Pro: The Role of Albumin in Pre–Liver Transplant Management
Abstract
The wait-list mortality of patients with decompensated cirrhosis awaiting liver transplantation remains elevated due to the occurrence of complications. Etiologic treatments improve patient survival and lower the incidence of complications when applied in compensated cirrhosis, but a decompensated disease does not improve or even progress despite a response to therapy in a substantial number of patients. Thus, disease-modifying treatments that reduce the incidence of complications and improve survival are most needed. Such treatments should be able to counteract one or possibly more pathophysiological mechanisms and thus lead to the proinflammatory and pro-oxidant milieu that characterizes decompensated cirrhosis. In this respect, albumin represents a potentially ideal agent. In fact, besides its ability to expand plasma volume, albumin possesses nononcotic properties, exerting potent antioxidant and immune-modulating effects. Recent studies have assessed the effect of longterm albumin administration in decompensated cirrhosis. Although the results of these studies may appear conflicting, their analyses suggest that albumin, if given in a sufficient amount and for a sufficient duration, can significantly reduce the incidence of life-threatening complications of cirrhosis and patient mortality. For these reasons, we favor albumin administration to patients with decompensated cirrhosis wait-listed for liver transplantation.
Abbreviations
-
- CTP
-
- Child-Turcotte-Pugh
-
- DAMP
-
- danger-associated molecular pattern
-
- HA
-
- human albumin
-
- HE
-
- hepatic encephalopathy
-
- HPS
-
- hepatopulmonary syndrome
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NF-κB
-
- nuclear factor kappa B
-
- PAF
-
- platelet-activating factor
-
- PAMP
-
- pathogen-associated molecular pattern
-
- QoL
-
- quality of life
-
- RNS
-
- reactive nitrogen species
-
- ROS
-
- reactive oxygen species
-
- SA
-
- serum albumin
-
- SBP
-
- spontaneous bacterial peritonitis
-
- SMT
-
- standard medical treatment
-
- TNF-α
-
- tumor necrosis factor α
-
- TXA2
-
- thromboxane A2
-
- VCAM1
-
- vascular cell adhesion molecule 1
Disease-Modifying Treatments of Patients Awaiting Liver Transplantation: An Unmet Need
Decompensated cirrhosis of the liver is a leading indication for liver transplantation (LT) worldwide.1, 2 Despite decreasing over recent years, wait-list patient mortality because of end-stage liver disease remains elevated. By analyzing the Organ Procurement and Transplantation Network registry,2 it appears that from 2007 to 2017, a mean of 13.9% of candidates per year died while on the waiting list, to which 11.8% should be added because they were removed from the list due to an excessive worsening of their disease. The occurrence of complications, such as ascites, renal dysfunction and hepatorenal syndrome, variceal bleeding, hepatic encephalopathy (HE), and bacterial infections, accounts for this adverse outcome. Moreover, in addition to favoring mortality or extreme worsening of clinical conditions, some complications, such as bacterial infections, require temporary delisting, further delaying the reach of LT, while others, such as hyponatremia and renal dysfunction, adversely affect the LT outcome.3-6 Thus, effective treatments able to prevent the occurrence of the complications of cirrhosis for patients waiting for LT are most warranted to improve both their survival and their post-LT outcome.
