Use of Steatotic Grafts in Liver Transplantation: Current Status
Abstract
In the field of liver transplantation, the demand for adequate allografts greatly exceeds the supply. Therefore, expanding the donor pool to match the growing demand is mandatory. The present review summarizes current knowledge of the pathophysiology of ischemia/reperfusion injury in steatotic grafts, together with recent pharmacological approaches aimed at maximizing the utilization of these livers for transplantation. We also describe the preclinical models currently available to understand the molecular mechanisms controlling graft viability in this specific type of donor, critically discussing the heterogeneity in animal models, surgical methodology, and therapeutic interventions. This lack of common approaches and interventions makes it difficult to establish the pathways involved and the relevance of isolated discoveries, as well as their transferability to clinical practice. Finally, we discuss how new therapeutic strategies developed from experimental studies are promising but that further studies are warranted to translate them to the bedside.
Abbreviations
-
- α-GST
-
- α-glutathione S-transferase
-
- ACE
-
- angiotensin-converting enzyme inhibitor
-
- ACE2
-
- angiotensin-converting enzyme 2
-
- ACTH
-
- adrenocorticotropic hormone
-
- Ang
-
- angiotensin
-
- ALT
-
- alanine aminotransferase
-
- ASK-1
-
- apoptosis signal-regulating kinase 1
-
- AST
-
- aspartate aminotransferase
-
- ATP
-
- adenosine triphosphate
-
- ATR
-
- angiotensin II receptor
-
- BD
-
- brain death
-
- BSP
-
- bromosulfophthalein
-
- cAMP
-
- cyclic adenosine monophosphate
-
- CCR2/5
-
- C-C chemokine receptor types 2/5
-
- cGMP
-
- cyclic guanosine monophosphate
-
- CIT
-
- cold ischemia time
-
- CoPP
-
- cobalt protoporphyrin
-
- CT
-
- computed tomography
-
- DAMP
-
- damage-associated molecular pattern
-
- eNOS
-
- endothelial nitric oxide synthase
-
- ER
-
- endoplasmic reticulum
-
- ET
-
- endothelin
-
- FA
-
- fatty acid
-
- FGF
-
- fibroblast growth factor
-
- FXR
-
- farnesoid X receptor
-
- GLDH
-
- glutamate dehydrogenase
-
- GLP-1
-
- glucagon-like peptide 1
-
- GLP-1R
-
- glucagon-like peptide 1 receptor
-
- GSH
-
- glutathione
-
- GSPx
-
- glutathione peroxidase
-
- H & E
-
- hematoxylin-eosin
-
- HFD
-
- high-fat diet
-
- HGF
-
- hepatocyte growth factor
-
- HMGB1
-
- high-mobility group box 1
-
- HO-1
-
- heme oxygenase 1
-
- HSC
-
- hepatic stem cells
-
- IL
-
- interleukin
-
- iNOS
-
- inducible nitric oxide synthase
-
- IP
-
- intraperitoneal
-
- I/R
-
- ischemia/reperfusion
-
- IRI
-
- ischemia/reperfusion injury
-
- IV
-
- intravenous
-
- KC
-
- Kupffer cell
-
- KLF2
-
- Kruppel-like factor 2
-
- LDH
-
- lactate dehydrogenase
-
- LDLT
-
- living donor liver transplantation
-
- LPS
-
- lipopolysaccharide
-
- LSEC
-
- liver sinusoidal endothelial cells
-
- LT
-
- liver transplantation
-
- LV
-
- lentiviral
-
- LXR
-
- liver X receptor
-
- mAChR
-
- muscarinic acetylcholine receptor
-
- MAPK
-
- mitogen-activated protein kinase
-
- Mas
-
- Mas receptor
-
- MDA
-
- malondialdehyde
-
- MDDP
-
- multidrug donor preconditioning
-
- MnSOD
-
- manganese-dependent superoxide dismutase
-
- MP
-
- machine perfusion
-
- mTOT
-
- mitochondrial target of thiazolidinediones
-
- NAD
-
- nicotinamide adenine dinucleotide
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NO
-
- nitric oxide
-
- NRF2
-
- nuclear erythroid 2 p45-related factor 2
-
- PAMP
-
- pathogen-associated molecular pattern
-
- PH
-
- partial hepatectomy
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PKC
-
- protein kinase C
-
- PMN
-
- polymorphonuclear leukocyte
-
- PPAR
-
- peroxisome proliferator-activated receptor
-
- RBP4
-
- retinol-binding protein 4
-
- ROS
-
- reactive oxygen species
-
- RT
-
- reperfusion time
-
- SGLT-2
-
- sodium-glucose cotransporter-2
-
- SIRT-1
-
- sirtuin 1
-
- SOD
-
- superoxide dismutase
-
- SREBP-1c
-
- sterol regulatory element-binding protein 1c
-
- TE
-
- transient elastography
-
- TGFβ
-
- tumor growth factor β
-
- TLR
-
- toll-like receptor
-
- TM
-
- thrombomodulin
-
- TNF-α
-
- tumor necrosis factor α
-
- TRIF
-
- toll/interleukin-1 receptor domain-containing adaptor inducing interferon β
-
- TUDCA
-
- tauroursodeoxycholic acid
-
- UCP2
-
- uncoupling protein 2
-
- US
-
- ultrasound
-
- vWF
-
- von Willebrand factor
-
- WIT
-
- warm ischemia time
-
- XDH
-
- xanthine dehydrogenase
-
- XOD
-
- xanthine oxidase
It is well known that the demand for liver allografts greatly exceeds the supply. Indeed, while organ shortage remains a major limitation in liver transplantation (LT), the demand for organs continues to increase worldwide. Aimed at expanding the liver donor pool, recent reports suggested the use of livers previously discarded for LT that were positive for hepatitis B virus and hepatitis C virus.1, 2 Liver steatosis is another factor to take into consideration when evaluating donor livers because it is highly frequent (30% in cadaveric and 20% in living donors) and may influence recipient outcomes.3 This is the case with nonalcoholic fatty liver disease (NAFLD) being a common cause of organ rejection. Ischemia/reperfusion injury (IRI) is another important cause of liver damage during LT, resulting in the induction of an acute inflammatory response that can lead to significant tissue damage and organ dysfunction.4
NAFLD grafts show increased vulnerability to ischemia/reperfusion (I/R) when they are transplanted. Moreover, different mechanisms of cell death in fatty versus nonfatty livers point to potential differences in the mechanisms involved in hepatic IRI. Accordingly, strategies that are effective in the healthy liver may not be useful in the presence of steatosis.5
In the present review, we summarize the current understanding of I/R pathophysiology in steatotic livers and describe those studies aimed at specifically developing therapies for this type of liver graft.
