Impact of Rifaximin Therapy on Ischemia/Reperfusion Injury in Liver Transplantation: A Propensity Score–Matched Analysis
Abstract
Intestinal microbiota is thought to play an important role in hepatic ischemia/reperfusion injury (IRI) after liver transplantation (LT). Rifaximin, a nonabsorbable antibiotic used to treat encephalopathy, exhibits antibacterial activity within the gut. We report the first study examining the impact of pre-LT rifaximin use on reducing hepatic IRI and inflammatory cell infiltration after LT. This retrospective single-center study included adult LT recipients from January 2013 through June 2016. Patients were divided into 2 groups based on duration of rifaximin use before LT: rifaximin group (≥28 days) and control group (none or <28 days). Patients receiving other antibiotics within 28 days of LT and re-LTs were excluded. Outcomes and messenger RNA (mRNA) expression in the graft were compared by 1:1 propensity score–matching and multivariate analyses. On 1:1 matching (n = 39/group), rifaximin patients had lower postoperative serum transaminase levels and lower early allograft dysfunction (EAD; 10.3% versus 33.3%; P = 0.014). Of the matched patients, 8 patients (n = 4/group) had postreperfusion liver biopsies (approximately 2 hours after reperfusion) available for mRNA analysis. Hepatic expression of CD86 (macrophage marker) and cathepsin G (neutrophil marker) was significantly lower in rifaximin patients than controls (P < 0.05). The multivariate analysis included 458 patients. Rifaximin treatment <28 days was identified as an independent risk factor EAD in all patients and those with high Model for End-Stage Liver Disease (MELD) score (MELD ≥35; n = 230). In conclusion, the propensity score–matched and multivariate analyses suggest a therapeutic role of rifaximin in reducing EAD. Pre-LT rifaximin administration exerted a protective function against early liver injury, potentially by suppressing inflammatory cell activation in the graft.
Abbreviations
-
- ALF
-
- acute liver failure
-
- ALT
-
- alanine transaminase
-
- AST
-
- aspartate transaminase
-
- BMI
-
- body mass index
-
- CI
-
- confidence interval
-
- CIT
-
- cold ischemia time
-
- DCD
-
- donation after circulatory death
-
- D-MELD
-
- donor Model for End-Stage Liver Disease
-
- DRI
-
- donor risk index
-
- EAD
-
- early allograft dysfunction
-
- EtOH
-
- ethanol
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HE
-
- hepatic encephalopathy
-
- IR
-
- ischemia/reperfusion
-
- IRI
-
- ischemia/reperfusion injury
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- mRNA
-
- messenger RNA
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- OR
-
- odds ratio
-
- POD
-
- postoperative day
-
- PT-INR
-
- prothrombin time–international normalized ratio
-
- ROC
-
- receiver operating characteristic
-
- WIT
-
- warm ischemia time
Liver transplantation (LT) is a well-established treatment for patients with end-stage liver disease.1, 2 Hepatic ischemia/reperfusion injury (IRI), resulting from innate immune-driven inflammation response, represents the predominant underlying cause of posttransplant organ dysfunction.3-6 The gut microbiota might be crucial in contributing to hepatic IRI because the liver has a dual blood supply arising from the hepatic artery and the portal vein, which in turn carries all mesenteric venous blood from the gut to the liver.
In humans, gut microbiota are composed of more than 100 trillion largely colon-restricted, autochthonous bacteria, that not only shape gut morphologic features/mucosal immunity but also contribute to the development of systemic inflammatory responses.7-9 A variety of diseases, such as inflammatory bowel disease,10 cardiovascular diseases,11 obesity,12, 13 diabetes,12 colorectal cancer,14 and nervous system diseases,15 are all thought to be affected by gut microbiota. The gut-liver axis, widely implicated in the pathogenesis of liver diseases, is increasingly becoming the focus of basic and clinical research,16, 17 including studies on hepatic IRI in rats,18, 19 and hepatic IRI in mouse LT models, where a correlation was drawn between gut bacterial density and Kupffer cell density, maturation status, and functionality. Hence, suppression of bacterial products might play a therapeutic role in prevention or treatment of hepatic inflammation. One potential therapeutic strategy incorporates antibiotics that can directly act by inhibition of harmful bacterial growth, leading to lower expression of proinflammatory cytokines.17, 20
Rifaximin is a nonabsorbable oral antimicrobial agent that exhibits broad-spectrum activity against both aerobic and anaerobic gram-positive and gram-negative microorganisms within the gut, with a low risk of inducing bacterial resistance.21, 22 First approved in the United States in 2004, rifaximin is now used for treatment of hepatic encephalopathy (HE) in many countries,23 improving survival and reducing the risk of hospitalization and portal hypertension complications.24, 25 Current American Association for the Study of Liver Diseases guidelines recommend rifaximin as an add-on therapy for the prevention of HE recurrence.26 Among candidates awaiting LT, many patients with a history of HE are treated with rifaximin.27, 28 By focusing on the risk of infections in LT recipients, Sun et al.27 showed a protective effect of pre-LT rifaximin use against posttransplant infections with no increase in multidrug-resistant bacterial infections. However, there has been no study linking rifaximin with posttransplant hepatocellular function or early allograft dysfunction (EAD) after LT.