Treatments antagonizing the underlying etiologic causes of cirrhosis, such as antivirals for hepatitis B and C, substantially improve patient survival and lower the incidence of complications. However, their effectiveness is maximal when applied during the compensated stage of the disease. Indeed, in 40%-60% of patients with decompensated cirrhosis who receive a successful etiologic treatment, their disease either does not improve or may even continue its course.7
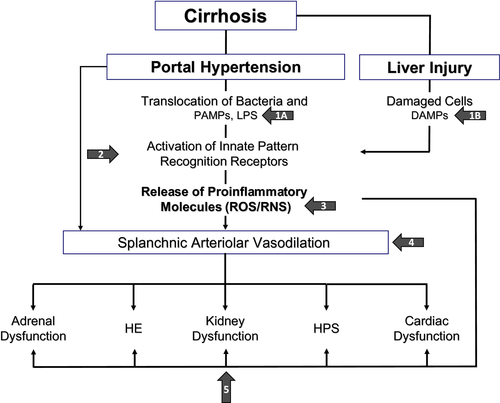
Thus, other strategies are needed that are able to slow down the course of decompensated cirrhosis and reduce the incidence of its most ominous complications. The recent advances in the knowledge of the pathophysiological background of decompensated cirrhosis provide the basis for the development of treatments directed at counteracting one or, possibly, more pathophysiological mechanisms.7 Over the last decade, it has become clear that patients with advanced cirrhosis present a sustained proinflammatory and prooxidant milieu. This results both from the systemic spread of bacteria and pathogen-associated molecular patterns (PAMPs) caused by abnormal translocation from the gut and also from the danger-associated molecular patterns (DAMPs) that are released from the diseased liver where inflammation, apoptosis, and cell necrosis take place. These events lead to the activation of immune cells, which are stimulated to synthetize and release proinflammatory cytokines and chemokines. This cascade of events leads to cardiocirculatory and extrahepatic organ dysfunctions that characterize decompensated cirrhosis (Fig. 1).8
Rationale for Longterm Administration of Albumin in Decompensated Cirrhosis
Among the molecules that at least theoretically could act as pathophysiological treatments in the setting of decompensated cirrhosis stands human albumin (HA).7 Indeed, besides its oncotic power that make it a very effective plasma expander, albumin possesses functional domains with important properties, such as the free cysteine residue in position 34, which exerts potent antioxidant and scavenging activities, the amino-terminal site that removes highly toxic reactive metal species, and many other domains binding a variety of endogenous and exogenous substances, including PAMPs, DAMPs, and endotoxin.9 Moreover, albumin carries immune-modulatory functions, protects capillary integrity, and influences acid-base balance and hemostasis (Table 1).9
| Colloid osmotic property | |
| Regulation of fluid partition within extracellular (intravascular; interstitial) compartments. | |
| Noncolloid osmotic properties | |
| Antioxidation |
Free cysteine residue at position 34 scavenging for different oxidative and nitrosative reactive species N-terminal binding and neutralizing free Cu2+ and iron that catalyze reactions in which free radicals are released Formation of albumin-heme complex that provides a lipid antioxidant effect |
| Binding/inactivation | Exogenous (drugs) and endogenous substances including PAMPs, DAMPs, endotoxin, bilirubin, bile acids, and prostaglandin E2 |
| Immunomodulation |
Increase in intracellular glutathione regulating NF-κB activation Inhibition of TNF-α–induced up-regulation of VCAM1 and NF-κB |
| Endothelial stabilization | Modulation of inflammation, reducing oxidative damage, interfering in neutrophil adhesion |
| Capillary permeability | Influence on vascular integrity and permeability by way of interactions with the extracellular matrix |
| Hemostatic effects | Binding arachidonic acid, inactivation of TXA2, binding PAF |
| Acid-base balance | Acting as a weak acid |
Reduced serum albumin (SA) concentration is frequent in advanced cirrhosis and is associated with poor survival.10 Moreover, it has become clear that SA in cirrhosis undergoes functional and structural changes.11-16 Damaged isoforms, such as cysteinylated, sulfydrylated, glycosylated, and truncated albumin, as well as homodimers of the molecule, are increased in patients with cirrhosis, leading to a substantial decline in the relative abundance of the intact “native” albumin. Such abnormalities become more evident in parallel with the severity of cirrhosis, predict survival better than total SA concentration, and are associated with specific complications. These findings clearly show that besides hypoalbuminemia, patients with cirrhosis present structural abnormalities of albumin, which endanger its nononcotic properties. Thus, the serum “effective” albumin concentration,9 which is not assessed by the methods currently employed in clinical practice, can be strikingly reduced.
