Successful Auxiliary Liver Transplant Followed by Hematopoietic Stem Cell Transplantation in X-Linked Lymphoproliferative Disease Type 1
Anil Dhawan consults for Promethera Biosciences and advises for Univar Solutions.
Marie-Eve Chartier wrote the manuscript and approved the final manuscript as submitted. Maesha Deheragoda provided the histology slides and associated comments as well as reviewed and revised the manuscript and approved the final manuscript as submitted. Michael Gattens, Anil Dhawan, Nigel Heaton, Claire Booth, and Nedim Hadžić reviewed and revised the manuscript and approved the final manuscript as submitted. Nedim Hadžić conceptualized the project and was the main supervisor. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Abbreviations
-
- AA
-
- aplastic anemia
-
- ALF
-
- acute liver failure
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- ATG
-
- anti-thymoglobulin
-
- CMV
-
- cytomegalovirus
-
- EBV
-
- Epstein-Barr virus
-
- H & E
-
- hematoxylin-eosin
-
- HIDA
-
- hepatobiliary iminodiacetic acid
-
- HLH
-
- hemophagocytic lymphohistiocytosis
-
- HSCT
-
- hematopoietic stem cell transplantation
-
- IgG
-
- immunoglobulin G
-
- IgM
-
- immunoglobulin M
-
- INR
-
- international normalized ratio
-
- LT
-
- liver transplantation
-
- MMF
-
- mycophenolate mofetil
-
- MRI
-
- magnetic resonance imaging
-
- PCR
-
- polymerase chain reaction
-
- PICU
-
- pediatric intensive care unit
-
- PID
-
- primary immunodeficiency
-
- PTLD
-
- posttransplant lymphoproliferative disease
-
- SAP
-
- signaling lymphocyte activation molecule–associated protein
-
- SLAM
-
- signaling lymphocyte activation molecule
-
- XLP1
-
- X-linked lymphoproliferative disease type 1
-
- XLP
-
- X-linked lymphoproliferative disease
X-linked lymphoproliferative disease type 1 (XLP1) is a rare, potentially life-threatening primary immunodeficiency (PID) with a range of clinical manifestations, including hemophagocytic lymphohistiocytosis (HLH), lymphoma, hypogammaglobulinemia, and autoimmune phenomena. It is caused by mutations in the SH2D1A gene, which encodes for the signaling lymphocyte activation molecule (SLAM)–associated protein (SAP).(1) The absence of SAP in XLP1 patients leads to multiple immunological defects including natural killer and T cell cytotoxicity,(2) the lack of natural killer T cell development, and defective CD4+ T-follicular cell (TFH) help,(3) which results in abnormal humoral function. The clinical disease phenotype is characterized by severe immune dysregulation, including development of lymphoma, T cell activation defects leading to HLH (or dysregulated immune response to infection), and abnormalities in immunoglobulin production and T cell-dependent humoral immune responses. SAP acts as a natural blocker of other Src homology 2 protein domain–containing inhibitory molecules from binding to SLAM family receptors. In the absence of SAP, SLAM family receptors switch function and mediate inhibitory signals.(4)
XLP1 is often regarded as an Epstein-Barr virus (EBV)–driven disease, and although they are susceptible to overwhelming EBV infection, up to 35% of patients develop symptoms in its absence, including HLH, lymphoma, lymphoid vasculitis, hepatitis, and cytopenias.(5, 6) Historically, outcomes for XLP1 patients were extremely poor with survival as low as 4% when associated with HLH.(7) However, with the advent of improved HLH and lymphoma protocols, anti-CD20 monoclonal antibodies, and immunoglobulin replacement, the mortality rate has fallen. Hematopoietic stem cell transplantation (HSCT) offers a curative treatment with survival reaching 80% with a well-matched donor.(5) Survival is reduced to ~50% in nontransplanted patients and in those requiring HSCT with a poorly matched donor and active disease at the time.(5)
Patient Report
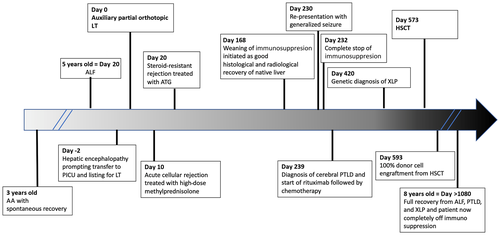
A 5-year-old Caucasian boy was admitted with acute liver failure (ALF) after a 2-week history of jaundice, abdominal pain, and lethargy. His past medical history included an episode of aplastic anemia (AA) at the age of 3 years. Diagnosis was confirmed by a markedly hypocellular bone marrow aspirate (<10% cellularity) without evidence of hemophagocytosis or infiltrative disease, and he received supportive treatment with blood products and intravenous immunoglobulin. He was negative for EBV, parvovirus B19, and human immunodeficiency virus but positive for cytomegalovirus (CMV) immunoglobulin G (IgG). HSCT was considered, but he recovered spontaneously.