In the present study, we aimed to determine the impact of pretransplant rifaximin use on post-LT (postreperfusion) liver damage and EAD. In addition, we evaluated inflammatory cell infiltration, including neutrophils and macrophages, in postreperfusion liver biopsies because these cells are key players in the development of liver IRI. We hypothesized that pre-LT rifaximin inhibition of harmful bacterial growth within the gut would result in reduced hepatic IRI and improved outcomes.
Patients and Methods
Patient Selection and Data Collection
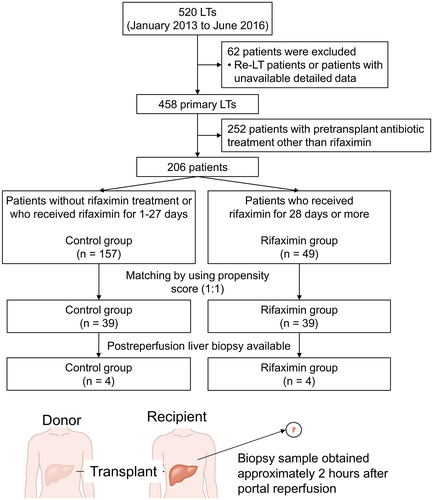
This study was approved by the University of California, Los Angeles institutional review board. Using a prospectively collected database, we performed a retrospective analysis of adult patients (age ≥18 years) who underwent LT from January 2013 through June 2016. In total, 62 patients who underwent re-LT in this period and patients with unavailable data were excluded. Among 458 primary LT patients, 252 patients underwent pre-LT treatment with an antibiotic other than rifaximin. Ultimately, 206 patients were included in the propensity score–matched analysis. All patients received prophylactic antibiotic therapy and immunosuppressive therapy per our institutional protocol during the perioperative period. All liver grafts, procured with standardized techniques from donation after brain death or donation after circulatory death (DCD) donors were stored in cold University of Wisconsin solution prior to implantation. We collected data including rifaximin and other preoperatively administrated antibiotics, recipient pretransplant demographics (age, sex, race, past history, indication for LT, length of pretransplant hospital stay, and Model for End-Stage Liver Disease [MELD] score), donor characteristics (age, sex, race, cause of death, DCD, past history, donor risk index [DRI], and laboratory data), cold ischemia time (CIT), warm ischemia time (WIT), and patient outcomes, including postoperative laboratory data in the first 7 post-LT days. Serum transaminase levels were used as a surrogate marker of hepatocellular injury. EAD was defined by the presence of 1 or more of the following: bilirubin level of ≥10 mg/dL on postoperative day (POD) 7, prothrombin time–international normalized ratio (PT-INR) ≥1.6 on POD 7, or aspartate transaminase (AST) and alanine transaminase (ALT) levels of >2000 IU/L within the first 7 days.
Patients were grouped into the rifaximin or control arm based on the presence or absence of daily rifaximin administration for the optimal duration in days. To identify the optimal duration of rifaximin in days needed to exert a beneficial effect, sensitivity and specificity for prevention of EAD were analyzed by receiver operating characteristic (ROC) curve at multiple time points: 1 day, 5 days, 7 days (1 week), 10 days, 14 days (2 weeks), 21 days (3 weeks), 28 days (4 weeks), 42 days (6 weeks), and 56 days (8 weeks; Supporting Table 1). Rifaximin was administrated at a dose of 550 mg twice daily.
