Deficits in Muscle Strength and Physical Performance Influence Physical Activity in Sarcopenic Children After Liver Transplantation
Abstract
Sarcopenia is a muscle disease characterized by reduced skeletal muscle mass (SMM), muscle strength, and physical performance. Reduced SMM has been identified in children after liver transplantation (LT), but no information related to muscle strength/physical performance or lifestyle factors contributing to sarcopenia is available. We hypothesized that sarcopenia, as determined by measures of SMM, muscle strength, and physical performance, is highly prevalent in children after LT and is related to poor diet quality (DQ) and physical inactivity. A cross-sectional study in post-LT children (n = 22) and age-matched healthy controls (n = 47) between the ages of 6 and 18 years examining body composition (dual energy X-ray absorptiometry and multiple skinfold), measures of muscle strength (handgrip, sit-to-stand, and push-ups), physical performance (6-minute walk test and stair climb test), diet (3-day food intake), and physical activity (accelerometer) was conducted. Low muscle strength/physical performance and SMM (SMM z scores ≤−1.5) were defined by values 2 standard deviations below the mean values for age- and sex-matched controls. Sarcopenia occurred in 36% of children who underwent LT, and they had significantly lower scores for muscle strength (sit-to-stand and push-up tests) and physical performance (stair climb test) than controls (P < 0.05). Deficits in physical performance in children with sarcopenia were predominantly revealed by longer stair climbing times (P = 0.03), with no differences in other muscle tests. Low SMM, muscle strength, and physical performance were associated with a lower amount of time spent in fairly and very active physical activity, but no associations with DQ were found. Sarcopenia is highly prevalent in children after LT and is related to lower moderate-to-vigorous physical activity. Development of effective rehabilitation strategies to treat sarcopenia are needed in post-LT children.
Abbreviations
-
- 6MWT
-
- 6-minute walk test
-
- ALD
-
- acute liver disease
-
- BA
-
- biliary atresia
-
- BMI
-
- body mass index
-
- BMI-z
-
- body mass index z score
-
- CST
-
- corticosteroid therapy
-
- DQ
-
- diet quality
-
- DXA
-
- dual energy X-ray absorptiometry
-
- ESLD
-
- end-stage liver disease
-
- FFM
-
- fat-free mass
-
- FFMI
-
- fat-free mass index
-
- FFMI-z
-
- fat-free mass index z scores
-
- HC
-
- heathy control
-
- height-z
-
- height z score
-
- ICC
-
- intraclass correlation coefficient
-
- IQR
-
- interquartile range
-
- LT
-
- liver transplantation
-
- METs
-
- metabolic equivalents
-
- MMF
-
- mycophenolate mofetil
-
- MUFA
-
- monounsaturated fatty acids
-
- NS
-
- not significant
-
- PUFA
-
- polyunsaturated fatty acids
-
- REM
-
- rapid eye movement
-
- SD
-
- standard deviation
-
- SMM
-
- skeletal muscle mass
-
- SMM-z
-
- skeletal muscle mass z scores
-
- SPO2
-
- peripheral capillary oxygen saturation
-
- VO2 max
-
- maximum rate of oxygen consumption measured during exercise
-
- weight-z
-
- weight z score
Malnutrition is prevalent in children and adults with end-stage liver disease (ESLD) awaiting liver transplantation (LT).1 Common factors influencing malnutrition include alterations in nutrient utilization, dietary intake, physical debilitation, hypermetabolism, and chronic inflammation.1 A common comorbid condition of malnutrition that has been identified in adults with ESLD is a condition called sarcopenia.2 Sarcopenia is a muscle disease, characterized by reduced muscle strength, loss of skeletal muscle mass (SMM), and low physical performance.3 Sarcopenia in adults with ESLD has been associated with increased risk of mortality and morbidity, such as increased length of hospital stay, postoperative complications, and risk for cardiometabolic dysregulation.4, 5 In contrast, in pediatrics, sarcopenia has only been recently identified in children with liver disease before and after LT and other disorders, such as inflammatory bowel disease.1, 6-12 Sarcopenia after LT has been reported to occur in up to 40% of children and has been associated with reduced rates of growth, prolonged perioperative length of stay, ventilator dependency, and rehospitalization in the longer term.9 However, the definition of sarcopenia in children has not been well defined.11 The interpretation of sarcopenia in pediatrics has been limited to SMM deficiency without the consideration of alterations in muscle strength or physical performance, which are important features in adult sarcopenia definitions.3, 7, 9, 10 The European Working Group on Sarcopenia in Older People defines sarcopenia as having reduced muscle strength accompanied by reduced SMM and/or physical performance (Fig. 1).3

There are several factors that may influence the expression of sarcopenia in children after LT. These factors include the use of immunosuppressive drugs (eg, tacrolimus, corticosteroids) that may adversely impact protein metabolism, pre-LT nutritional status, and lifestyle factors (diet and physical inactivity) that may contribute to suboptimal nutritional status and skeletal muscle function.1, 13, 14 In adults, reduced protein and vitamin D intakes have been identified as important dietary factors that contribute to sarcopenia risk.1 A recent study in newly diagnosed children with inflammatory bowel disease identified suboptimal vitamin D status to be more prevalent in those with sarcopenia.8 Poor diet quality (DQ), physical inactivity, and reduced aerobic fitness levels have frequently been observed in children after LT, which also contributes to sarcopenia risk.13, 15, 16
There is a need to explore the prevalence of sarcopenia in children after LT on the basis of a broader definition of sarcopenia and to evaluate the contribution of modifiable lifestyle risk factors to sarcopenia risk. The study objectives were, first, to describe the prevalence of sarcopenia in children after LT using a definition of sarcopenia that includes the evaluation of fat-free mass (FFM), muscle strength, and physical performance, and second, to describe the lifestyle factors (diet and physical activity) associated with sarcopenia prevalence. We hypothesized that sarcopenia is highly prevalent in children after LT and is related to poor DQ and physical inactivity.