NAFLD, a Common Disease Affecting the Use of Steatotic Grafts for LT: Current Knowledge, Pharmacological Strategies, and Future Perspectives
Because of the increasing prevalence of NAFLD, this pathology has become a major focus of interest for researchers in the liver field. Previous reviews have comprehensively described NAFLD pathophysiology, potential treatment strategies, and ongoing clinical trials,6-8 and the main findings are summarized in Fig. 1. In the scenario of LT, treatments for NAFLD donors may aim to reduce fatty infiltration, ameliorate injury, and/or improve the profile of several metabolic risk factors before the transplant procedure, especially in living donor liver transplantation (LDLT). Nevertheless, current therapies for NAFLD require longterm intervention (ranging from 6 to 72 weeks),9-11 which represents a limitation in the case of clinical LT from cadaveric donors. Thus, further investigations to optimize therapies and reduce the pretreatment times during this emergency procedure are necessary.

There is now evidence in LDLT showing that bariatric surgery performed in recipients before, during, or after LT improves survival by reducing obesity-related comorbidities as well as reducing the incidence of recurrent NAFLD/nonalcoholic steatohepatitis (NASH).12 Among the different effects, there is a decrease in portal influx of free fatty acids (FAs) following surgery, which may be related both to feeding restrictions and to metabolic changes in visceral insulin sensitivity.13 On the other hand, adhesions, distortions of bowel continuity complicating biliary reconstruction, and nutritional deficiencies perpetuated by a bypass procedure have also been reported. A longer-term concern is the prevalence of weight regain, which may also have a significant effect on NAFLD.14 The pharmacological treatments of NAFLD as well as bariatric surgery may provide a solution for patients with steatosis to be successful liver donors. This would not only benefit the recipients, who would receive better functioning livers, but it would also reduce the metabolic syndrome in the donors, allowing them to have a faster recovery from the transplant procedure.9 A remedy for steatotic livers in living donor candidates will also increase the organ pool. Indeed, the first successful case of a right lobe living donor hepatectomy in a patient who previously underwent bariatric surgery for obesity was recently published.15
On the other hand, in our view, further research is required to select drugs that can regulate hepatic fatty infiltration with minimal adverse effects and to optimize such potential treatments (ie, dose and pharmacokinetics) before being translated into treatments for human disease. In our opinion, drugs or surgical procedures that reduce hepatic steatosis should be considered with caution in clinical liver surgery due to the potential adverse effects of these drugs or the potential risk associated with bariatric surgery. In this sense, bariatric surgery is not currently recommended for NAFLD/NASH patients because mortality in those patients is dictated by cardiovascular events.16 In addition, different results have been reported on the role of lipids in liver regeneration. Data based on genetic or pharmacological approaches indicate that the presence of steatosis is beneficial17, 18 or at least may not impact19, 20 regeneration following partial hepatectomy (PH). However, the interruption of the development of steatosis during the regenerative process negatively impacts liver regeneration.21, 22 In our view and in line with that reported previously by different authors,19, 20 fat accumulation alone is not sufficient to inhibit liver regeneration. Differences in the experimental surgical conditions, the approaches evaluated to modify lipid accumulation, and the forms of steatosis, which may result in different regenerative signaling pathways, should also be considered. In this sense, recent clinical experience also supports this idea, showing similar postoperative outcomes in steatotic and nonsteatotic LT, suggesting that additional genetic or environmental factors impact the effects of steatosis on hepatic regeneration.23 Preclinical studies and larger clinical trials should be carried out that are aimed at evaluating whether the reduction in fat infiltration affects both the donor’s and the recipient’s postoperative outcomes.
Alternatively, new therapeutic options to reduce IRI in NAFLD donors are being investigated. This review will discuss reported studies in this sense.
- Whether methods to evaluate the type and degree of fatty infiltration are appropriate.
- Whether a fatty liver graft should be transplanted.
- Whether the fatty content of the liver is really the only factor responsible for initial dysfunction or initial nonfunction after LT.24
In this regard, the limited donor population is a current concern that requires further characterization to improve the procedure for preconditioning steatotic livers and to expand the donor pool to fit the growing demand for LT.25 Indeed, a growing focus in LT research relates to the use of so-called extended criteria donor grafts,26 with steatosis being a frequent reason to define a graft as extended criteria donor. The degree and type of hepatic steatosis, inflammation, fibrosis, and damage should be evaluated before surgery in potential donor grafts as well as in patients in the immediate postoperative period after LT.
At present, there are no known clinical or biochemical markers able to estimate the degree of fatty infiltration of the liver.27, 28 Several reports have demonstrated that liver steatosis is associated with the elevation of certain clinical and biochemical markers28-30 with low predictive potential. Indeed, transaminases and specifically alanine aminotransferase (ALT) commonly used as liver damage markers are not 100% reliable because some patients suffering from NAFLD do not present high levels of ALT.31 Recent studies evaluating other biomarkers including circulating microRNA reported promising results,32 although larger studies are still necessary to validate them in the future.
During procurement of the graft in the donor, the gold standard to assess hepatic steatosis is a histological analysis.33 The practice of transplant programs varies between hospitals because some perform liver biopsies on all potential donors, whereas others perform them only when significant steatosis cannot be ruled out.25 Nevertheless, it is important to note that liver biopsy is an invasive procedure that has a significant interobserver variability among experts for both quantitative and qualitative assessments of the histologic features of liver steatosis and is not suitable for continuous monitoring. Unfortunately, current imaging methods are inaccurate and inadequate for quantification of liver steatosis and do not distinguish clearly between the microvesicular and the macrovesicular types.34 Magnetic resonance techniques can enable more direct assessment of the amount of fat in the liver than can either computed tomography (CT) or ultrasound (US).35 In a study applying state-of-the-art equipment for US, CT, and magnetic resonance imaging, Saadeh et al. reported that only a hepatic fat accumulation above 25%-30% can be reliably detected radiologically.36 Also, none of these modalities was able to either distinguish steatosis from steatohepatitis or to detect individual pathologic features required to establish steatohepatitis such as necroinflammatory changes, hepatocyte ballooning, and fibrosis.29 Other tools, such as biochemical impedance and transient elastography (TE) have been shown to predict steatosis/fibrosis, and the use may be extended in assessments of the donor liver. TE has been validated as a screening tool for advanced fibrosis in NAFLD, though it cannot be used to rule out inflammation of the liver.37 The use of noninvasive, rapidly available, sensitive, specific, and cost-effective biomarkers could simplify liver injury assessment and improve the time to management of postoperative complications in steatotic livers that undergo surgery.