Human Liver Sample Collection and Quantitative Polymerase Chain Reaction Analysis
Protocol Tru-Cut (CareFusion, SanDiego, CA) needle biopsies were obtained intraoperatively from the left lobe approximately 2 hours after portal reperfusion (prior to surgical closure of the abdomen) and snap-frozen, as previously described.29, 30 RNA extracted with RNase Mini Kit (Qiagen, Germantown, MD) was reverse-transcribed into complementary DNA. Quantitative polymerase chain reaction was performed using Quant Studio 3 (Applied Biosystems, Foster City, CA). The primer sequences are listed in Supporting Table 2. The expression of the target gene was normalized to the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical Analysis
Descriptive statistics were reported for the entire study cohort. Categorical variables were summarized as numbers and percentages, and continuous variables were summarized as medians and ranges. Groups were compared using Pearson’s chi-square/Fisher’s test for categorical variables and the Mann-Whitney U test for continuous variables. The propensity score–matching method (1:1) was used to control confounding factors and selection bias between the rifaximin and control groups. Survival curves were generated by the Kaplan-Meier method, and differences in survival rates were analyzed using the log-rank test. To identify risk factors of EAD, logistic regression modeling was used, and all significant variables in univariate analyses were subsequently included in the multivariate analysis. All tests were 2-sided, and P < 0.05 was considered statistically significant. All analyses were performed using SPSS, version 25 (IBM, Armonk, NY).
Results
Demographics of the patient cohort (n = 206) after exclusion of patients receiving antibiotics treatment other than rifaximin and re-LT patients are shown in Table 1. The median recipient age was 60 (20-75) years, with males constituting approximately 70% of the patients. The most common recipient race was Hispanic (n = 95, 46.1%), followed by white patients (n = 68, 33.0%). Hepatitis C virus (HCV)–related hepatic disease was the most predominant indication for LT (n = 107, 51.9%), followed by nonalcoholic steatohepatitis (NASH; n = 28, 13.6%) and alcohol (ethanol, EtOH)–related liver disease (n = 23, 11.2%). Concomitant hepatocellular carcinoma (HCC) occurred in 128 patients (62.1%). The median (range) MELD score was 14 (6-47). Median (range) donor age was 38 (7-76) years, and approximately 60% of the donors were male. Almost 50% of donors were white (n = 102, 49.5%), and the rate of DCD donor graft use was 4.4% (n = 9). Median CIT was 440 minutes, and median WIT was 48 minutes.
| Variables | Patients (n = 206) |
|---|---|
| Recipient | |
| Age, years | 60 (20-75) |
| Sex | |
| Female | 59 (28.6) |
| Male | 147 (71.4) |
| Race | |
| White | 68 (33.0) |
| Hispanic | 95 (46.1) |
| African American | 14 (6.8) |
| Asian | 22 (10.7) |
| Others | 7 (3.4) |
| Indication for LT | |
| HBV | 13 (6.3) |
| HCV | 107 (51.9) |
| EtOH | 23 (11.2) |
| NASH | 28 (13.6) |
| ALF | 9 (4.4) |
| Others | 26 (12.6) |
| Concomitant HCC | 128 (62.1) |
| MELD | 14 (6-47) |
| Donor | |
| Age, years | 38 (7-76) |
| Sex | |
| Female | 78 (37.9) |
| Male | 128 (62.1) |
| Race | |
| White | 102 (49.5) |
| Hispanic | 64 (31.1) |
| African American | 25 (12.1) |
| Asian | 12 (5.8) |
| Others | 3 (1.5) |
| DCD | 9 (4.4) |
| DRI | 1.4 (0.9-2.8) |
| CIT, minutes | 440 (163-878) |
| WIT, minutes | 48 (23-176) |
NOTE:
- Data are given as n (%) or median (range).
Classification According to Rifaximin Therapy
We selected 28 days as a cutoff value because it had the maximum sum of sensitivity and specificity within its candidates. There were 49 patients (rifaximin group, 23.8%) who received rifaximin for 28 days or more prior to LT, and the remaining 157 patients did not receive rifaximin or received rifaximin for 1-27 days (control group). The perioperative clinical features of 206 patients were analyzed according to the presence or absence of the continuous administration of rifaximin for at least 28 days before LT (Table 2).