Current international guidelines provide specific indications for the use of albumin in cirrhosis: prevention of paracentesis-induced circulatory dysfunction and its clinical consequences, prevention of renal dysfunction in patients with spontaneous bacterial peritonitis (SBP), and treatment of hepatorenal syndrome associated with the administration of vasoconstrictors.17, 18 The beneficial effects of albumin in these settings have been traditionally attributed to its capacity to expand plasma volume because these complications are characterized by an abrupt worsening of effective volemia. However, evidence has emerged that these effects may be also related to the nononcotic properties of the molecule. Indeed, albumin but not hydroxyethyl starch infusion to patients with SBP increased peripheral vascular resistance and cardiac work19; albumin but not hydroxyethyl starch or saline restored heart contractility in rats with cirrhosis and ascites20; albumin is currently used as a toxic adsorbent in extracorporeal liver assist devices21; and finally, albumin improves immune-competence by binding free circulating prostaglandin E2, whose excess hampers macrophage function.22
These pieces of information can offer novel perspectives to the use of albumin in decompensated cirrhosis, besides the well-established indications reported previously. In light of the pathophysiological network of decompensated cirrhosis, the longterm administration of albumin, besides promoting plasma volume expansion or at least protecting effective volemia, could simultaneously act on several abnormalities by binding offending molecules, modulating immune responses, exerting antioxidant activity, improving cardiac function, and restoring endothelial integrity. Therefore, prolonged albumin administration may potentially represent an effective multitarget treatment (Fig. 1).7
Available Evidence on the Effects of Longterm Albumin Administration in Decompensated Cirrhosis
Until recently, data on the effect of longterm albumin administration were quite limited. In one study,23 the prolonged administration of albumin (25 g weekly for 1 year and then 25 g every 2 weeks) to hospitalized patients with cirrhosis and ascites reduced the recurrence of ascites after discharge and the need of hospital readmission but had no effect on survival during a mean follow-up of 20 months (Table 2). The same research group then reported that albumin administration for a longer period through a median follow-up of 84 months also improved survival.24 The small patient sample size, however, precluded to reach a definitive conclusion.
| References | Number of Patients | Population Characteristics | Dose of HA Infused | Follow-up Length | Effect on SA | Main Results |
|---|---|---|---|---|---|---|
| Gentilini et al.23 (1999) | 126 |
Patients hospitalized for ascites decompensation Nonresponder to bedrest and low sodium diet CTP score: 9-10 (MELD score not available) |
12.5 g/day (in-hospital phase) 25 g/week (first year) 25 g every 2 weeks (second and third year) |
Up to 3 years (mean follow-up: 20.0 ± 1.9 months) |
Increase (0.2-0.3 g/dL) |
Improvement in response to diuretics Prevention of recurrence of ascites No difference in survival |
| Romanelli et al.24 (2006) | 100 |
First onset ascites Median CTP score: 9-10 (MELD score not available) |
25 g/week (first year) then 25 g every 2 weeks |
Median follow-up: 84 months | Not available |
Decrease of ascites recurrence Improvement in overall survival |
| Caraceni et al.25 (2018), ANSWER Study | 431* |
Patients with uncomplicated ascites (not refractory) Ongoing treatment with at least 200 mg/day of antialdosteronics and 25 mg/day of furosemide Median CTP score: 8 Median MELD score: 12-13 |
40 g twice per week (for the first 2 weeks) then 40 g/week |
Up to 18 months (median follow-up: 11.5 months, SMT; 17.6 months, HA) |
Increase (0.6-0.8 g/dL) |
Reduction of overall and liver-related mortality Improvement in ascites control Reduction of incidence of complications of cirrhosis QoL benefit Cost-effective treatment |
| Solà et al.27 (2018), MACHT Study | 173* |
Patients with ascites included on the waiting list for LT Mean MELD score: 16-17 |
40 g every 2 weeks |
Up to 1 year (median follow-up: 80 days) |
No differences |
No difference in incidence of complications No difference in survival Slight decrease in plasma renin activity and aldosterone |
| Di Pascoli et al.26 (2018) | 70 |
Patients with refractory ascites Mean CTP score: 9 Mean MELD score: 15 |
20 g twice per week |
Up to 2 years (mean follow-up: 408 ± 394 days) |
Not available |
Reduction of mortality Reduction of hospitalizations for the occurrence of complications |
- *The number of patients considered for the full analysis set (ANSWER study and MACHT study randomized 440 and 196 patients, respectively).
