiologies of liver injury can also have moderate or dense CD8 staining on liver biopsy, including viral hepatitis, HLH, macrophage activation syndrome, and drug-induced hypersensitivity reactions.[21, 35, 36] This notable overlap among immune-mediated processes with hepatic manifestations calls for further investigation into the specific immune mediators that drive the various forms of PALF, even beyond the indeterminate form.
New research has also shown a subset of patients with indeterminate PALF may be subclassified by the combination of high perforin and granzyme expression in CD8 lymphocytes in conjunction with elevated soluble interleukin 2 (IL2) and an elevated absolute CD8+ T cell count as summarized by Figure 2.[37] This discovery highlights the multifaceted and complex interaction of immune mediators and exemplifies the interrelatedness of the intrahepatic immune system. Modeling approaches including machine-learning algorithms may be required to further understand these nuanced relationships. Zamora et al. emphasized that the intricate immune characteristics of PALF differ not only based on underlying etiology and ultimate patient outcome but also by patient age. Possible biomarkers responsible for the mediation of PALF progression include high mobility group box 1 protein (HMGB1), monokine induced by gamma interferon (MIG), interferon gamma inducible protein 10 (IP10), and monocyte chemotactic protein 1 (MCP1), with the highest dynamic complexity of immune composition observed in children aged 4–13 years compared with children of other ages.[38] Expansion of these approaches to further identify and understand the innerworkings of the immunologic components of PALF will enhance clinicians' ability to predict the clinical course and may also offer possibilities for future medical interventions.
Predictive models of clinical outcomes
Today, there remains a lack of accurate prediction models regarding patient outcomes of PALF. This means that patients who may have otherwise recovered without invasive intervention will instead undergo organ transplantation. Determining which patients may recover versus those who would die without LT continues to be the focus of ongoing research to protect patients from unnecessary transplantation and the sequalae of lifelong immunosuppression. It is difficult to use subjective manifestations of disease, such as presence or progression of hepatic encephalopathy as a reliable marker of overall prognosis. Accurate and predictive biomarkers could dramatically change the diagnosis and management of PALF. As the CD8-dense inflammatory subset of indeterminate PALF has been identified, it likely that the immune landscape of PALF may represent a more definitive approach to establish a clinical trajectory as well as identify possible therapeutic targets.
CONCLUSIONS
PALF continues to be a rare but potentially lethal disease process in otherwise healthy children. Its clinical trajectory remains unpredictable and requires further characterization to establish reliable management algorithms. The reasoning process for when to commit to LT also remains undefined. Given the necessity for rapid decisions to list patients for transplant, teams must weigh the risks associated with transplant surgery and lifelong immunosuppression with the risk of decompensating acute liver failure. Data show improved overall graft and patient survival following LT for PALF, regardless of the type of graft, and the use of technical variant grafts has significantly reduced waitlist mortality. LDLT also reduces patient wait times and may provide higher quality organs than DDLT. Research into newer techniques to characterize the immunologic features of PALF may help clarify the diagnostic process, establish predictive models, and aid in the clinical management of these patients.
FUNDING INFORMATION
Johanna M. Ascher Bartlett was supported by University of Southern California Stem Cell's Broad Clinical Research Fellows Program and the Children's Hospital Los Angeles Core Pilot Grant from the Single Cell, Sequencing and CyToF (Cytometry by Time of Flight) Core. Juliet Emamaullee was supported by a K08 from the National Cancer Institute (K08CA245220); American Society of Transplant Surgeons Faculty Development Grant; American Society for the Study of Liver Diseases Clinical, Translational, and Outcomes Research Award; and a Liver Scholar Award from the Gilead Research Foundation.
CONFLICT OF INTEREST
Nothing to report.