| Variables | Control Group (n = 157) | Rifaximin Group (n = 49) | P Value |
|---|---|---|---|
| Recipient | |||
| Age, years | 60 (20-75) | 59 (21-68) | 0.095 |
| Sex | 0.462 | ||
| Female | 47 (29.9) | 12 (24.5) | |
| Male | 110 (70.1) | 37 (75.5) | |
| Race | 0.044 | ||
| White | 58 (36.9) | 10 (20.4) | |
| Hispanic | 67 (42.7) | 28 (57.1) | |
| African American | 12 (7.6) | 2 (4.1) | |
| Asian | 17 (10.8) | 5 (10.2) | |
| Others | 3 (1.9) | 4 (8.2) | |
| Indication for LT | 0.016 | ||
| HBV | 13 (8.3) | 0 (0.0) | |
| HCV | 83 (52.9) | 24 (49.0) | |
| EtOH | 14 (8.9) | 9 (18.4) | |
| NASH | 17 (10.8) | 11 (22.4) | |
| ALF | 9 (5.7) | 0 (0.0) | |
| Others | 21 (13.4) | 5 (10.2) | |
| Past history | |||
| Smoking | 73 (46.5) | 22 (44.9) | 0.845 |
| Hypertension | 73 (46.5) | 23 (46.9) | 0.957 |
| Diabetes | 55 (35.0) | 17 (34.7) | 0.965 |
| Coronary artery disease | 24 (15.3) | 11 (22.4) | 0.244 |
| Concomitant HCC | 109 (69.4) | 19 (38.7) | <0.001 |
| Pretransplant AST, IU/L | 59 (20-1918) | 59 (20-197) | 0.963 |
| Pretransplant ALT, IU/L | 33 (9-3705) | 29 (11-134) | 0.125 |
| MELD | 13 (6-47) | 28 (9-43) | <0.001 |
| Preoperative hospital stay, days | 1 (0-136) | 1 (0-48) | 0.159 |
| Preoperative ICU stay | 16 (10.2) | 6 (12.2) | 0.684 |
| Donor | |||
| Age, years | 38 (7-76) | 38 (12-70) | 0.973 |
| Sex | 0.409 | ||
| Female | 57 (36.3) | 21 (42.9) | |
| Male | 100 (63.7) | 28 (57.1) | |
| Race | 0.002 | ||
| White | 77 (49.0) | 25 (51.0) | |
| Hispanic | 54 (34.4) | 10 (20.4) | |
| African American | 13 (18.3) | 12 (24.5) | |
| Asian | 12 (7.6) | 0 (0.0) | |
| Others | 1 (0.6) | 2 (4.1) | |
| Cause of death | 0.435 | ||
| Head trauma | 60 (38.2) | 14 (28.6) | |
| Cerebrovascular accident | 59 (37.6) | 20 (40.8) | |
| Anoxia | 38 (24.2) | 15 (30.6) | |
| DCD | 6 (3.8) | 3 (6.1) | 0.492 |
| Past history | |||
| Hypertension | 47 (29.9) | 17 (34.7) | 0.530 |
| Diabetes | 18 (11.5) | 9 (18.4) | 0.211 |
| Coronary artery disease | 7 (4.5) | 3 (6.1) | 0.636 |
| AST, IU/L | 43 (9-747) | 38 (13-294) | 0.390 |
| ALT, IU/L | 35 (7-286) | 31 (9-165) | 0.273 |
| Total bilirubin, mg/dL | 0.8 (0.2-11.5) | 0.7 (0.2-2.7) | 0.864 |
| PT-INR | 1.2 (0.9-16.1) | 1.2 (0.9-2.8) | 0.933 |
| DRI | 1.5 (0.9-2.3) | 1.4 (1.0-2.8) | 0.565 |
| CIT, minutes | 439 (163-878) | 463 (211-760) | 0.970 |
| WIT, minutes | 48 (23-176) | 49 (27-81) | 0.185 |
NOTE:
- Data are given as n (%) or median (range). A total of 49 patients (rifaximin group) received rifaximin for 28 days or more prior to LT, and the other 157 patients (control group) did not. The races of the recipients and donors were significantly different between the 2 groups (P < 0.05). In the rifaximin group, the rate of HCC was lower and MELD score was significantly higher as compared with the control group (P < 0.001).
As shown in Table 2, recipient race distribution, indication for LT, presence of concomitant HCC, MELD score, and distribution of donor race were significantly different between the 2 groups before matching (P < 0.05). The rate of patients who had HCC in the rifaximin group was significantly lower than those in the control group (38.7% versus 69.4%; P < 0.001). Patients in the rifaximin group had significantly higher median (range) MELD scores than those in the control group (28 [9-43] versus 13 [6-47]; P < 0.001). There was no significant difference in pretransplant serum AST (59 [20-197] versus 59 [20-1918] IU/L; P = 0.963) and ALT (29 [11-134] versus 33 [9-3705]; P = 0.125) levels. Posttransplant transaminase levels and the rate of EAD before matching are shown in Supporting Figs. 1 and 2. Serum ALT levels at/from POD 0 (approximately 6 hours after operation) to POD 7 and AST levels at POD 0-4 and 6-7 in the rifaximin group were significantly lower than those in the control group (P < 0.05). The rate of EAD was also significantly lower in the rifaximin group (14.3% versus 29.9%; P = 0.030).