Various studies have evaluated the possible association between the percentage of steatosis and/or the pattern of fat distribution and the postoperative outcomes after LT. In microvesicular steatosis, the cytoplasm of the hepatocytes contains tiny lipid vesicles without nuclear dislocation.38 However, macrovesicular steatosis is characterized by a single, bulky fat vacuole in the hepatocytes, which displaces the nucleus to one side. On the other hand, in microvesicular steatosis, multiple small droplets are finely dispersed in the cytoplasm without nuclear displacement. Livers with macrovesicular steatosis are more intolerant to ischemic injury than those with microvesicular steatosis.33 The triglycerides deposition as intracytoplasmic fat droplets is associated with an increase in hepatocellular volume, which may induce distortion and narrowing of the sinusoid, with a reduction in the luminal diameter by up to 50% compared with that in the healthy liver.39 Increases in liver fat infiltration are consistent with the appearance of severe alterations in liver blood flow and hepatic microcirculation, respectively. In addition, macrovesicular steatosis is associated with increased risk of acute cellular rejection and bile duct loss suggestive of chronic ductopenic rejection in allograft recipients.40 These observations are in accordance with previous reports, indicating that transplantation outcomes are not affected by hepatic microvesicular steatosis regardless of its severity. Therefore, such livers can be safely used, assuming there are no other donor or recipient risk factors.41 In contrast, some transplant programs exclude donors with macrovesicular steatosis exceeding 10%-15%.25 The use of grafts with moderate steatosis (>30% and 60%) is indeed controversial because these may impose a relative risk on posttransplant outcomes. Previous reports have shown an increased incidence of primary nonfunction after LT from donors with moderate steatosis compared with nonsteatotic livers,42 whereas other authors have reported excellent results.43 Donor livers with severe macrovesicular steatosis (>60%) are typically ruled out for transplantation because of an expected high risk of graft failure.33 Consequently, from our point of view, and as reported by other authors,24, 25 it should be considered that transplant dysfunction is multifactorial, depending on factors like advanced donor age, cold ischemia time (CIT), and intensive care stay, suggesting that isolated graft steatosis is not the only cause of dysfunction or initial nonfunction after LT.24 In fact, a large study (5051 patients) showed that when cold ischemia extends beyond 11 hours, a low degree of macrovesicular steatosis (20%) is associated with an increased risk of graft loss.44 In this sense, it has been recommended that organs with >30% steatosis may only be used if other factors are controlled (ie, donor age <40 years, short CIT of <5 hours, and noncirculatory cause of death).25, 45
In summary, in the absence of clear evidence on the postoperative outcomes when using steatotic liver grafts and considering the difficulties involved in assessing the degree of steatosis among potential liver donors, the utilization of steatotic donor livers has been highly variable among surgeons, centers, and countries, with some surgeons discarding livers that may have had acceptable outcomes.46 Instead of discarding steatotic grafts for transplant, the community should look for new therapeutic strategies in order to minimize or modify factors that could further endanger graft quality and increase the potential use of steatotic livers for transplant. In this context, pharmacological or surgical strategies aimed at reducing the vulnerability of steatotic liver grafts to I/R should be considered. This could increase the availability in the organ pool and consequently reduce the waiting list for transplantation. It should be noted, however, that evaluation of the percentage of steatosis using a noninvasive method is difficult and the criteria for the use of livers for transplantation differ between surgeons, centers, and countries.
Animal Models of LT From Steatotic Grafts
Multiple experimental studies have been performed with the aim of increasing the number of available liver grafts and improving the postoperative outcomes in steatotic LT. In our view, animal models of LT should be reproducible, reliable, simple, affordable, and technically available with minimal disadvantages.
In the specific situation of NAFLD and given the complexity of human behavior and biology, key factors, such as physical activity, social environment, psychological stress factors, and genetics, should be kept in mind when selecting an animal model to study NAFLD (because they may also be important for the development of the disease in humans) and also when interpreting data obtained from these experimental models.47 In this sense, an ideal animal model of the disease should reflect the hepatic histopathology and pathophysiology of human NAFLD/NASH. Some animal models of NASH recapitulate the liver injury observed in humans but in a different metabolic context (nutritional deficiencies or insulin sensitivity), whereas others were developed by considering the metabolic context of NAFLD but without the full development of steatohepatitis. More recently, models of overnutrition have been achieved through high-fat feeding, eg, forced caloric overload in genetically hyperphagic mice fed a high-fat diet (HFD). These models appear most suited to study the complex biological interactions that are involved in the development of fatty liver disease.48 However, there is not an agreement on what comprises steatohepatitis in experimental models, thus hampering the research done in NASH and limiting the interpretation of results about therapeutic approaches and metabolic pathways.
A variety of animal models of liver steatosis can be applied when investigating the impact of steatosis on liver surgery. Mice and rats have mostly been used as experimental animals in models of NAFLD/NASH, which can be classified into genetic, nutritional, and a combination of genetic and nutritional models.49 Large animals are more expensive and less available because of the requirements of special breeding facilities. In addition, studies have demonstrated problems in inducing reliable steatosis in pigs. For instance, the model described by Lee et al. did not show high levels of triglycerides, and the livers did not present macrovesicular steatosis.50 Rodent hepatic steatosis can be induced by a genetic leptin mutation (ie, Zucker rats, ob/ob mice) or by modulation of nutritional factors. The genetically modified rodents overeat due to the lack of the controlling effect of leptin and, consequently, develop combined microvesicular and macrovesicular steatosis without inflammatory changes. The nutritional models are based either on diets with a high percentage of saturated fat or with amino acid deficiency (ie, choline and methionine), which is essential for hepatic lipid excretion.51 We recently reported a new rat model of NASH that fulfills the major characteristics of human disease.52 However, its applicability in the field of LT remains unexplored.
