Graft Programmed Death Ligand 1 Expression as a Marker for Transplant Rejection Following Anti–Programmed Death 1 Immunotherapy for Recurrent Liver Tumors
This study was supported by National Key R&D Program of China (2019YFC1315800 and 2019YFC1315802), Shanghai Municipal Key Clinical Specialty, the National Natural Science Foundation of China (81972232, 81830102, and 81772578), and the Shanghai Municipal Natural Science Foundation (18410720700).
Monica M. Bertagnolli has a travel grant with the Chinese Medical Education Association.
Potential conflict of interest: Nothing to report.
Abbreviations
-
- AG
-
- nab-paclitaxel and gemcitabine
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- HCC
-
- hepatocellular carcinoma
-
- ICB
-
- immune checkpoint blockade
-
- ICC
-
- intrahepatic cholangiocarcinoma
-
- IHC
-
- immunohistochemistry
-
- PD1
-
- programmed death 1
-
- PD-L1
-
- programmed death ligand 1
-
- PEI
-
- percutaneous ethanol injection
-
- RFA
-
- radiofrequency ablation
-
- TACE
-
- transcatheter arterial chemoembolization
Solid organ transplant patients have a significantly higher risk of developing malignancy, likely due to longterm immunosuppression. Recently, immune checkpoint blockade (ICB) has improved outcomes for patients with a variety of solid tumors, including melanoma, squamous cell, lung, gastrointestinal, and liver cancers.(1, 2) Solid organ transplant recipients have been excluded from these therapies because of concern for graft rejection, which is reported as ranging from 25% to 60% for heart, lung, kidney, and liver transplant recipients.(3)
Liver transplantation is one of the standards of care for patients with early stage hepatocellular carcinoma (HCC), providing an opportunity for longterm disease-free survival as well as treatment of underlying liver disease.(3) Intrahepatic cholangiocarcinoma (ICC) is also an indication for transplantation under certain conditions. Tumor recurrence following transplantation occurs in 15%-50% of recipients, depending upon the type and extent of disease prior to transplant, and systemic adjuvant therapy has not been shown to reduce this risk or to extend overall survival.(3) When posttransplant tumor recurrence occurs, treatment involves the use of regimens that show efficacy in patients with advanced disease at initial presentation. Recently, early phase trials for patients with advanced HCC demonstrated increased response rates and better overall survival when standard therapy was combined with agents inhibiting the immunosuppressive activity of immune checkpoint programmed death 1 (PD1)/programmed death ligand 1 (PD-L1).(1)
Promising results from early studies of immune checkpoint inhibitor therapy for liver cancer led us to investigate methods to determine which patients may safely receive anti-PD1/PD-L1 immunotherapy following liver transplantation, which is a setting where immune suppression is essential for graft survival. We reviewed the experience from a large liver cancer treatment center (Zhongshan Hospital, Fudan University, Shanghai, China) and retrospectively examined PD-L1 expression levels in transplanted livers from 2 patients with recurrent HCC who developed acute graft rejection after receiving anti-PD1 inhibitor therapy for tumor recurrence. Both patients demonstrated positive PD-L1 expression in the liver biopsy that was obtained to pathologically confirm graft rejection. A similar result was reported for 2 patients treated at the Mattel Children’s Hospital of the University of California, Los Angeles. Each of these patients received anti-PD1 therapy for posttransplant recurrence of HCC, followed by graft rejection, and liver biopsy at the time of rejection showed positive PD-L1 expression in the graft.(3) In a recent retrospective study from the Mayo Clinic, Arizona, 5 patients who received PD1 inhibitors following liver transplant underwent graft biopsy. Of these 5 patients, 2 developed graft rejection, and biopsy at the time of rejection also showed PD-L1–positive cells.(3) The other 3 patients who did not develop graft rejection had liver biopsies without evidence of PD-L1 expression.
These cases provide promising signs that the liver transplant patients reported here with PD-L1–negative grafts were all safely treated with anti-PD1 therapy. However, to date all of the graft biopsy results reported for patients with immune checkpoint inhibitor–associated graft rejection were obtained from transplanted livers in the clinical setting of acute rejection. It is not clear, therefore, whether the absence of PD-L1 expression in the graft prior to treatment can indicate a low risk of PD1 inhibitor–associated rejection, and this must be rigorously tested using pretreatment biopsies if this can be used as a marker to guide therapy.