At presentation with ALF, he had grade 1 encephalopathy, jaundice (total bilirubin, 227 μmol/L; conjugated bilirubin, 171 μmol/L), elevated transaminases (alanine aminotransferase [ALT], 1006 IU/L; aspartate aminotransferase [AST], 1374 IU/L), and abnormal synthetic function (international normalized ratio [INR], 1.83; ammonia, 70 μmol/L; albumin, 35 g/L). He was initially started on intravenous vitamin K, broad-spectrum antibiotics, and antifungal treatment. Ultrasound showed no splenomegaly or portal hypertension, but the liver parenchyma appeared abnormal. Etiological workup was negative for hepatitis A, B, C, and E; CMV; adenovirus; enterovirus; parvovirus B19; and human herpesvirus 6. He had positive EBV IgG, but EBV immunoglobulin M (IgM) and polymerase chain reaction (PCR) RNA were negative. Two weeks earlier, EBV capsid IgG and IgM as well as EBV nuclear antigen antibodies were negative. He had mildly positive serum anti-nuclear antibodies (titer 1:40), whereas serum IgG levels were slightly decreased at 4.72 g/L (normal, 4.9-16.1 g/L) with normal IgM and IgA levels. The remainder of his autoantibody panel was negative for anti-smooth muscle, anti-mitochondrial, anti-gastric parietal cell, and anti-liver-kidney microsome antibodies. His lymphocyte count varied between 6 and 13 ×109/L (normal, 2-9 ×109/L). Serum ferritin was raised to 2088 ng/mL (normal, 20-300 ng/mL) with borderline low fibrinogen of 1.3 g/L (normal, 1.5-4.5 g/L), but he had normal triglyceride of 1.1 mmol/L (normal, 0.5-1.7 mmol/L). Given his background of AA and weakly positive autoantibodies, he was empirically started on methylprednisolone (2 mg/kg/day) for possible autoimmune hepatitis. Three days later, as INR increased to 2.5, a transjugular liver biopsy was performed. Histopathology showed a portolobular hepatitis with interface activity and early portal fibrosis that was compatible with partially treated autoimmune hepatitis (Fig. 1A,B). Given the poor biochemical response to steroids, he received 1 dose of anti-CD20 monoclonal antibody (rituximab 400 mg/m2) 12 days later.
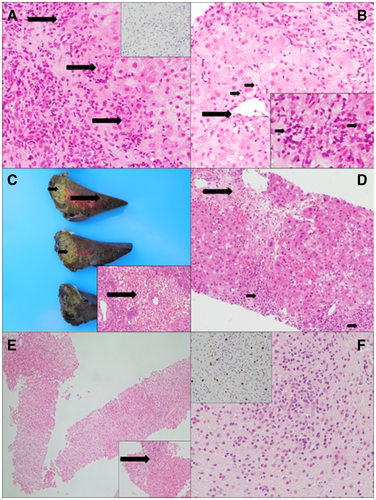
On day 18 after admission, he developed grade 2/3 encephalopathy, with ammonia of 170 μmol/L, INR of 3.15, and profound hypoglycemia. He was intubated, transferred to the intensive care unit, and listed for emergency liver transplantation (LT). Two days later, he received an auxiliary partial orthotopic LT with a left lateral segment from a cadaveric adult EBV+/CMV– donor. Auxiliary grafting was performed based on the absence of macroscopic evidence of chronic liver disease (Fig. 1C) and presumed indetermined etiology of ALF, according to the standard practice in our unit.(8)
The immediate postoperative course was complicated by severe T cell–mediated rejection requiring high-dose methylprednisolone (10 mg/kg/day for 3 days) followed by rabbit anti-thymoglobulin (ATG; 1.5 mg/kg/day for 10 consecutive days), as a repeated biopsy, 1 week later, showed persistent T cell–mediated rejection with bile duct damage (Fig. 1D). As his liver enzymes improved with ATG, no liver biopsy was repeated at the end of treatment. During the rejection episode, he was found to have significant ascites with high abdominal drain output, hypoalbuminemia (22 g/L), and hypogammaglobulinemia (IgG 1.07 g/L). Observed pancytopenia prompted a bone marrow aspirate showing hypocellularity, but no hemophagocytosis, which was interpreted as likely secondary to the mycophenolate mofetil (MMF) that had been added as a second immunosuppressant to tacrolimus. The pancytopenia improved after converting MMF to azathioprine within 3 days.