To our knowledge, only a few studies have combined the use of steatotic grafts with orthotopic LT and were mainly developed in rat models of either nutritionally or genetically induced moderate steatosis.53 Additionally, livers procured following brain death (BD) currently constitute the majority of transplanted organs,54 and steatosis occurs in 9%-26% of biopsied donor livers,55 which are both characteristic risk factors for LT. Thereby new experimental conditions should focus not only on liver graft damage associated with transplantation but also on BD donors to most closely mimic the clinical situation of LT and ultimately develop effective therapeutic strategies in this setting.56
Experimental findings support clinical evidence that BD impairs the viability of organs for transplantation, triggering hemodynamic, hormonal, and inflammatory responses. BD itself induces hypoperfusion in the mesenteric microcirculation, thereby leading to increased local inflammation and organ dysfunction.56, 57 The effects of BD in steatotic LT have been evaluated with the finding that liver damage and inflammatory response were increased in steatotic livers compared with nonsteatotic ones.56
Mechanisms Involved in IRI in Steatotic LT
From a microcirculatory point of view, now we know that all sinusoidal cells suffer significant deregulations leading to their dysfunction, ultimately contributing to IRI. Indeed, liver sinusoidal endothelial cells (LSEC) are seriously compromised in both warm and cold ischemia associated with LT. The lack of biomechanical stimuli occurring during procurement and preservation deteriorates the protective phenotype of LSEC by down-regulating the expression of the transcription factor Kruppel-like factor 2 (KLF2), which orchestrates the transcription of a variety of protective genes including the endothelial nitric oxide synthase (eNOS), the antithrombotic molecule thrombomodulin (TM)m or the nuclear erythroid 2 p45-related factor 2 (NRF2).58 Impairment of the microcirculation is considered a major cause of reperfusion injury in steatotic livers.59 Fatty accumulation in the cytoplasm of hepatocytes is associated with an increase in cell volume that reduces the size of the hepatic sinusoid space and may contribute to a partial or complete obstruction of the hepatic sinusoid space, which causes a reduction in hepatic blood flow.59 An imbalance between vasoconstrictors (ie, endothelin [ET] 1) and vasodilators (ie, nitric oxide [NO]) negatively affects the hepatic microcirculation in steatotic livers (Fig. 2). Blebs and solidified fat globules released into the sinusoidal space further compromise the microvascular space and impair hepatic microcirculation.59
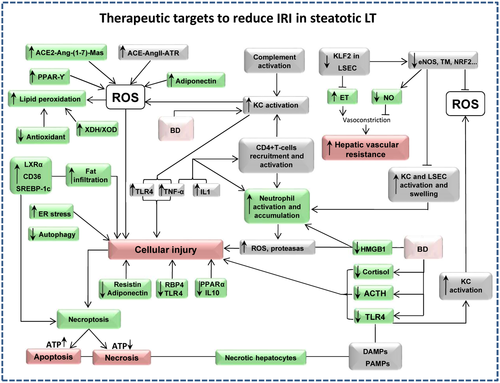
Steatotic livers are more susceptible to lipid peroxidation because of either their lower antioxidant defenses, superoxide dismutase (SOD), and glutathione (GSH), or their greater production of reactive oxygen species (ROS) from the mitochondria and/or xanthine/xanthine oxidase (XOD) system or both.60 Neutrophils and endoplasmic reticulum (ER) stress have been implicated in the increased vulnerability of steatotic livers to IRI.61 Differences were also observed when we analyzed the role of the renin-angiotensin (Ang) system, as the angiotensin-converting enzyme inhibitor (ACE)–AngII–angiotensin II receptor (ATR) and angiotensin-converting enzyme 2 (ACE2)–Ang–(1-7)–Mas receptor (Mas) axis plays a major role in nonsteatotic and steatotic grafts, respectively.5 It is well known that Kupffer cells (KCs) suffer from a profound activation process that is promoted by the release of damage-associated molecular patterns (DAMPs) from neighboring necrotic hepatic cells and pathogen-associated molecular patterns (PAMPs). Toll-like receptors (TLRs) recognize both PAMPs and DAMPs, leading to activation of downstream signaling cascades.42 Reduced retinol-binding protein 4 (RBP4) and TLR4 levels and increased peroxisome proliferator-activated receptor (PPAR) γ levels were observed in steatotic livers compared with nonsteatotic livers.53, 62 The vulnerability of steatotic livers subjected to ischemia is also associated with reduced adiponectin and resistin levels.63 It has been reported that hepatocytes with fatty infiltration develop massive necrosis after IRI, rather than apoptosis observed in nonsteatotic livers. This may be due to low adenosine triphosphate (ATP) production and dysfunction of regulators of apoptosis (B cell lymphoma 2, B cell lymphoma extra large, and B cell lymphoma 2–associated X protein).60 The up-regulation of sterol regulatory element-binding protein 1c (SREBP-1c) and the transcription factor liver X receptor (LXR) α in steatotic liver grafts contributes to the increased de novo synthesis of FA. It has been also reported that the hepatic uptake of FAs is facilitated by cell surface receptors, including CD36.64 Finally, it is worth considering recent findings indicating that BD exacerbates hepatic I/R damage in steatotic liver grafts when compared with nonsteatotic livers.57 The injurious effects of BD are mainly mediated through KC activation, leading to tumor necrosis factor α (TNF-α) amplification. BD induction resulted in a reduction in adrenocorticotropic hormone (ACTH), cortisol, high-mobility group box 1 (HMGB1), and TLR4 in steatotic LT compared with nonsteatotic LT.53, 57, 65, 66 All of these mechanisms increased the vulnerability of steatotic livers to IRI.
Effects of Steatosis and BD on Regeneration in LT
The I/R inherent to LT negatively affects regenerative responses, and BD further exacerbates the I/R process in LT. Alterations in the proteins regulating hepatic cell proliferation and differentiation have been reported under BD conditions.56, 57 After BD, the perfusion of abdominal organs is compromised. Alterations in specific mitochondrial functions may lead to impaired production of ATP, which leads to an acceleration of glycolysis and the accumulation of lactate.56 This is associated with the up-regulation of inflammatory cytokines (interleukin [IL] 6, IL10, TNF-α, tumor growth factor β [TGFβ], interferon γ, and monocyte chemoattractant protein-1α), oxidative stress, microcirculatory diseases, neutrophil accumulation, and KC activation in liver grafts.67 Thus, the events induced by BD exacerbate the hepatic damage that already occurs by I/R and, consequently, increase the risk of regenerative failure.