On the basis of these preliminary findings, we hypothesized that PD-L1 expression might serve as an effective biomarker for predicting graft rejection following PD1 inhibitor therapy. We therefore designed a prospective single-arm study of anti-PD1 immunotherapy for patients with recurrent malignancy following liver transplantation whose grafts were shown to lack PD-L1 expression. The primary objective of the study was to compare the graft rejection rate for transplant recipients whose grafts were PD-L1 negative to that of a historical cohort comprising liver transplant recipients with unknown PD-L1 status.
Patients and Methods
Patients Enrolled
Participants who received a liver transplantation for HCC or biliary tract cancers and experienced tumor recurrence or secondary primary hepatic malignancy were screened for the study. No organs from executed prisoners were used in the present study. All patients underwent a core needle biopsy of the liver graft prior to study enrollment (NCT03966209). The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Zhongshan Hospital, Fudan University.
Tissue PD-L1 Expression
Tissue specimens were fixed in formaldehyde, embedded in paraffin, and sectioned at 4 μm followed by staining with hematoxylin-eosin. Reagents for PD-L1 (22C3 pharmDx and 28-8 pharmDx) immunohistochemistry (IHC) assays were purchased from Dako.com (Carpinteria, CA), and tissue sections were processed using the manufacturer’s staining protocol. Tonsil tissues were used as a positive control for PD-L1 expression. The biopsies were reviewed and scored by a single study pathologist according to the standard criteria.(1, 2)
The number of PD-L1–positive cells (liver cells, lymphocytes, and Kupffer cells)/total number of viable cells were counted, and ≥1% in sections stained with either PD-L1 22C3 or 28-8 antibody was defined as positive PD-L1 expression.(1, 2)
Assessment of Graft Rejection
Patients who developed elevations of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥2 times the upper limit of normal underwent liver core biopsies, which were scored according to the Banff system. Graft rejection was defined as a Banff score ≥4.
Study Treatment and Statistical Method
Study patients, who had negative PD-L1 expression in the graft, received 240 mg of toripalimab (Junshi Biosciences, Shanghai, China) every 3 weeks until the development of graft rejection or until grade 4 or higher toxicity indicated a need to discontinue PD1 inhibitor therapy. All patients received sirolimus or everolimus as the main agents for immunosuppression. Any patient experiencing pathologically proven graft rejection would have toripalimab discontinued, followed by treatment with methylprednisolone and/or mycophenolate sodium enteric-coated tablet or tacrolimus/cyclosporine.
The primary objective of the study was to compare the graft rejection rate for liver (PD-L1–negative graft) transplant recipients receiving PD1 inhibitors to historical controls (liver transplant recipients with unknown PD-L1 status). Assuming the rejection rate in unselected liver transplant patient is 35% (P0), a sample size of 20 achieves 86.7% power to detect a difference (P1-P0) of −.25 using a one-sided binomial test. P1 is the ture rejection rate under the alternative hypothesis. The target significance level is 0.05. The actual significance level achieved by this test is 0.04.
Results
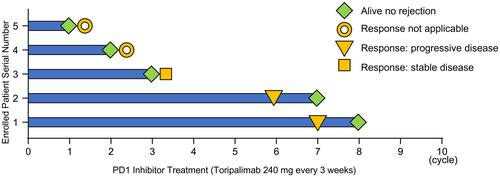
As of January 2020, 18 patients with posttransplant tumor recurrence received screening biopsies. A total of 14 patients received liver transplantation due to HCC, 2 patients had ICC, and 2 patients had mixed HCC and ICC. Among all the screened patients, 50% of patients had positive PD-L1 expression in the graft (Supporting Table 1). Among the 9 patients with negative PD-L1 expression, 5 patients were enrolled into the current study and received treatment with anti-PD1 therapy (Fig. 1). The other 4 patients were excluded due to bowel obstruction, gastrointestinal bleeding, or because they declined participation.