Patients and Methods
We prospectively recruited participants aged 6-18 years (n = 22) from the Pediatric Liver Transplant Clinic at the Stollery Children’s Hospital who had undergone LT a minimum of 1 year prior and presented with normal liver allograft function and no acute organ rejection within 3 months after LT. The exclusion criteria were participants <6 years old with any known episodes of acute rejection within the past 3 months or with bone or muscular disorders/injuries that precluded the ability to participate in muscle function assessments or who underwent LT due to an inborn error of metabolism. Age-matched healthy controls (n = 47) were recruited from the community. Selection bias was minimal because 2 (8%) of 24 patients who met the inclusion criteria declined participation due to the large geographical distance from the center. Informed consent and assent were obtained from participants and parents prior to study entry. This study was approved by the Human Research Ethics Board, University of Alberta (number Pro00076244).
Primary outcomes included body composition measurements using dual energy X-ray absorptiometry (DXA; Hologic QDR 4500A/Apex System 2.4.2, Hologic Inc., Waltham, MA), multiple skinfolds, and 5 muscle function tests assessing muscle strength and physical performance. Demographic data (eg, sex, age, age at LT), etiology of LT, immunosuppression, laboratory data (eg, liver function test, tacrolimus/sirolimus trough level, 25-hydroxy vitamin D, urea, creatinine, estimated glomerular filtration rate17), anthropometric data (eg, weight, height, and body mass index [BMI]) were collected at LT assessment, LT, and at time of study.
Definition of Sarcopenia
- Probable sarcopenia—low muscle strength assessed by handgrip, sit-to-stand, or push-up tests.
- Sarcopenia—low muscle strength plus low muscle quantity.
- Severe sarcopenia—low muscle strength plus low muscle quantity plus low physical performance assessed by the 6-minute walk test (6MWT) or stair climb test.
Low muscle strength and physical performance were defined by values 2 standard deviations (SDs) below the mean values for age- and sex-matched healthy controls (HCs). Low muscle mass was defined as skeletal muscle mass z scores (SMM-z) ≤−1.5 for LT participants (DXA). Low FFM was defined by using fat-free mass index (FFMI; fat-free mass index z score [FFMI-z] ≤−1.5) in both groups (skinfold measure).
Body Composition
Body composition was evaluated using 2 methods: multiple skinfold measures and DXA. Because of ethical restraints related to radiation exposure associated with DXA, only LT children underwent DXA, which is performed as a part of routine clinical care. Total and segmental body composition for fat mass and lean soft tissue mass (absolute and z scores) were reviewed. SMM was calculated against age- and sex-matched normative data, and SMM-z were determined.18 Low muscle quantity was defined as SMM-z ≤−1.5.
Skinfold measurement (at the triceps, biceps, subscapular, supraspinal, suprailiac, abdominal, and calf) was performed by 1 trained investigator (P.H.O.) according to the International Society for the Advancement of Kinanthropometry methodology using a Lange skinfold caliper (Beta Technology, Santa Cruz, CA).19 The intrasubject coefficient of variation for all of the individual skinfolds measured for the LT and HC groups ranged between 1% and 3% (P = 0.78). FFM from 4 skinfolds (at the biceps, triceps, subscapular, and suprailiac) were calculated according to a predictive equation.20 A Bland-Altman plot was used to assess agreement in FFM determinations between DXA and multiple skinfold measures.21 FFMI (FFMI = FFM/height2 [absolute/z score]) was determined according to normative data.22 A FFMI-z score ≤−1.5 was defined as reduced FFM.23
Measures of Muscle Strength and Physical Performance
Muscle strength was assessed by handgrip, bent-knee push-up, and sit-to-stand tests, whereas physical performance was evaluated by the 6MWT and stair climb tests. All muscle tests were performed by 1 trained investigator (P.H.O.) using standardized protocols that indicated the order of test and rest time (1 minute) between muscle test repetitions.11 The 5 muscle tests were demonstrated by the investigator, followed by practice by the participant to ensure that the technique was consistent and performed accurately. Test and retest reliability of each muscle test was performed in an independent sample of controls (n = 9) on 2 separate occasions within a median of 0.9 weeks (interquartile range [IQR], 0.7-1.1 weeks). Reliability testing for muscle tests was evaluated using intraclass correlation coefficient (ICC), where ICC <0.50, 0.50-0.75, >0.75-0.90, >0.90 were indicative of poor, moderate, good, and excellent reliability, respectively.24
Handgrip strength was assessed using a JAMAR Hand Dynamometer (Patterson Medical, Mississauga, ON, Canada) according the standardized methodology.25 Three measurements were taken for each hand (dominant and nondominant hands) in an alternative manner with brief breaks (<1 minute) between testing. The average of 3 scores was used to evaluate handgrip strength.