On the other hand, steatotic livers show differences in the regenerative response and less tolerance to damage in comparison with nonsteatotic livers.57, 68, 69 Data from preclinical studies indicate that impaired regeneration in steatotic livers was associated with interruption of the normal IL6 signaling pathway and failure of signaling at the level of the G1/S phase transition in the cell cycle during hepatocyte proliferation.70, 71 This arrest has been proposed to be due to a combination of factors, such as the inhibition of induction of the cyclin D1 gene and mitogen-activated protein kinase (MAPK) in the G1 phase of cell cycle.72 Fatty livers are more susceptible to oxidative stress, which cause direct damage to mitochondrial DNA, further exacerbating ATP depletion and mitochondrial failure. Mitochondrial cytochrome c oxidase-IV, ATP synthase-β, and reduced nicotinamide adenine dinucleotide (NAD) dehydrogenase-3 markedly decreased in fatty partial grafts.73, 74 In addition, the inflammatory response in steatotic livers following surgery due to microcirculatory disturbances, KC dysfunction, and increased leukocytes negatively affect liver regeneration.42
Therapeutic Strategies Applied in Steatotic Livers for Transplantation
Numerous experimental studies have focused on inhibiting the harmful effects of the I/R-associated inflammatory response.75 The difficulty of blocking the inflammation related to this process must be considered because, among other factors, many mediators and cell types are involved in this kind of inflammatory response.76 Interestingly, and although LDLT is an established treatment for end-stage liver disease, we have not found many studies reporting strategies aimed at using steatotic grafts from these donors. In clinical practice, only a few pharmacological approaches based on benzafibrate, an activator of PPARα and PPARβ/δ, have been used to treat living donors for LT. Other drugs that could potentially be taken by LDLT presented significant adverse effects that limited their use.77 This is a point to bear in mind because living donors could potentially increase the number of available grafts and therefore help reduce waiting lists. Further studies on this aspect are necessary.
Table 1 summarizes the experimental studies describing pharmacological approaches in experimental models of steatotic liver surgery associated with hepatic resections and LT covering the period 2011-2018. Please note that previous reviews based on this topic summarized the potential therapeutics described before 2011.61, 90, 91 Although there is much heterogeneity between methods, most of the therapeutic approaches have dedicated their efforts to improving pathophysiological events by reducing inflammation and oxidative stress and maintaining ATP levels and mitochondrial integrity, as well as molecular pathways (ie, phosphoinositide 3-kinase [PI3k]/Akt activation). In other words, studies have focused on the mechanisms that have traditionally been considered responsible for the vulnerability of the steatotic grafts to IRI. However, there are no conclusive studies that describe in detail the signaling pathways involved in this vulnerability. On the other hand, only a few studies have focused on evaluating the repercussions of drug administration and adverse effects. In this sense, just 1 study81 compared whether the effect of the drug used is dependent on the type of steatosis, although it was performed in a normothermic condition rather than LT. Furthermore, the majority of drugs/treatments were administered in donors before IRI57, 63-66, 76, 78-81, 83, 84, 87, 88; it would be interesting to evaluate whether these drugs are equally effective when applied to recipients which would make their clinical use easier. In addition, in order to ensure effective strategies, the drug pretreatment times tend to be long in donors. This is an obvious difficulty due to the low feasibility of longterm drug administration for some I/R processes. This is particularly problematic in LT from cadaveric donors because of the very short period of time available in which to pretreat them in this emergency procedure. Many of the previously mentioned studies (13 of 23) were performed in rat models with moderate steatotic livers.53, 57, 62, 63, 65, 66, 76, 78-80, 84, 86, 87 Of these studies,11 used fatty livers from Zucker rats53, 57, 62, 63, 65, 66, 68, 77, 78, 87-89; 5 used a methionine-choline deficient diet53, 68, 82, 83, 89 and 5 a HFD,64, 79-81, 85, 88 both models of steatohepatitis. Finally, in 2 studies steatohepatitis was induced by alcohol.64, 76 Only 3 experimental studies used steatotic grafts from previously induced BD donors.57, 65, 66 Considering the complexity of the mechanisms involved in hepatic IRI, the experimental conditions used will have a great impact, eg, type of research (in vitro, ex vivo, or in vivo), type of ischemia applied (warm or cold), period of ischemia (ranging from minutes to days), extent of hepatic ischemia (partial or total), or the subclinical situation of the graft (healthy, steatotic, aged, and so on). The effectiveness of a certain strategy could differ depending on the surgical conditions evaluated. Consequently, protective strategies that work under some conditions may be ineffective or even deleterious when these conditions change.