Propensity Score–Matching Analysis Between the Rifaximin and Control Groups
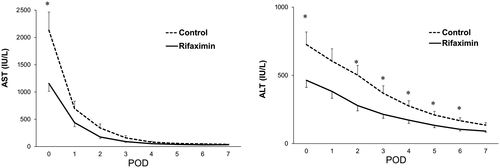
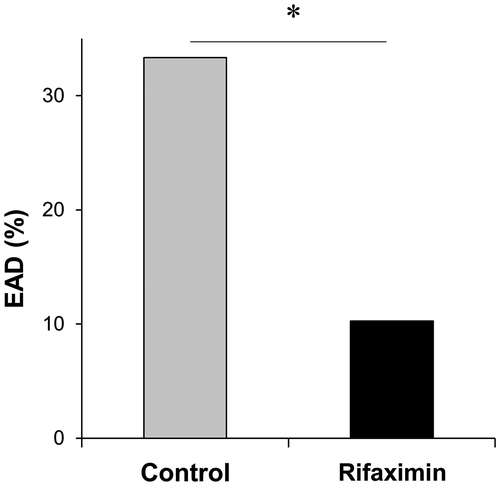
The rifaximin and control groups were matched in a 1:1 ratio to the nearest propensity scores, and 39 patients in each group were selected. Table 3 shows the results comparing the 2 groups after propensity matching. This analysis controlled for all significant differences in recipient and donor factors between the 2 groups. After the propensity matching, the value of posttransplant serum transaminase levels and the rate of EAD were compared between the rifaximin and control groups. As shown in Fig. 1, patients in the rifaximin group had significantly lower serum AST and ALT levels at POD 0. Moreover, serum ALT levels at POD 2-6 in the rifaximin group were significantly lower than those in the control group (P < 0.05). Despite similar trends, serum levels of AST at POD 1-7 and ALT at POD 1 and 7 did not reach statistical significance. Notably, the rifaximin group had a significantly lower rate of EAD after matching compared with the control group as was the case before matching (10.3% versus 33.3%; P = 0.014; Fig. 2). There were no differences in patient and graft survival between the 2 groups (Supporting Fig. 3).
| Variables | Control Group (n = 39) | Rifaximin Group (n = 39) | P Value |
|---|---|---|---|
| Recipient | |||
| Age, years | 57 (21-72) | 59 (37-68) | 0.519 |
| Sex | 0.604 | ||
| Female | 9 (23.1) | 11 (28.2) | |
| Male | 30 (76.9) | 28 (71.8) | |
| Race | 0.986 | ||
| White | 10 (25.6) | 10 (25.6) | |
| Hispanic | 21 (53.8) | 20 (51.3) | |
| African American | 1 (2.6) | 2 (5.1) | |
| Asian | 4 (10.3) | 4 (10.3) | |
| Others | 3 (7.7) | 3 (7.7) | |
| Indication for LT | 0.106 | ||
| HBV | 4 (10.3) | 0 (0.0) | |
| HCV | 23 (59.0) | 20 (51.3) | |
| EtOH | 4 (10.3) | 5 (12.8) | |
| NASH | 3 (7.7) | 10 (25.6) | |
| ALF | 4 (10.3) | 4 (10.3) | |
| Others | 1 (2.6) | 0 (0.0) | |
| Past history | |||
| Smoking | 23 (59.0) | 18 (46.2) | 0.257 |
| Hypertension | 16 (41.0) | 16 (41.0) | >0.999 |
| Diabetes | 14 (35.9) | 12 (30.8) | 0.631 |
| Coronary artery disease | 4 (10.3) | 8 (20.5) | 0.209 |
| Concomitant HCC | 22 (56.4) | 18 (46.2) | 0.365 |
| Pretransplant AST, IU/L | 50 (20-885) | 63 (20-197) | 0.285 |
| Pretransplant ALT, IU/L | 30 (9-1170) | 31 (11-134) | 0.780 |
| MELD | 17 (6-43) | 25 (9-40) | 0.206 |
| Preoperative hospital stay, days | 1 (0-35) | 1 (0-47) | 0.726 |
| Preoperative ICU stay | 5 (12.8) | 4 (10.3) | 0.723 |
| Donor | |||
| Age, years | 36 (8-65) | 35 (12-70) | 0.853 |
| Sex | 0.352 | ||
| Female | 13 (33.3) | 17 (43.6) | |
| Male | 26 (66.7) | 22 (56.4) | |
| Race | 0.541 | ||
| White | 17 (43.6) | 21 (53.8) | |
| Hispanic | 14 (35.9) | 10 (25.6) | |
| African American | 7 (17.9) | 8 (20.5) | |