The average age of the 5 patients enrolled in this trial was 55.8 years (46-66 years), with a duration of anti-PD1 treatment ranging from 1 to 8 cycles (Fig. 2). These 5 patients received liver directed therapy (radiofrequency ablation [RFA] and transcatheter arterial chemoembolization [TACE]), or other systemic therapies as reported in Table 1. None of the patients demonstrated evidence of graft rejection at the time of this report, with a median observation time of 2 months. Except for 2 patients who did not reach the scheduled assessment time, objective treatment responses could be determined for 3 patients, one of which remained stable disease at the last follow up (2 months). The other 2 patients experienced disease progression at 4 and 5 months after anti-PD1 therapy, respectively (Fig. 2). At the time of this report, all 5 patients continue to receive anti-PD1 therapy.
| Screening Number | Study Number | Age, years | Sex | Disease | Transplant Date | Recurrence Date | Site of Recurrence | Treatment Prior to Anti-PD1 |
|---|---|---|---|---|---|---|---|---|
| 10 | 1 | 59 | Male | ICC | 18 June 2017 | 29 October 2018 | Liver | AG [nab-paclitaxel (Celgene Corporation, NJ) and gemcitabine (Eli Lilly Company, Indianapolis, IN)], tegafur, and tomotherapy |
| 13 | 2 | 46 | Male | HCC | 25 September 2018 | 18 June 2019 | Lung | Sorafenib and lenvatinib |
| 14 | 3 | 46 | Male | HCC | 13 July 2017 | 27 September 2018 | Liver, peritoneum, and lung | TACE, PEI, resection, sorafenib, lenvatinib (Eisai Co. Ltd, Tokyo, Japan), and Avastin (Roche Co., Basel, Switzerland) |
| 15 | 4 | 62 | Male | HCC | 24 November 2017 | 12 November 2018 | Liver | Sorafenib (Bayer Co., Leverkusen, Germany), lenvatinib, TACE, and RFA with PEI |
| 18 | 5 | 66 | Male | HCC | 6 September 2018 | 28 August 2019 | Liver and peritoneum | Sorafenib, lenvatinib, and regorafenib (Bayer Co., Leverkusen, Germany) |
| 11* | — | 62 | Female | HCC | 7 February 2018 | 18 July 2019 | Lung and peritoneum | Lenvatinib |
- * This patient was treated off-study at her request.
A single patient who was screened for the study and found to have positive PD-L1 expression in the graft biopsy went on to receive anti-PD1 therapy. Although she was not eligible for this study, she had exhausted other potential effective options and strongly requested therapy. After being fully informed of the significant risks associated with anti-PD1 therapy, the patient was treated with toripalimab off protocol. This patient developed pathologically confirmed graft rejection 7 days after PD1 inhibitor treatment. Although she was treated promptly by discontinuing toripalimab and administering systemic steroids and immunosuppressive agents, she died of graft rejection and liver failure 146 days after anti-PD1 therapy.
Discussion
Immune checkpoint molecules are T cell surface proteins that modulate immune function, providing a mechanism for inhibiting an activated response when circumstances warrant this. PD1 is a coinhibitor receptor that interacts with its ligands, PD-L1 and programmed death ligand 2, to suppress antigen-specific T cell activation. PD-L1 is expressed on many cells, including Kupffer cells, other antigen-presenting cells, and tumor cells.(4) Immune checkpoint PD1/PD-L1 plays an important role in immune tolerance in patients with malignancy. This has been clearly demonstrated by those dramatic anti-tumor responses that are sometimes seen in response to agents that block the PD1–PD-L1 interaction.(4)
HCC is considered to be an immunogenic tumor, arising in the setting of chronic inflammation, such as that associated with viral infection, alcoholic cirrhosis, or steatosis. PD-L1 expression is observed in HCC, together with infiltration by CD8-positive T lymphocytes that demonstrate increased levels of PD1 and PD-L1, suggesting that this relationship mediates immune tolerance to HCC.(1) PD-L1 expression is present in 20%-80% of HCCs, and elevated PD-L1 expression levels correlate with HCC stage and with poor prognosis.(1) Initial phase 1 and 2 studies of anti-PD-L1 and anti-PD1 antibodies have shown durable objective response rates of 16%-20% in patients with advanced HCC.(1, 2) Although phase 3 studies have not yet achieved primary endpoints, the enthusiasm for anti-PD1 treatment of HCC has not weakened, and a number of trials are in progress testing combinations of immune checkpoint inhibitors with tyrosine kinase inhibitors (eg, sorafenib, lenvatinib), cytotoxic chemotherapy, or mammalian target of rapamycin inhibitors.(1, 2) Cholangiocarcinoma has not been extensively examined, but studies with relatively small sample numbers show PD-L1 expression in 9%-72% of tumors and 46%-63% of tumor-infiltrating immune cells.(5) Patient reports have described significant anti-tumor responses for treatment of advanced ICC with anti-PD1–based immunotherapy plus lenvatinib, and several phase 1 and 2 trials of this and similar regimens are underway.(5) These results have made checkpoint-directed immunotherapy an important new direction for therapeutic development in HCC and ICC. Our preliminary results also showed that 3 patients with at least one time of tumor assessment had 2-5 months of progression-free survival from anti-PD1 therapy, though these findings need to be verified in this clinical study with sufficient follow-up time.