Prior to testing, a standardized picture of a bent-knee push-up was shown to participants, followed by a 3-minute warm-up session (with forward and backward arm circles, biceps stretch, and triceps stretch; 1 minute each).26 Participants were asked to be face down on a soft nonmovable mat, with hands placed underneath the shoulders, elbows slightly apart, arms straight, and knees touching the mat. Participants were given 30 seconds to complete as many push-ups as possible, and the number of executed push-ups was recorded.
The sit-to-stand test was done on a straight back chair without armrests (with a seat 43-cm high). Participants were asked to sit on the chair with hips and knees flexed and feet flat on the floor. Participants were instructed to fold their arms across their chests, stand up, and sit down as quickly as possible in 30 seconds, while ensuring the full extension of the trunk and lower extremities.27, 28 In younger children who were unable to position their feet directly on the floor (n = 4 in the LT group and n = 8 in the control group), a modification was used by the inclusion of a stool (with a seat height ~20 cm). The number of repetitions in 30 seconds was recorded, and the average number from 2 measurements was taken. No differences were observed in the number of repetitions between children using either method (P < 0.05).
The 6MWT was performed on a 30-meter indoor track on a flat and hard surface according to standard methodologies.29 Blood pressure, heart rate, peripheral capillary oxygen saturation (SPO2), and perceived exertion (using a modified Borg scale) were measured and rated before and after the test. The walking distance (in meters) was measured at the end of the test.
For the stair climb test, participants were instructed to walk up and down the staircases (12 steps, 17-cm high) as fast as they could without running, jumping, or skipping steps from a standardized starting point. Participants were free to choose to use the handrail for support when ascending and descending the staircases. Use of the handrail was recorded by the investigator (handrail use, n = 4 in the LT group versus n = 2 in the control group; P = 0.07). The time taken (seconds) to walk up, turn around on the top platform, and then walk down the stairs with both feet touching the starting point was recorded.27, 28, 30 A shorter time taken to complete the test represented better physical performance.30
Anthropometric Data
Weight and height to the nearest 0.1 kg/cm were recorded by trained personnel with participants wearing light clothing and no shoes. BMI was given by kg/m2. Weight z score (weight-z), height z score (height-z), and body mass index z score (BMI-z) were determined based on World Health Organization standards.31 Weight-z and height-z in LT children were examined at LT assessment, LT, and at time of study.
Dietary Intake
Dietary intake was assessed using 3-day food records (2 weekdays and 1 weekend day). Food records were analyzed using food processor software (ESHA Research 2015; version 10.15.41, Salem, OR). DQ was determined using the healthy eating index for children,32 where scores of <60, 60-80, >80 were referred to as poor, needs improvement, and good DQ, respectively. Underreporting or overreporting of intake was determined by the ratio of energy intake to basal metabolic rate, where energy intake/basal metabolic rate values outside the 95% confidence intervals were indicative of misreporting.33
Physical Activity: Accelerometry
Habitual physical activity (ie, number of steps, distance walked, number of floors climbed, active minutes, and heart rate) and sleep patterns (ie, total sleep/stages) were assessed using an accelerometer (Fitbit Charge 2, FitBit Inc, San Franciso, CA). Participants were instructed to wear the accelerometer around their wrist for 2 weeks (24 hours/day). Hourly reminders to move given by the accelerometer were turned off to ensure that habitual physical activity was captured. Accelerometers were synced with an electronic device to the Web-based server, and data were downloaded by investigators. The number of steps, number of active minutes (ie, sedentary, lightly active, moderately active, and very active minutes), total sleep hours and sleep stages (ie, percentage of time awake or in rapid eye movement [REM], light, or deep stages), and resting heart rates were compared with normative data.34-37 Moderate-to-vigorous physical activity minutes were determined using the sum of fairly active and very active minutes and were compared with current guidelines.34 The cardio fitness score is an estimate of the maximum rate of oxygen consumption measured during exercise (VO2 max), which was predetermined by the device using resting heart rate, age, sex, and weight and was classified into poor (score 1) to excellent (score 6) fitness levels based on published data.38 As an accelerometer only records the heart rate if it is placed on a participant’s wrist, adherence rates were determined by the presence of heart rate data for at least 20 hours/day of wear time.39
Statistical Analysis
Data analysis was completed using SAS, version 9.4 (SAS Institute Inc., Cary, NC). Data were expressed as mean ± SD or median and IQR, unless otherwise specified. The Shapiro-Wilk test was conducted to assess the normality of distribution. Independent t or Kruskal-Wallis tests with post hoc Dunn tests were conducted to compare the differences between controls and LT children for body composition, muscle strength, physical performance, and anthropometric and demographic data. Chi-square and Fisher’s exact tests were used to measure differences in categorical data. Multivariate analysis (analysis of variance) was conducted to examine the potential interrelationships between measures of muscle mass (SMM-z or FFMI-z), tests of muscle strength, physical performance, and lifestyle factors (eg, diet and physical activity). A P value <0.05 was considered significant.