| Asian | 0 (0.0) | 0 (0.0) | |
| Others | 1 (2.6) | 0 (0.0) | |
| Cause of death | 0.961 | ||
| Head trauma | 14 (35.9) | 13 (33.3) | |
| Cerebrovascular accident | 14 (35.9) | 14 (35.9) | |
| Anoxia | 11 (28.2) | 12 (30.8) | |
| DCD | 3 (7.7) | 2 (5.1) | 0.644 |
| Past history | |||
| Hypertension | 13 (33.3) | 13 (33.3) | >0.999 |
| Diabetes | 3 (7.7) | 8 (20.5) | 0.104 |
| Coronary artery disease | 2 (5.1) | 2 (5.1) | >0.999 |
| AST, IU/L | 45 (9-483) | 42 (13-294) | 0.487 |
| ALT, IU/L | 40 (7-193) | 32 (9-165) | 0.171 |
| Total bilirubin, mg/dL | 0.8 (0.3-4.0) | 0.8 (0.2-2.7) | 0.756 |
| PT-INR | 1.2 (0.9-2.0) | 1.2 (0.9-2.8) | 0.631 |
| DRI | 1.5 (1.0-2.3) | 1.3 (1.0-2.3) | 0.385 |
| CIT, minutes | 429 (263-842) | 463 (211-760) | 0.355 |
| WIT, minutes | 53 (25-73) | 51 (27-81) | 0.656 |
NOTE:
- The rifaximin and control groups were matched in a 1:1 ratio, and 39 patients in both groups were selected. The propensity matching reduced differences between the 2 groups that existed before the matching.
Evaluation of Inflammatory Cell Activation in Postreperfusion LT
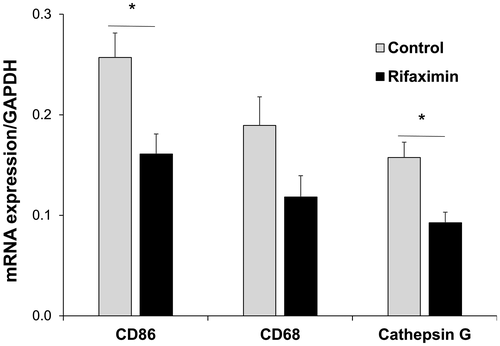
Liver biopsy samples, obtained approximately 2 hours after portal reperfusion from 4 patients in each group after matching (Fig. 3), were evaluated for the activation of infiltrating inflammatory neutrophils and macrophages, as well as screening messenger RNA (mRNA) coding for CD86, CD68, and cathepsin G. CD86 is a costimulatory molecule on monocytic cells, including macrophages; CD68 is a macrophage marker; whereas cathepsin G is typically stored in neutrophils and released by a variety of stimuli, including ischemia/reperfusion (IR) stress. As shown in Fig. 4, the expression of mRNA coding for CD86 and cathepsin G in the rifaximin group was significantly lower than in the control group (CD86: 0.16 ± 0.02 versus 0.26 ± 0.02; P = 0.029; cathepsin G: 0.09 ± 0.01 versus 0.16 ± 0.02; P = 0.029), whereas CD68 expression did not reach statistical significance between the 2 groups despite showing similar trends (0.12 ± 0.02 versus 0.19 ± 0.03; P = 0.200).
Univariate and Multivariate Analyses for EAD in all Patients and Patients With High MELD Scores
Univariate and multivariate analyses were used to determine the impact of pre-LT rifaximin treatment on EAD in all patients (n = 458) as well as in high MELD patients (MELD ≥35, n = 230) only. On univariate analysis for all patients, longer CIT/WIT, older donor age, higher donor body mass index (BMI), higher DRI, and rifaximin treatment <28 days were identified as risk factors for the development of EAD (P < 0.05; Supporting Table 3). On univariate analysis in high MELD patients, longer WIT, older donor age, higher donor BMI, and rifaximin treatment <28 days were identified as risk factors for the development of EAD (P < 0.05; Supporting Table 4).