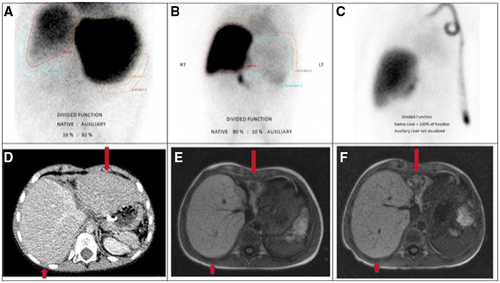
He was discharged home 2 months after LT but continued having fluctuating transaminases (ALT, 70-445 IU/L; AST, 48-484 IU/L). One month after LT, elective hepatobiliary scintigraphy (hepatobiliary iminodiacetic acid [HIDA]) showed that only 18% of the liver function and biliary excretion was performed by the native liver, while 3 months later this increased to 90% (Fig. 2A,B). Six months after LT, a liver biopsy demonstrated good regeneration and minimal lobular inflammation of the native liver (Fig. 1E), whereas the left transplanted liver showed moderate rejection. Computed tomography volumetry confirmed that the native liver was increasing in size while the graft was getting smaller (Fig. 2D). With the evidence of a good radiological and functional recovery of the native liver, we initiated gradual reduction in immunosuppression with target tacrolimus levels between 2 and 4 μg/L.
Seven months after LT, the boy presented again with prolonged generalized tonic-clonic seizure, fever, and maculopapular rash. Brain magnetic resonance imaging (MRI) revealed 2 focal lesions in the right and left temporal regions. Herpes simplex virus PCR and bacterial cultures were negative, but he was highly EBV viremic (EBV DNA 4,100,000 copies/mL). A brain biopsy revealed heavy mononuclear CD20+ infiltrates and Epstein-Bar virus-encoded RNA (EBER) expressing B-lymphoid infiltrate, supporting the diagnosis of posttransplant lymphoproliferative disease (PTLD; Fig. 1F). Immunosuppression was discontinued, and a course of anti-CD20 monoclonal antibody (rituximab 400 mg/m2/week for 4 weeks) was given. However, the brain lesions progressed radiologically, prompting treatment escalation with chemotherapy (cytarabine, methotrexate, and etoposide). Because of the rarity of cerebral PTLD after LT and his background of AA and ALF of indeterminate etiology, he underwent genetic testing that identified a pathogenic c.245 duplication (Asn82Lysfs*22) mutation of the SH2D1A gene, confirming the diagnosis of XLP1.
Following clinical remission of PTLD, the boy underwent evaluation for HSCT. By then, his native liver had fully recovered, as documented on repeated HIDA scan, MRI volumetry (Fig. 2B,E), and liver biopsy, which showed only minimal lobular inflammation and mild portoseptal fibrosis (Fig. 1E). Therefore, his liver function was deemed sufficiently recovered for the challenges of the next transplant. He was conditioned with fludarabine, treosulfan, and alemtuzumab, and he underwent a 10/10 matched unrelated donor hematopoietic stem cell transplant 18 months after his LT. Twenty days later, 100% donor cell engraftment was documented. He remained EBV viremic during and after the HSCT but without further complications. Post-HSCT immunosuppression was maintained with cyclosporin and MMF because he required no medications for the liver graft. His liver function tests remained normal. With excellent engraftment, immune recovery, and no evidence of graft-versus-host disease, MMF was stopped after 4 months, followed by cyclosporin tapering 8 months after HSCT. At 30 months after LT and 1 year after HSCT, the boy remains completely well, off immune suppression, and cured from life-threatening ALF, cerebral lymphoma, and, most importantly, underlying XLP1. See Fig. 3 for the complete timeline of the critical events.