Results
Demographic, Anthropometric, and Clinical Characteristics and Body Composition
Demographic and anthropometric characteristics are presented in Table 1. The majority of children had weight-z, height-z, and BMI-z within normal reference ranges (>95%). LT children with low SMM-z (≤–1.5) had lower weight-z than those with SMM-z within normal reference ranges (>1.5). There were no significant differences in demographic and clinical characteristics (age, sex, liver disease type, and height-z, weight-z, or BMI-z at LT), laboratory parameters, or immunosuppressive therapies (eg, type, dose, or trough levels) between children with SMM-z above or below −1.5 (P > 0.05). All LT children had liver biochemistries within normal reference ranges (ie, aspartate aminotransferase, alanine aminotransferase, gamma glutamyltransferase, bilirubin, albumin, international normalized ratio, and partial thromboplastin time; data not shown). The majority of LT children had height-z (80%) and weight-z (100%) within the normal reference ranges, which is indicative of normal growth patterns. No differences in adipose indices were noted in the LT children with or without sarcopenia (Supporting Table 1).
| Variables | Cohorts by Transplantation | LT Cohort by Sarcopenia Prevalence*, *, * | ||||
|---|---|---|---|---|---|---|
| LT Group (n = 22) | Control Group (n = 47) | P Value†, †, † | Sarcopenia Group (n = 8) | Nonsarcopenia Group (n = 14) | P Value†, †, † | |
| Sex | NS | NS | ||||
| Male | 11 | 20 | 6 | 5 | ||
| Female | 11 | 27 | 2 | 9 | ||
| Age, years | 12.1 ± 3.5 | 12.2 ± 3.5 | NS | 11.1 ± 3.5 | 12.7 ± 3.5 | NS |
| z scores at time of study | ||||||
| Weight-z | 0.28 ± 0.85 | 0.11 ± 1.03 | NS | −0.24 (−0.51 to 0.63) | 0.53 (−0.13 to 0.68) | NS |
| Height-z | −0.19 ± 1.01 | 0.44 ± 1.04 | 0.02 | −0.30 ± 1.27 | −0.13 ± 0.87 | NS |
| BMI-z | 0.52 ± 0.98 | −0.15 ± 1.05 | 0.01 | 0.37 ± 1.12 | 0.60 ± 0.92 | NS |
| z scores at LT | ||||||
| Weight-z | −0.34 ± 1.09 | — | — | −0.50 ± 1.00 | −0.25 ± 1.17 | NS |
| Height-z | −0.36 ± 1.63 | — | — | −0.77 ± 1.78 | −0.01 ± 1.53 | NS |
| BMI-z | 0.11 ± 0.85 | — | — | 0.20 ± 0.60 | 0.02 ± 1.06 | NS |
| Age at LT, years | 1.5 (0.9-8.0) | — | — | 4.6 (1.0-9.4) | 1.3 (0.7-3.8) | NS |
| Time after LT, years | 8.0 ± 4.4 | — | — | 5.7 ± 3.5 | 9.1 ± 4.5 | NS |
| Liver etiology | NS | |||||
| BA | 12 (55) | — | — | 5 (62) | 7 (50) | |
| ALD | 2 (9) | 1 (12) | 1 (7) | |||
| Others‡, ‡, ‡ | 8 (36) | 2 (25) | 6 (43) | |||
| Immunosuppression§, § | NS | |||||
| Tacrolimus | 19 (86) | — | — | 7 (88) | 12 (86) | |
| Sirolimus | 1 (5) | 0 | 1 (7) | |||
| Tacrolimus plus MMF | 1 (5) | 0 | 1 (7) | |||
| Tacrolimus plus CST | 1 (5) | 1 (12) | 0 | |||
| Re-LT||, || | 3 (14) | — | — | 1 (12) | 2 (14) | NS |
| Number of rejections | 0 (0-1) | — | — | 0 (0-1) | 0 (0-0) | NS |
| Tacrolimus trough, µg/L | 4.4 (3.5-4.8) | — | — | 4.4 (3.9-4.8) | 3.8 (3.1-4.8) | NS |
NOTE:
- Data are given as n (%), mean ± SD, or median (IQR).