On multivariate analysis for all patients, pre-LT rifaximin treatment <28 days (odds ratio [OR], 2.096; 95% confidence interval [CI], 1.298-3.382; P = 0.002) and longer CIT (OR, 1.003; 95% CI, 1.002-1.005; P < 0.001) were identified as independent risk factors for EAD (Table 4). Additionally, multivariate analysis in patients with high MELD scores showed that pre-LT rifaximin treatment <28 days (OR, 2.015; 95% CI, 1.071-3.792; P = 0.03) and longer WIT (OR, 1.052; 95% CI, 1.026-1.079; P < 0.001) were independent risk factors for EAD (Table 5).
| Factors | OR | 95% CI | P Value |
|---|---|---|---|
| Pre-LT rifaximin <28 days | 2.096 | 1.298-3.382 | 0.002 |
| CIT (per minute) | 1.003 | 1.002-1.005 | <0.001 |
| WIT (per minute) | 1.012 | 0.998-1.027 | 0.096 |
| Donor age (per year) | 1.016 | 0.995-1.038 | 0.135 |
| Donor BMI (per kg/m2) | 1.029 | 0.991-1.069 | 0.136 |
| DRI (per point) | 0.858 | 0.392-1.879 | 0.701 |
NOTE:
- Pre-LT rifaximin <28 days and longer CIT were identified as independent risk factors for EAD.
| Factors | OR | 95% CI | P Value |
|---|---|---|---|
| Pre-LT rifaximin <28 days | 2.015 | 1.071-3.792 | 0.03 |
| WIT (per minute) | 1.052 | 1.026-1.079 | <0.001 |
| Donor age (per year) | 1.014 | 0.992-1.035 | 0.21 |
| Donor BMI (per kg/m2) | 1.027 | 0.968-1.090 | 0.377 |
NOTE:
- Pre-LT rifaximin <28 days and longer WIT were identified as independent risk factors for EAD.
Discussion
IRI is associated with acute cellular damage, cell death, and a severe hepatocellular inflammatory response.31-33 EAD, which is considered to be largely due to IRI, is associated with increased graft failure and mortality. In this retrospective propensity score–matched analysis, we document that preoperative rifaximin treatment improved hepatocellular function after LT. Patients who received continuous rifaximin therapy for at least 28 days prior to surgery had diminished serum AST/ALT levels and lower rates of EAD after LT. To the best of our knowledge, this study is the first to report the influence of rifaximin therapy on posttransplant graft function in LT recipients. Because the elevation of serum transaminases is often used as a surrogate marker of hepatic IRI,34, 35 our results indicate that unlike controls, rifaximin attenuated IRI in LT.
In the propensity score–matched analysis (n = 206), there were some notable differences in patient demographics. Patients in the rifaximin group had higher MELD scores and a lower rate of concomitant HCC compared with the control group. Consistent with a previous report examining the protective effect of rifaximin against posttransplant infections,27 our study showed that patients with pretransplant rifaximin treatment had a higher acuity level reflected by significantly higher MELD scores compared with controls. Another study by Wong et al.36 reported that patients with a history of HE had higher MELD scores compared with patients without HE. In the present study, the rate of patients with a history of HE in the rifaximin group was significantly higher than that in the control group (13.4% versus 100%; P < 0.001). These previous studies support the observed MELD score differences in our cohort before matching. Additionally, there was a lower incidence of HCC among patients treated with rifaximin. This is consistent with previous reports27, 28 and is not surprising because most patients awaiting LT for HCC have relatively compensated liver function and, thus, do not suffer from HE. This also accounts for the difference in MELD score between the 2 groups. These studies as well as the present analysis reflect the inherent medical acuity differences between patients likely to be on rifaximin and those who do not medically require it prior to LT. This important difference, however, was mitigated by use of a propensity score–matching analysis to verify the true effect of rifaximin.
We observed no differences between donor graft quality in any of the donor parameters measured including DRI before or after propensity matching. This is noteworthy given the possible confounding effect resulting from potentially using better-quality grafts for the higher-acuity recipients and grafts more likely to be predisposed to IRI in the lower-acuity patients with HCC or in those well enough not to require rifaximin before transplant. Furthermore, our matching model reduced differences including donor factors between the 2 groups.
IRI may occur at several key time points of LT, starting in the donor, continuing during cold storage, and when the organ is reperfused with the recipient’s blood. During these events, not only do donor-derived damage-associated molecular patterns, which are mainly released by damaged liver cells, readily stimulate the immune system, but so do pathogen-associated molecular patterns, which are primarily secreted from recipient gut microbiota. These events all contribute to the severity of IRI.33 The effect of pretransplant rifaximin administration is considered to impact liver IRI at the time of hepatic reperfusion.