| Drug Name | Reference | Experimental Model | Effects | |||||
|---|---|---|---|---|---|---|---|---|
| Administration | Species | Steatosis Model | Steatosis Degree | Type of I/R | I/R Times | |||
| SQ22536 (cAMP inhibitor) | Jiménez-Castro et al.78 (2011) | 300 µg/kg (IP) in donor, 5 minutes before surgery | Rat | Genetic, Zucker | Moderate macrovesicular and microvesicular steatosis (Oil Red O staining) | Ex vivo LT | CIT: 6 hours | ↓Lethality, ↓hyaluronic acid, ↓vascular permeability, ↓edema, ↓endothelial cell damage, ↓microvascular diseases, ↓hexose 6-phosphates, ↓lactate, ↓MDA, ↓nitrotyrosine, ↓XOD activity, ↓cAMP, ↑GSH, ↑ATP, ↓damage score |
| RT: 4 hours | ||||||||
| Recombinant adiponectin or resistin | Jiménez-Castro et al.63 (2013) | Adiponectin: 1.5 mg/kg (IV) in donor, 20 minutes before surgery | Rat | Genetic, Zucker | Moderate macrovesicular and microvesicular steatosis (Oil Red O staining) | In vivo LT | CIT: 6 hours | ↑PI3K/Akt pathway, ↓ALT, ↓AST, ↓α-GST, ↑Ki-67–positive hepatocytes, ↓lethality, ↓damage score |
| Resistin: 100 µg/kg (IV) in donor, 10 minutes before surgery | RT: 4 hours | |||||||
| Recombinant RBP4 or PPARy antagonist (GW9662) | Casillas-Ramírez et al.62 (2011) | Recombinant RBP4: 150 µg/kg (IV) in donor, 30 minutes before surgery | Rat | Genetic, Zucker | Moderate macrovesicular and microvesicular steatosis (Oil Red O staining) | In vivo LT | CIT: 6 hours | ↓PPARγ, ↓ALT, ↓AST, ↓damage score |
| GW9662: 1000 µg/kg (IP) in donor, 1 hour before surgery | RT: 4 hours | |||||||
| Tauroursodeoxycholic acid (TUDCA) | Jiménez-Castro et al.53 (2012) | 100 mg/kg (IP) in donor, 10 minutes before surgery | Rat | Genetic, Zucker, and nutritional choline-deficient diet | Moderate macrovesicular and microvesicular steatosis (Oil Red O staining) | In vivo LT | CIT: 6 hours | ↓PPARγ, ↓ALT, ↓AST, ↓damage score, ↑TLR4, ↑TRIF, ↓lethality |
| RT: 4 hours | ||||||||
| LXRα-RNAi-lentiviral (LV) (lentiviral-mediated RNA interference of LXRα) | Zhao et al.64 (2015) | 7×107 TU LXRα-RNAi-LV/rat (intraportal) in donor, 72 hours before surgery | Rat | Nutritional, HFD, and 56% alcohol | Severe macrovesicular steatosis (H & E staining) | In vivo LT | CIT: 46 minutes | ↓SREBP-1c, ↓CD36, ↓FA accumulation, ↓ALT, ↓AST, ↓IL1β, TNF-α, ↓apoptosis, ↑survival |
| RT: 2 and 24 hours | ||||||||
| Acetylcholine | Jiménez-Castro et al.57 (2015) | 500 µg/kg (IV) in donor, just after BD induction | Rat | Genetic, Zucker | Moderate macrovesicular and microvesicular steatosis (Oil Red O staining) | In vivo LT from BD donors | CIT: 6 hours | ↓ALT, ↓AST, ↓damage score, ↑PKC ↑SOD, ↑GSH, ↑GSPx levels, ↓MDA, ↓neutrophil accumulation |
| RT: 2, 4, and 16 hours | ||||||||
| Recombinant HMGB1 (reduced form) | Cornide-Petronio et al.66 (2016) | 0.6 mg/kg (IP) in donor, just after BD induction | Rat | Genetic, Zucker | Moderate macrovesicular and microvesicular steatosis (Oil Red O staining) | In vivo LT from BD donors | CIT: 6 hours | ↓ALT, ↓AST, ↓damage score, ↓MDA, ↓nitrotyrosines, ↓neutrophil accumulation, ↑PI3K/Akt, ↑survival |
| RT: 4 hours | ||||||||
| Cortisol | Jiménez-Castro et al.65 (2017) | 5 mg/kg (IP) in donor, 15 minutes before BD induction | Rat | Genetic, Zucker | Moderate macrovesicular and microvesicular steatosis (Oil Red O staining) | In vivo LT from BD donors | CIT: 6 hours | ↓AST, ↓ALT, ↑PI3K-PKC, ↓MDA, ↓neutrophil accumulation, ↑ACTH |
| RT: 4 hours | ||||||||
| Multidrug donor preconditioning (MDDP) II (Multidrug): Pentoxyphylline, glycine, deferoxamine, N-acetylcysteine, erythropoietin, melatonin, and simvastatin | von Heesen et al.79 (2012) | Pentoxyphylline: 50 mg/kg (IP); glycine: 100 mg/kg (IP); deferoxamine: 30 mg/kg (IP); N-acetylcysteine: 150 mg/kg (IP); erythropoietin: 1000 UI (IP); melatonin: 10 mg/kg (IP), simvastatin: 5 mg/kg (intragastric), before surgery | Rat | Nutritional, high-carbohydrate, fat-free diet | Moderate macrovesicular steatosis (Sudan staining) | Ex vivo LT | CIT: 24 hours | ↓MDA, ↑bile flow, ↓ALT, ↓AST, ↓IL1, ↓apoptosis, ↓GLDH, ↓LDH |
| RT: 1 hours | ||||||||
| Acetazolamide (carbonic anhydrase inhibitor) | Bejaoui et al.77 (2015) | 30 mg/kg (IV), 10 minutes before liver procurement | Rat | Genetic, Zucker | Moderate macrovesicular and microvesicular steatosis (H & E staining) | Ex vivo LT | CIT: 24 hours | ↓ALT, ↓AST, ↑bile production, ↑BSP clearance, ↓free cholesterol, ↓triglycerides, ↓vascular resistance, ↓MAPK |
| RT: 2 hours | ||||||||
| CoPP (HO-1 inducer) | Kim et al.76 (2012) | 5 mg/kg (IP), 24 hours before surgery | Rat | Nutritional, chronic ethanol feeding | Moderate steatosis | Ex vivo LT | CIT: 24 hours | ↑Bile output, ↓LDH, ↓MDA, ↑GSH, ↓portal pressure, ↑hyaluronic acid clearance |
| Type of steatosis and staining not indicated | RT: 2 hours | |||||||
| Recombinant MnSOD | Hide et al.80 (2014) | 150 µg/kg (IV), 30 minutes before liver isolation | Rat | Nutritional, HFD | Severe macrovesicular steatosis (Oil Red O staining) | Ex vivo LT | CIT: 16 hours | ↓AST, ↓ALT, ↓LDH, ↑SOD, ↓vWF, ↓MDA, ↓nitrotyrosines, ↑NO |
| RT: 1 hour | ||||||||
| Simvastatin | Gracia-Sancho et al.81 (2013) | 1 mg/kg (IV), 30 minutes before liver procurement | Rat | Nutritional, HFD | Moderate and severe macrovesicular and microvesicular steatosis (Oil Red O staining) | Ex vivo LT | CIT: 16 hours | ↓AST, ↓ALT, ↓apoptosis, ↓LDH, ↑eNOS, ↑cGMP, ↑microcirculation |
| RT: 1 hour | ||||||||
| Y-27632 (Rho-kinase inhibitor) | Kuroda et al.82 (2015) | 0.1 mg/kg of liposomes (IV), 30 minutes before ischemia | Rat | Nutritional, choline-deficient diet | Not indicated | In vivo total warm ischemia | WIT: 30 minutes | ↓AST, ↓ALT, ↑survival |