- * Sarcopenia refers to probable sarcopenia, defined as low muscle strength (low handgrip/sit-to-stand/push-ups) based on revised European consensus on sarcopenia guidelines from Cruz-Jentoft et al.3
- † P values <0.05 are considered statistically significant.
- ‡ Other liver etiologies: children with probable sarcopenia (Crigler-Najjar syndrome, n = 1; alpha-1-antitrypsin deficiency, n = 1); children without probable sarcopenia (Crigler-Najjar syndrome, n = 2; primary sclerosing cholangitis, n = 2); progressive familial intrahepatic cholestasis type 3, n = 1; and unknown, n = 1.
- § Immunosuppression: n = 1 on sirolimus 1 mg once daily with a level of 3.7 µg/L; n = 1 on tacrolimus 4 mg once daily and MMF 8.5 mL twice daily; and n = 1 on tacrolimus 0.8 mg twice daily and prednisolone 15 mg daily. All tacrolimus trough levels were within the therapeutic range (3-5 µg/L).
- || Re-LT indicates liver retransplantation in children with first graft failure.
Muscle Strength and Physical Performance
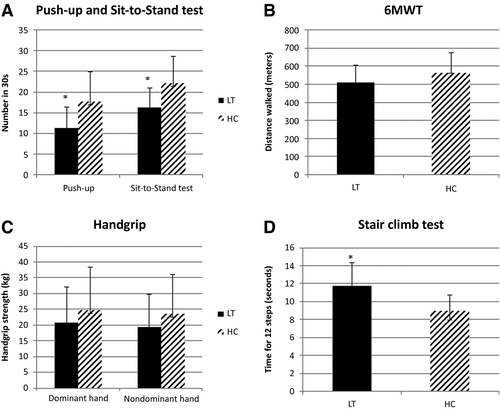
Figure 2 illustrates the differences in muscle strength and physical performance between the LT and control groups. LT children had lower mean performance in all 5 muscle tests than HC children with significant differences observed in 3 out of 5 tests (ie, push up, sit-to-stand, and stair climb tests). There were no differences (pretest versus posttest) in blood pressure, heart rate, SPO2, and perceived exertion (Borg scale) related to the 6MWT in LT versus control children and in LT children with or without sarcopenia. With the exception of the sit-to-stand test (ICC, 0.66; moderate), test-retest reliability of all the other muscle tests showed good to excellent repeatability (ICC, 0.84-0.99).
Sarcopenia Prevalence
The prevalence of sarcopenia was 36%. Out of the 8 children with sarcopenia, 2 children were classified as severely sarcopenic, with the remaining being classified as probable. None of the controls had sarcopenia. Of the LT children, 29% had low SMM defined by SMM-z ≤–1.5, and 55% had low muscle strength and low physical performance. Deficits in physical performance in children with sarcopenia were predominantly revealed by longer stair climbing times (P = 0.03), with no differences in 6MWT, sit-to-stand test, and number of push-ups performed. Handgrip was significantly higher in children with FFMI-z >–1.5 versus FFMI-z ≤–1.5 (27.9 ± 13.7 kg versus 17.8 ± 9.3 kg; P = 0.02).
Lifestyle Factors and Sarcopenia
Diet
The majority of LT (68%) and control (66%) children had DQ characterized by needs improvement, while 27% of LT children and 28% HC had intake characterized by poor DQ. There were 5% and 6% of children who had good DQ in the LT and control groups, respectively (Table 2). LT children with sarcopenia had lower fat and monounsaturated and polyunsaturated fatty acid intakes and higher protein intake than children without sarcopenia (2.4 ± 0.6 g/kg/day versus 1.7 ± 0.4 g/kg/day, respectively; P = 0.005). These differences were independent of age, sex, liver disease diagnosis, or misreporting of dietary intake.