In attempting to elucidate the putative mechanism of rifaximin-mediated hepatoprotection against IRI, we focused on inflammatory cell graft infiltrates because neutrophils and macrophages are key players in the pathophysiology of liver IRI.4-6 The mRNA levels coding for CD86 and cathepsin G, markers of macrophages and neutrophils, respectively, were lower in LT of rifaximin-treated versus control patients. Patients with end-stage liver disease have a relatively low concentration of bile acids in the gut, which is believed to contribute to chronic inflammation because of an overgrowth of pathogenic bacteria, increased endotoxin levels, and secondary stimulation of a potent inflammatory response.37, 38 Numerous studies indicate that inflammatory cells, such as neutrophils and macrophages, respond to bacterial products via nuclear factor kappa B and production of proinflammatory cytokines/chemokines, suggesting that these cells would be responsive to physiologically relevant levels of microbial products that reach the liver.39 Rifaximin directly affects bacterial growth, leading to a lower proinflammatory response.16, 17 In a recent study examining patients with nonalcoholic fatty liver disease (NAFLD), 28 days of treatment with rifaximin was shown to exert beneficial effects in early clinical trials, lowering endotoxemia and reducing transaminases.40 Another study in HE patients suggested that rifaximin therapy has a systemic and local effect on the microbiota, metabolome, endotoxemia, and cognition, and a significant improvement in endotoxemia was observed with a modest change in stool microbiota composition.37 Hence, the mechanism of action of rifaximin on hepatic IRI, based on these studies and our current findings, may involve modulation of microbiota inflammatory function leading to reducing neutrophil/macrophage activation in IR-stressed liver grafts.
Furthermore, it is suspected that an innate immune pathologic response occurs with subsequent bacterial translocation of organisms from the gut in patients with a history of HE and portal hypertension that may result in chronic endotoxemia.41 This cascade culminates in a local milieu of proinflammatory cytokines/chemokines that up-regulate adhesion receptors and activate neutrophils.41 Although there is no reliable evidence regarding the difference in the inflammatory condition of HE patients before and during administration of rifaximin compared with patients without HE, patients in the rifaximin group might undergo LT with a high degree of underlying inflammation. Nevertheless, lower levels of neutrophil/macrophage infiltration in their IR-stressed livers may support the protective effect of rifaximin against gut-derived hepatic inflammation.
Some limitations to this study include the fact that it is a single-center retrospective analysis, thereby providing for an inherent difference in the presence of HE between the 2 groups even after matching. The median (range) MELD score of all 78 recipients included in the matching study was 21 (6-43), ie, relatively low compared with a median MELD of 35 (6-51) for 458 primary LT patients at our institution during the study period. This is mainly a result of excluding many high MELD patients requiring concomitant antibiotic therapy other than rifaximin for infections, such as spontaneous bacterial peritonitis or septic shock. Unfortunately, propensity score–matching in all patients was not possible because the differences between the groups were too large to properly match (Supporting Table 5). Therefore, we used multivariate analysis for EAD to determine the impact of rifaximin treatment. Multivariate analysis identified rifaximin use <28 days as an independent risk factor for EAD. This finding was also present in the high MELD patient cohort suggesting that rifaximin treatment may potentially protect against liver injury in high MELD patients as well. In fact, although we used 28 days of rifaximin treatment as the minimum duration of therapy based on ROC curve and previous reports,24, 40 the rate of patients receiving rifaximin for at least 1 day was nearly 60% at our institution (data not shown). Therefore, although rifaximin use may have provided a protective benefit against IRI and EAD in those patients, this was difficult to ascertain given the simultaneous use of other antibacterial agents.
The exact effect of rifaximin administration on graft injury and its optimal duration in high acuity patients are yet to be fully delineated. To better evaluate the effect of sole pretransplant rifaximin administration, prospective studies, including randomized controlled trials, are needed. It is also important to note that in the present study, rifaximin was used only in the pretransplant period and was not continued after transplant. Whether continuing administration of rifaximin in the immediate postoperative period can further decrease the rate of EAD after LT or not is unknown. Further experiments in animal LT models might assist in developing a better mechanistic appreciation of the specific molecular signaling pathways by which rifaximin may exert this protective function.
In conclusion, pretransplant rifaximin administration exerted a protective effect against EAD, while suppressing neutrophil/macrophage activation in IR-stressed human LT. These propensity score–matched and multivariate analyses suggest a therapeutic potential for preoperative gut microbiota alteration by rifaximin against IRI in LT patients. Additional studies are needed to further elucidate this relationship and analyze underlying mechanisms.