At the time of this report, a review of the available literature shows a total of 17 reported cases of patients following liver transplantation receiving anti-PD1 therapy,(3) with 6 (35%) patients developing treatment-associated graft rejection (Supporting Table 2). In the clinical settings of liver transplantation, PD-L1 is expressed by hepatocytes, cholangiocytes, and along the sinusoids in posttransplant liver allografts.(4) PD-L1 expression is probably up-regulated by proinflammatory cytokines, such as interferon γ, tumor necrosis factor α, and interleukin 6.(4) Graft PD-L1 status was reported for 8 of these patients. Four patients showed positive PD-L1 expression in the graft, and each of these patients developed graft rejection. Rejection was not encountered in any of the 4 patients whose graft lacked PD-L1 expression. The current study reports an additional 5 patients without rejection who lacked graft PD-L1 expression. Compared with the traditional graft rejection rate of up to 35%, our results show that transplant recipients whose grafts are PD-L1 negative as a screening standard for the use of PD1 inhibitor will effectively avoid the incidence of graft rejection after liver transplantation. Our experience also adds another case to those patients with positive PD-L1 expression in the graft who developed rejection following anti-PD1 therapy. It is well acknowledged that PD1/PD-L1 immune checkpoint plays a critical role in induction and maintenance of immune tolerance.(4) Anti-PD1 inhibitor-based ICB usually results in immune overactivation and graft rejection after liver transplantation. Importantly, our findings indicate that determination of graft PD-L1 status prior to PD1 inhibitor therapy may predict treatment-associated risk of transplant rejection.
Solid organ transplantation is used to treat a range of conditions, and it has become increasingly common over recent years, with a total of 36,528 transplants in the United States in 2018. Unfortunately, patients receiving a solid organ transplant have a 2- to 5-fold higher risk of developing malignancy, particularly nonmelanoma skin cancer, non-Hodgkin lymphoma, and liver cancers, than the general population due to immunosuppression.(3, 4) These individuals also develop common malignancies at a rate similar to the nontransplant population. For a significant number of these tumors, immunotherapy utilizing checkpoint inhibitors has become the standard of care. It is therefore important to determine whether and under what conditions agents such as anti-PD1 antibody can be administered to transplant recipients. Our results indicate that graft PD-L1 expression may be an important biomarker for the safety in using anti-PD1 agents in patients with solid organ transplantation. The exact mechanism that the intragraft PD-L1–negative patients will not develop graft rejection after anti-PD1 therapy remains to be uncovered. PD1/PD-L1 immune checkpoint plays a critical role in induction and maintenance of immune tolerance in patients with positive PD-L1. But, in patients with negative PD-L1, some undetermined factors, such as B- and T-lymphocyte attenuator, CD160, erpesvirus entry mediator, and LIGHT (a ligand for the lymphotoxin [LT] beta receptor) probably play an important role in the maintenance of immune tolerance.(4) Admittedly, our study has limitations, such as the small sample size and the short follow-up time. This possibility should continue to be rigorously studied because a large number of patients stand to benefit from immunotherapy used to treat tumor recurrence or secondary malignancy.