| Variables | Cohorts by Transplantation | LT Cohort by Sarcopenia Prevalence*, *, * | ||||
|---|---|---|---|---|---|---|
| LT Group (n = 22) | Control Group (n = 47) | P Value†, †, † | Sarcopenia Group (n = 8) | Nonsarcopenia Group (n = 14) | P Value†, †, † | |
| Protein, g | 37 (33-43) | 39 (35-45) | NS | 42 ± 8 | 36 ± 7 | NS |
| Carbohydrate, g | 130 ± 15 | 133 ± 15 | NS | 135 ± 18 | 128 ± 13 | NS |
| Fat, g | 37 ± 7 | 35 ± 7 | NS | 33 ± 6 | 39 ± 6 | 0.03 |
| Saturated fat, g | 13 ± 3 | 12 ± 3 | NS | 13 ± 3 | 14 ± 3 | NS |
| MUFA, g | 13 ± 3 | 12 ± 3 | NS | 11 ± 3 | 14 ± 3 | 0.04 |
| PUFA, g | 6 (5-8) | 6 (5-8) | NS | 5 ± 1 | 7 ± 2 | 0.02 |
| Sugar, g | 55 ± 16 | 44 ± 14 | 0.01 | 56 ± 12 | 54 ± 19 | NS |
| Dietary vitamin D, IU | 97 (67-164) | 75 (43-107) | NS | 160 ± 86 | 98 ± 76 | NS |
| Participants on vitamin D supplement | 17 (77) | 15 (32) | <0.001 | 7 (88) | 10 (71) | NS |
| Total vitamin D including supplement, IU | 509 (209-747) | 95 (52-332) | <0.001 | 650 ± 282 | 390 ± 272 | 0.05 |
| Dietary calcium, mg | 563 ± 209 | 467 ± 162 | 0.04 | 640 ± 128 | 519 ± 237 | NS |
| Participants on calcium supplement | 3 (14) | 5 (11) | NS | 1 (12) | 2 (14) | NS |
| Total calcium including supplement, mg | 575 ± 211 | 471 ± 162 | 0.03 | 641 ± 127 | 537 ± 243 | NS |
| DQ score‡ | 65 ± 12 | 66 ± 11 | NS | 68 (64-72) | 66 (58-69) | NS |
NOTE:
- Data are given as mean ± SD, median (IQR), or n (%). All nutrients were expressed on a per 1000 kcal basis, except DQ, which was based on absolute energy intake.
- * Sarcopenia refers to probable sarcopenia, defined as low muscle strength (low handgrip/sit-to-stand/push-ups) based on revised European consensus on sarcopenia guidelines from Cruz-Jentoft et al.3
- † P values <0.05 are considered statistically significant.
- ‡ DQ is a scoring system based on dietary adequacy, moderation, and variety. DQ scores range from 0 to 100, in which <60, 60-80, >80 referred to poor, need improvement, and good DQ, respectively.
Habitual Physical Activity and Sleep
Accelerometers were worn for 13 (12-14) days in excess of 20 hours/day. Table 3 represents the habitual physical activity and sleep data between LT groups and between LT children with and without sarcopenia. Time spent in moderate-to-vigorous physical activity and average fairly active minutes and very active minutes were positively correlated with measures of muscle strength (handgrip; P < 0.001) and inversely correlated with FFMI-z (P < 0.05). In particular, FFMI-z <–1.5 was associated with less time spent in moderate-to-vigorous physical activity (15.6 ± 16.7 versus 39.5 ± 30.5 minutes/day; P = 0.006), fairly active physical activity (12.5 ± 13.5 versus 28.3 ± 22 minutes/day; P = 0.002), and very active physical activity (3.1 ± 0.6 versus 11.2 ± 11.5 minutes/day; P = 0.002) than children with FFMI-z >–1.5. This difference occurred in both groups.
| Variables | Cohorts by Transplantation | LT Cohort by Sarcopenia Prevalence*, *, * | ||||
|---|---|---|---|---|---|---|
| LT Group (n = 18) | Control Group (n = 47) | P Value†, †, † | Sarcopenia Group (n = 8) | Nonsarcopenia Group (n = 10) | P Value†, †, † | |
| Physical activity‡, ‡, ‡ | ||||||
| Number of steps/day | 9236 ± 2609 | 10,011 ± 2706 | NS | 8447 ± 2402 | 9867 ± 2715 | NS |
| Distance walked, km/day | 5.1 (4.1-6.9) | 6.4 (4.9-7.5) | NS | 5.3 ± 2.4 | 5.8 ± 1.6 | NS |
| Number of floors climbed/day | 9 (5-16) | 11 (6-16) | NS | 8 (5-10) | 12 (5-17) | NS |
| Resting heart rate, beats per minute | 79 ± 8 | 69 ± 5 | <0.0001 | 80 ± 9 | 77 ± 7 | NS |
| Sedentary time, minutes/day§, § | 622 ± 104 | 604 ± 117 | NS | 653 ± 112 | 596 ± 95 | NS |
| Active time, minutes/day||, || | ||||||
| Lightly active | 299 ± 87 | 297 ± 77 | NS | 275 ± 75 | 318 ± 95 | NS |
| Fairly active | 16 (4-19) | 15 (7-36) | NS | 11 ± 9 | 15 ± 11 | NS |
| Very active | 2 (0-7) | 2 (1-11) | NS | 2 (0-17) | 3 (0-7) | NS |
| Moderate-to-vigorous activity¶ | 21 (5-30) | 24 (9-48) | NS | 20 ± 19 | 19 ± 13 | NS |
| Cardio fitness scores# | 3 (2-4) | 4 (3-6) | 0.03 | 3 ± 2 | 3 ± 1 | NS |
| Participants meeting age-specific recommendations, %** | ||||||
| Number of steps/day | 11 | 22 | NS | 12 | 10 | NS |
| Moderate-to-vigorous physical activity minutes/day | 0 | 16 | NS | 0 | 0 | — |
| Total sleep | 6 | 9 | NS | 0 | 10 | NS |
| Sleep stages†† | ||||||
| Awake | 94 | 100 | NS | 100 | 89 | NS |
| REM | 100 | 98 | NS | 100 | 100 | — |
| Light sleep | 100 | 100 | — | 100 | 100 | — |
| Deep sleep | 88 | 86 | NS | 75 | 100 | NS |
NOTE:
- Data are given as mean ± SD or median (IQR) unless otherwise noted. Data are available for n = 18 participants in the LT group.