| RT: 3 hours | ||||||||
| Hydrolyzed whey peptide (nutritional supplement) | Nii et al.83 (2014) | 4 mL (intragastric), immediately after reperfusion and every 6 hours thereafter | Rat | Nutritional, choline-deficient diet | Severe steatosis | In vivo total warm ischemia | WIT: 30 minutes | ↓AST, ↓ALT, ↓TNF-α, ↓IL6, ↓iNOS, ↓UCP2, ↑survival, ↓necrotic areas |
| Type of steatosis: not indicated (H & E staining) | RT: 6 and 12 hours | |||||||
| IL10 | Sutter et al.84 (2014) | 1 µg/rat (IP), 30 minutes before surgery | Mouse | Genetic, B6. V-Lepob/J | Moderate steatosis | In vivo total warm ischemia | WIT: 15 minutes | ↑Survival, ↑IL10 mRNA, ↓IL1β mRNA, ↓ALT |
| Type of steatosis and staining not indicated | RT: 6 and 24 hours | |||||||
| Glucose or lipid emulsion | Mendes-Braz et al.68 (2014) | 5 mL glucose or 5 mL lipid solution (IV), 4 hours after surgery | Rat | Genetic, Zucker, and nutritional choline-deficient diet | Severe macrovesicular and microvesicular steatosis | Partial warm ischemia + PH | WIT: 60 minutes | ↓AST, ↓ALT, ↑ratio sphingosine-1-phosphate/ceramide, ↑phospholipid, ↑HGF, ↑proliferation, ↑ATP, ↑IL6, ↓TGFβ |
| (Oil Red O staining) | RT: 12, 24, and 48 hours | |||||||
| Ankaflavin (food additive) | Yang et al.85 (2015) | 0.624 mg/kg (gavage), once a day for 1 week | Mouse | Nutritional, HFD | Moderate steatosis | Partial warm ischemia | WIT: 1 hour | ↓Steatosis, ↓ALT, ↓AST, ↓apoptosis, ↓lipid peroxidation, ↓TNF-α, ↓IL6,↓IL1β |
| Type of steatosis not indicated | RT: 3 hours | |||||||
| Taurine (Kupffer cell inhibitor) and glycine (endogenous antioxidant radical scavenger) | Bruns et al.86 (2011) | Taurine or glycine solution, 300 mM (IV), 10 minutes before surgery | Rat | Nutritional, ethanol via gavage | Moderate steatosis | Partial warm ischemia | WIT: 60 minutes | ↓Leukocyte, ↓platelet-endothelium interactions, ↓AST, ↓ALT, ↓LDH |
| Type of steatosis and staining not indicated | ||||||||
| Melatonin (hormone) | Kireev et al.87 (2013) | 10 mg/kg (IP and orally), 24 hours before and after surgery | Rat | Genetic, Zucker | Moderate macrovesicular and microvesicular steatosis (H & E staining) | Partial warm ischemia | WIT: 35 minutes | ↓AST, ↓ALT, ↓apoptosis, ↓lipid peroxidation, ↓TNF-α, ↓iNOS |
| Exendin 4 (GLP-1 analogue) | Gupta et al.88 (2014) | 20 µg/kg (IV), 2 hours before and after surgery | Mouse | Nutritional, HFD | Moderate macrovesicular and microvesicular steatosis | Partial warm ischemia | WIT: 20 minutes | ↓ALT, ↓mitochondrial damage, ↓autophagy |
| Staining not indicated | RT: 24 hours | |||||||
| Anti-visfatin antibodies or resistin | Elias-Miró et al.89 (2014) | Anti-visfatin antibodies: 500 µg/kg (IV), 30 minutes before surgery | Rat | Genetic, Zucker, and nutritional choline-deficient diet | Severe macrovesicular and microvesicular steatosis (Oil Red O staining) | Partial warm ischemia + PH | WIT: 60 minutes | ↓AST, ↓ALT, ↓TGFβ, ↓NAD, ↓L-arginine, ↓urea, ↑regeneration |
| Resistin: 100 µg/kg (IV), 10 minutes before surgery | RT: 24 hours | |||||||
| M3 mAChR antagonist | Cornide-Petronio et al.69 (2017) | 5 mg/kg (IP), 10 minutes before surgery | Rat | Genetic, Zucker | Severe macrovesicular and microvesicular steatosis (Oil Red O staining) | Partial warm ischemia + PH | WIT: 60 minutes | ↓AST, ↓ALT, ↑PI3K/Akt, ↑HGF, ↓TGFβ, ↑survival |
| RT: 16 and 24 hours | ||||||||
Moreover, most of the reported studies have been performed in normothermic hepatic I/R models with or without hepatic resection68, 69, 82-89 conditions that greatly differ from the clinical practice of LT. Additionally, mechanisms involved in hepatic IRI may differ depending on the method used to induce experimental steatosis. For example, as opposed to other models of steatosis, both dietary and alcohol exposure induce the production of SOD/catalase-insensitive ROS, which may partly explain steatotic failure after LT. Neutrophils, which have been implicated in the increased vulnerability of alcohol-induced steatotic livers to IRI, would not account for I/R damage in nonalcoholic steatotic livers. Furthermore, hepatocytes and LSEC are more vulnerable to I/R than other hepatic cells and, in addition, show varying sensibility to different ischemic conditions.92 Given this, it would be extremely useful to make a clear distinction between the different types of steatosis (obesity/nutritionally induced versus alcoholic) for each surgical situation in order to design therapies that demonstrate its effectiveness under experimental conditions similar to those in the clinical setting. Moreover, few studies have closely mimicked the clinical practice of steatotic LT.53, 62-64, 66 In contrast, most studies reported I/R under normothermic conditions with or without hepatic resection.58, 62, 68, 76, 77, 79, 81-83, 85, 87-89 Importantly, if the aim of a certain study is to investigate the role of inflammation in hepatic IRI, it is required to select an appropriate experimental model of genetically induced steatosis because it is not associated with inflammation that occurs in experimental models of steatosis induced by diet. Indeed, choline-deficient diet is associated with inflammation, which may conceal the inflammation associated with BD by itself and that induced by hepatic I/R.53 Under these conditions, it is impossible to evaluate the mechanisms responsible for the inflammation associated with I/R in LT and hepatic resections.