Discussion
We describe a highly unusual presentation of XLP1, which required combined interventions to treat ALF, cerebral PTLD, and immune dysregulation. Our patient became immunosuppression independent after bridging his ALF with auxiliary LT and subsequent successful HSCT engraftment.
According to a recent multicenter study of 91 XLP1 patients,(5) HLH was the most common presenting feature (31.9%), with a further 22% having dysgammaglobulinemia and 14.3% having lymphoma. Less than 10% presented with other symptoms such as anemia, gastritis, vasculitis, or hepatitis. It appears that EBV-negative XLP1 patients are less likely to have HLH as the first presentation.(5) Our patient initially presented with AA and negative EBV serology. This emphasizes that a comprehensive immunological assessment, ideally also including assessment for XLP, should be considered in all AA patients with negative initial investigations.
The clinical management of our patient was driven by symptoms. After he developed progressive ALF with encephalopathy and failed to respond to an empirical course of steroids for a presumed autoimmune etiology, he received emergency LT. Intraoperatively, it was decided to perform auxiliary partial LT because the potential for regeneration of the native liver was anticipated.
It is well established that patients with XLP1 can present with fulminant hepatitis. Markin et al. described 61 patients who died from EBV infection (30 with sporadic infectious mononucleosis and 31 with XLP1).(6) A total of 58% of XLP1 patients died of fulminant hepatitis, at a mean of 7 weeks after onset of symptoms. On necropsy, their liver biopsy typically showed prominent portal infiltrate composed of lymphocytes, plasma cells, immunoblasts, and histiocytes.(6) The inflammatory component was similar to that reported in our patient’s transjugular biopsy, misleading us to suspect autoimmune hepatitis given the previous history of AA and low-titer seropositivity for anti-nuclear antibodies. Therefore, differential diagnosis between ALF secondary to autoimmune hepatitis or XLP1 may not be straightforward. PIDs, including XLP, should be excluded in all indeterminate ALFs of childhood, particularly in the presence of pancytopenia, dysgammaglobulinemia, and supportive history.
The immediate post-LT course of our patient was complicated by episodes of T cell–mediated rejection, which could lead to increased abdominal drain losses due to the graft stiffness. Thus, he received standard treatment with high-dose steroids followed by ATG for steroid-resistant rejection because ATG by depleting T cells can be effective in treating solid organ steroid-resistant rejection(9) and late cholestatic rejection in pediatric LT recipients.(10) This would have made him even more susceptible to EBV-related injury during the primary exposure to the virus, which was likely acquired from the adult graft donor. The hypogammaglobulinemia observed after LT was initially interpreted as secondary to immunoglobulin losses from the significant abdominal drain output, but in retrospect, it is more likely to have been related to XLP1.
The most unusual complication in our patient was the development of cerebral PTLD 7 months after LT. PTLD ranges from nondestructive lesions (follicular hyperplasia) to malignant forms (lymphoma-like lesions) as a result of chronic immunosuppression in transplant recipients. PTLD in paediatric patients is EBV-driven in approximately 70% cases, especially early after transplant. One study reported a mean onset latency for brain PTLD of 31 months (range, 3-131 months) after transplantation.(11) With our experience, it is mandatory to exclude PIDs in all children developing cerebral PTLD after LT because their ultimate treatment could require additional management, including HSCT.
PTLD remission was achieved after chemotherapy, providing a window for potentially curative treatment. HSCT can be associated with a number of hepatic complications, such as graft-versus-host disease, sinusoidal obstructive syndrome, drug-induced liver injury, or sepsis. Our patient underwent elective assessments of native liver function, including ultrasound studies, MRI volumetry, HIDA, and histology, all of which indicated that liver regeneration was likely to be adequate for the challenges of HSCT. He indeed had no hepatic complications and remains in good health after his minimal immunosuppression was completely withdrawn 1 year after HSCT.
In conclusion, a diagnosis of XLP1 links all previous symptoms including cytopenias, ALF, and EBV-driven brain PTLD. Our unusual experience demonstrates that LT can be lifesaving in patients with XLP1 who present with ALF, but vigorous diagnostic attempts should be made in all indeterminate cases to rule out PIDs. Had the diagnosis been reached earlier, HSCT could have been performed sooner to avoid additional complications associated with PIDs.