- * Sarcopenia refers to probable sarcopenia, defined as low muscle strength (low handgrip/sit-to-stand/push-ups) based on revised European consensus on sarcopenia guidelines from Cruz-Jentoft et al.3
- † P values <0.05 are considered statistically significant.
- ‡ Adherence to wearing the accelerometer device was determined using heart rate data ≥20 hours/day. Any days within 2 weeks with heart rate data <20 hours were eliminated from the data analysis.
- § Sedentary time refers to inactivity for 10 consecutive minutes.
- || Active minutes (lightly, fairly active, and very active) were recorded if participants participated in continuous activities at or above 3 metabolic equivalents (METs) (indicator of exercise intensity based on heart rate) for 10 minutes.
- ¶ Moderate-to-vigorous physical activity minutes/day refers to fairly active minutes plus very active minutes compared with the guideline.
- # Cardio fitness score is an estimate of VO2 max predetermined by the accelerometer using resting heart rate, age, sex, and weight. Score classification: 1 = poor, 2 = fair, 3 = average, 4 = good, 5 = very good, and 6 = excellent.
- ** Recommendations: steps, ≥12,000 steps/day (Colley et al.35); moderate-to-vigorous physical activity, ≥60 minutes/day (Canadian Society for Exercise Physiology34); and total sleep for patients 6-13 years, 540-660 minutes/day and for patients 14-17 years, 480-600 minutes/day (Hirshkowitz et al.36).
- †† Sleep stages are based on age-specific recommendations (Ohayon et al.37).
No other major differences in physical activity (sedentary) or sleep patterns (total; percentage of light, deep, and REM sleep; or number of awakenings) were observed between groups.
Discussion
This is the first study that has examined the prevalence of sarcopenia in children after LT using definitions that included measures of body composition, muscle strength, and physical performance and that examined lifestyle factors (diet and physical activity) that may contribute to sarcopenia. We confirmed that sarcopenia was highly prevalent in children up to 10 years after LT9 and was characterized by deficits in muscle strength and physical performance, particularly in the lower limbs. In addition, children with sarcopenia had low habitual levels of moderate-to-vigorous physical activity. DQ was poor following LT but was not related to sarcopenia.
Poor DQ in post-LT children is not an unexpected finding. DQ in LT recipients is similar to HCs and often does not meet current dietary guidelines.13, 15 Although suboptimal protein and vitamin D intake has been associated with sarcopenia prevalence in adults, we did not see any associations. Children with sarcopenia had higher protein intake (>2.5 g/kg/day) when compared with children without sarcopenia (1.7 g/kg/day) and were equally sufficient in vitamin D due to the high prevalence of vitamin D supplementation (>95% in LT). However, both groups had protein intakes in excess of recommended protein requirements and had no major differences in the absolute grams of protein consumed. Differences may have been due to the increasing emphasis on protein intake by families with children who experienced protein-energy malnutrition in the pre-LT period. Vitamin D deficiency is known to increase oxidative stress in the skeletal muscle, which leads to mitochondrial dysfunction and muscle atrophy.40 Emerging evidence in the adult population suggests that vitamin D supplementation increases vitamin D receptor concentration in skeletal muscle, increases muscle fiber size, and contributes to myoblast proliferation.40, 41 We have previously shown that branched-chain amino acid requirements are significantly higher in LT recipients when compared with healthy children,42 suggesting that routine branched-chain amino acid supplementation may be an important component of rehabilitation in children with sarcopenia.42
Suboptimal aerobic fitness in children after LT has been well documented, with early muscle fatigue and reduced self-efficacy related to participation in physical activity commonly reported.16, 43 Findings provide preliminary evidence that reductions in muscle strength and physical performance in pediatric sarcopenia may influence physical activity in LT children. In particular, children with sarcopenia spent less time in active physical activity than children without sarcopenia, and levels of active physical activity were related to measures of muscle strength, particularly in the lower limbs. Reductions in lower limb muscle mass would potentially exacerbate physical inactivity potentially posing barriers to participating in routine physical activity. This has important implications for the development of effective rehabilitation strategies to treat sarcopenia in children after LT. Recent evidence in adults with cirrhosis and sarcopenia indicate that the utilization of resistance exercise to treat sarcopenia is a safe, feasible, and effective method to elicit improvements in muscle strength.44-47 Evaluation of this approach as a potential rehabilitation strategy is warranted in children with sarcopenia.