As discussed previously, we must distinguish between types of ischemia (warm and cold) because of the existing controversy about the pathophysiological mechanisms of cold ischemia in comparison to warm. Only 1 study aimed to evaluate the effect of the same drug (simvastatin, a hepatic and vascular protector) in preclinical situations of ex vivo cold storage using normal and steatotic grafts and also during in vivo warm IRI.81
Intensive research in small LT is required to expand liver donor pool. In this clinical situation, we should consider liver regeneration inherent to the surgical procedure and the mechanisms of hepatic damage derived from the removal of the tissue. Hepatic artery thrombosis and biliary complications as well as problems associated with small-for-size syndrome and regenerative failure have been extensively reported in split-liver transplantation.93, 94 However, clinical data suggest that the use of split LT may be an excellent option to expand the cadaveric donor pool because the patient survival is actually similar to recipients of whole organs.95, 96 Indeed, several countries have implemented guidelines that allow for an increase in the liver-splitting rates.97-99
For LDLT, periods of ischemia range from 40 to 60 minutes; this range may not be accurate for cadaveric donor LT. Additionally, in experimental models of the perfused liver, the liver is isolated from the influence of blood and other tissues, and this is not the case with cadaveric donor LT.90
In LDLT, the potential use of grafts with undetermined tumors is not only an ethical issue but also indicates a high risk for recipients, which may experience rapid dysfunction of their liver grafts. On the basis of studies in the literature, grafts with benign tumors are feasible in some conditions, but intensive preclinical studies and more studies with longterm follow-up are needed to evaluate the safety of these types of liver grafts. We believe that approaches designed to limit mechanisms involved in I/R damage may be capable of reducing the incidence of metastasis following surgery. This is because the mechanisms involved in hepatic I/R damage negatively affect tumor growth. Postischemic microcirculatory disturbances accelerated the outgrowth of colorectal liver metastases after I/R.100 Hepatic I/R results in microcirculatory disturbances, macrophages, and excessive inflammation, which induce polymorphonuclear neutrophils, vascular cell adhesion molecule 1, matrix metalloproteinase 9, and ET-1, all of which may also directly stimulate tumor cell proliferation and promote tumor invasion and metastasis. Thus, inhibition of the cytokines involved in hepatic I/R damage may represent a potential approach to limiting metastasis following hepatic I/R.101 First, inflammatory cytokines may promote adhesion of tumor cells and promote angiogenesis. Resveratrol, rosiglitazone, a potent PPARγ agonist, the heat shock protein 90 inhibitor (alvespimycin), atrasentan, and L-arginine—all of which reduce hepatic I/R mechanisms—have been reported as effective anticancer therapies for the prevention of hepatic tumor growth and metastasis.100-102 However, before clinical application, intensive investigations will be required with pharmacological strategies aimed at increasing liver regeneration (for instance, hepatocyte growth factor [HGF] or insulin-like growth factor), TNF-α, and IL6 because these may also directly stimulate tumor cell proliferation.
In addition to the pharmacological approaches described in the present review, it should be mentioned that machine perfusion (MP) has emerged as a suitable strategy for preserving liver grafts with promising data over the past decade, especially when marginal organs, such as steatotic livers, are used for transplantation. The benefits of normothermic MP have been reported in LT from non–heart-beating donors.103 Recently, a new preservation modality was described that combines MP under subnormothermic conditions with a new hemoglobin-based oxygen carrier solution, triggering regenerative and cell protective responses resulting in improved allograft function.104 Hypothermic MP is increasingly being used as an alternative method to static cold storage for the preservation of grafts obtained from nonoptimal donors, demonstrating improved preservation by providing oxygen to livers. Whether liver MP will find its way into widespread clinical application remains uncertain. MP has been criticized for its complicated logistics (ie, portability) and for possibly damaging the organ and vital structures such as the endothelium.105
Conclusion
- Minimize the adverse effects of IRI, thus improving outcomes in steatotic LT.
- Increase the number of both suitable transplantation grafts and patients who successfully recover from LT.
However, much needs to be done to transfer experimental results to clinical practice. Indeed, we still do not know precisely how steatosis (type, grade, and so on) affects the postoperative results and graft survival. In addition, the success or dysfunction of liver grafts may be affected by other factors, including donor age, CIT, and length of intensive care stay. Furthermore, a method for determining the extent of steatosis remains imprecise and inconsistently reported. All these premises imply that new preclinical studies in the field of LT must be performed in experimental models as close as possible to the real clinical conditions (ie, coming from cadaveric or living donors, with proper ischemia times, presence of steatosis, age of the donor, or type of death). Indeed, the extent and time of ischemia, the type of donor and liver submitted to I/R, and the presence of liver regeneration all influence the mechanisms of hepatic IRI and, thus, the effects of therapeutic strategies. However, few advances merging the presence of steatosis and the different conditions of donors have been made in recent years. Future preclinical studies and larger clinical studies aimed at evaluating whether the reduction in fatty infiltration affects both the donor and the recipient postoperative outcomes should be examined. Nevertheless, drugs or surgical procedures that reduce hepatic steatosis should be considered with caution in clinical liver surgery by the potential adverse effects of these drugs or the potential risks associated with bariatric surgery. On the other hand, there is a high heterogeneity between methods and therapeutic options for reducing IRI, the majority of them being administered to donors before IRI and not to recipients, which would facilitate their clinical applicability. In addition, there is no consensus on which experimental model should be used, especially given that the effectiveness of a certain strategy could differ depending on the surgical conditions evaluated. Thus, as researchers, we should focus on the use of standardized experimental models of LT with steatotic grafts that resemble clinical practice as much as possible in order to consider and evaluate the relevance of all of the variables involved. Of course, the selection of an appropriate experimental model of steatosis is required if the aim is to evaluate the effects of the presence of fatty infiltration (by itself without associated inflammation) on the underlying mechanisms responsible for either inflammation associated with I/R or regeneration. Only under these premises will we be able to solve the current clinical problem with steatotic grafts and transfer experimental results to clinical practice.