We used multiple skinfold measures to determine FFM in the control and LT groups, and we found a high level of agreement between these 2 measures (data not shown). Although use of multiple skinfold measures is simple and easy to perform, it depends on technical precision and the use of normative equations to calculate FFM.11 In addition, these equations may not be the best measures to determine muscle mass in some clinical populations. Although the use of magnetic resonance imaging or computed tomography has been identified as a gold standard in sarcopenia assessment, those methods have the potential to expose a child to radiation, are expensive, require specialized technical support, and may not be routinely available.1, 11
Age and sex did not have any observable effects on the categorization of sarcopenia based on the functional tests or body composition in this cohort. However, variations in age affect the performance of muscle function tests, suggesting that the inclusion of more than 1 muscle test in sarcopenia diagnosis in children may be important. Our present findings contrast with previous data, where younger (<10 years) female children after LT were at higher risk of developing sarcopenia.9 Data have shown that males have higher lean body mass after puberty than females do,48 which may confer a benefit related to lean mass depletion in older male children. However, controversies exist for lean body mass differences between sexes in the prepuberty period.49, 50 There is a need to develop age- and sex-specific cutoffs for low skeletal muscle and to consider growth in the diagnosis of sarcopenia in children.11 Other factors that may have influenced the study findings include the use of immunosuppressive therapies such as tacrolimus and corticosteroid therapy (CST), which have been shown to influence protein turnover and induce muscle fiber changes that may potentially contribute to changes in muscle strength and performance.1 In particular, tacrolimus was found to up-regulate myostatin expression in skeletal muscle, which is an inhibitor to muscle synthesis and transformations of slow-to-fast twitch muscle fibers.51, 52 Similar to our earlier findings,9 both tacrolimus and CST were not associated with sarcopenia prevalence, likely due to the fact that all patients had stable tacrolimus concentrations that were within targeted therapeutic levels (3-5 µg/L) and only 1 patient with autoimmune hepatitis was on CST. Uniquely, our center implemented a CST minimization protocol in 2007 that has effectively reduced CST use, enabling the study of skeletal muscle physiology in a relatively CST-free environment.
The inclusion of muscle strength and physical performance measures were conferred as strengths in this study. The muscle strength and physical performance measures from the control group were well within the range of published normative data and were indicative of the representativeness of healthy children.28, 30, 53 The reliability testing demonstrated a high concordance with low intrasubject and test-retest variation, suggesting that the testing environment was reproducible and consistent. All muscle tests used were simple to perform, reliable, short in duration, and required minimal equipment. Assessment of physical activity using an accelerometer was an important consideration of examining habitual physical activity. Although the accelerometer used has shown validity and reliability in step counts with comparable heart rate estimates in children, there is a potential for underestimates or overestimates in total sleep time, sleep stages, distance walked, and vigorous physical activity.54-56 The accelerometer also tends to underestimate physical activity in participants who participated in water sport or other physical training that required the removal of the device (eg, soccer and hockey). Despite its limitations, the use of an accelerometer is easy and inexpensive and could be a satisfactory measure rather than self-reporting physical activity levels and sleep patterns.56 In addition, the potential for variations in adherence to wearing the device may be a challenge for children and youth. In this study, participants wore the device for 2 weeks to account for variations in activity due to the day of the week (weekend/weekday). Our cohorts had good compliance rates in excess of 90%.
Our study was limited by a small sample size and participants with wide age range and number of years after LT. Despite a smaller sample size, a post hoc analysis revealed sufficient power (β > 95%) to detect differences in primary outcomes of interest (muscle strength/physical performance) between groups. When adjusted for age (above/below median age of 12.5 years) and years after LT (above/below 7.6 years [median] and ≤10 and >10 years), we did not find any difference in the prevalence of sarcopenia. This is in line with our earlier longitudinal review, where sarcopenia in children occurred 1-10 years after LT.9 Although heterogeneity of liver disease diagnosis may have influenced sarcopenia expression, the majority of children had biliary atresia (BA; >50%), which is typically the most common reason for pediatric LT.
In summary, post-LT children have a high prevalence of sarcopenia, which included deficits in lean mass, muscle mass, and physical performance that was associated with low levels of moderate-to-vigorous physical activity. These findings highlight the importance of examining childhood sarcopenia from a broader perspective beyond muscle quantity measures because variations in routine physical activity may play a large impact on overall muscle development, strength, and physical functioning in children. High-quality randomized control trials examining the impact of different rehabilitation approaches to prevent and treat sarcopenia after LT are warranted in children.45
Acknowledgments
The authors wish to thank Don Breakwell (Medical Imaging Consultants), April Nguyen, Ruby Bhutani, and Amber Hager for their assistance with data collection and data auditing.