Three studies assessing the effects of longterm albumin administration to patients with decompensated cirrhosis have been published in the current year. The ANSWER study, a multicenter, investigator-initiated, open-label, randomized trial enrolled 431 patients with cirrhosis and uncomplicated ascites requiring the daily administration of 200 mg of an antialdosteronic drug and 25 mg of furosemide.25 Patients were randomized to receive either standard medical treatment (SMT), which included albumin administration for well-established indications, or SMT plus 40 g of albumin twice a week for the initial 2 weeks and then 40 g once a week. Patients were then followed up for 18 months or to LT, to transjugular intrahepatic portosystemic shunt insertion, or to a severity of ascites refractoriness requiring 3 or more paracentesis procedures per month. The overall mortality rate, which represented the primary endpoint of the study, was significantly lower in patients receiving the longterm administration of albumin with a 38% reduction in the mortality hazard ratio. In addition to ease the control of ascites, as witnessed by a 54% reduction in the incidence rate of paracentesis and a reduced incidence of refractory ascites, prolonged treatment with albumin reduced the cumulative incidence rate of severe complications of cirrhosis, including SBP, non-SBP bacterial infections, episodes of renal dysfunction as defined by serum creatinine >1.5 mg/dL, hepatorenal syndrome type 1, and severe HE grade III or IV. Potential diuretic-induced adverse effects, such as hyponatremia and hyperkalemia, were also lessened, whereas gastroesophageal variceal bleeding did not differ between the 2 arms of the study. Besides these clinical effects, longterm albumin administration also led to a significant reduction in the need of patient hospitalizations and the number of days spent in the hospital. This result, coupled with a favorable impact on patient quality of life (QoL), led the treatment with albumin to be cost-effective.
The pragmatic nature of this trial could not clarify the exact mechanisms that led to the favorable effects of albumin. However, both hemodynamic and anti-inflammatory/antioxidant effects were likely involved. In fact, the incidence of events closely influenced by altered hemodynamics, such as ascites formation, renal dysfunction, and electrolyte abnormalities, as well as others likely related to systemic inflammation and immune dysfunction, such as HE and bacterial infections other than SBP, were significantly reduced.
A similar albumin administration schedule (20 g twice per week) has been employed in a prospective, single-center, nonrandomized trial that enrolled patients with refractory ascites.26 With respect to patients who received standard medical care, those who also received albumin experienced a lower incidence of complications requiring hospitalization, such as overt HE, ascites, and bacterial infections including SBP. Moreover, the cumulative incidence of 24-month mortality was significantly lower in patients treated with albumin.
Contrasting results emerged from the MACHT study, a multicenter, double-blind, randomized trial that enrolled patients with decompensated cirrhosis on the waiting list for LT.27 The full analysis set included 173 patients who received SMT plus either albumin (40 g/15 days), plus midodrine (15 mg/day, elevated to 30 mg/day if the increase in mean arterial pressure with respect to baseline values was <10 mm Hg), or the respective placebos. The primary endpoint was the incidence of complications of cirrhosis, including renal failure, hyponatremia, bacterial infections, HE, and gastrointestinal bleeding. Secondary endpoints were mortality, activity of endogenous vasoconstrictor systems, and plasma cytokine levels. Despite a small but significant decrease in plasma renin activity and aldosterone, suggesting a favorable effect on systemic hemodynamics, both the probability of developing complications of cirrhosis during the follow-up, even though the episodes of hyponatremia and renal failure were more severe in patients enrolled in the placebo group, and 1-year mortality did not differ between the 2 arms of the study. No effects of albumin administration on the circulating levels of proinflammatory cytokines were seen.
There are several aspects of the MACHT study that could explain its variant results with respect to the ANSWER study. Patients enrolled in the MACHT study were more severely ill, as witnessed by the higher Model for End-Stage Liver Disease (MELD) score (17 versus 13) and by the more advanced clinical stage at baseline. Moreover, the very high LT rate, a peculiarity of the area where the study was performed, determined a low wait-list mortality (approximately 6%) and a quite rapid interruption of the study for many patients so that only few of them completed the 1-year study treatment.
However, we think that the most relevant aspect is related to the different impact of the 2 schedules of albumin administration on the SA concentration: In the MACHT study, the SA concentration of the treated group remained similar to that observed in the control group throughout all the follow-up, whereas in the ANSWER study, a sustained significant increase of approximately 0.8 g/dL was obtained. This likely resulted from the different amounts of albumin administered in the 2 trials. Indeed, in contrast to those patients enrolled in the ANSWER study, those enrolled in the MACHT study received half the amount, and no loading dose of HA was administered. It is reasonable to assume that an improvement of clinical endpoints should hardly be expected if SA concentration is not influenced by the exogenous supplementation.
In conclusion, taken together, all these findings indicate that several aspects of longterm albumin administration still need to be clarified. For example, further investigations are warranted in order to determine whether a threshold value of SA concentration has to be reached to obtain favorable results, whether different degrees of severity of cirrhosis require different amounts of albumin to raise SA concentration, or whether there are patient subgroups who most benefit from albumin administration. Meanwhile, we think that the available results are strong enough to be in favor of longterm albumin administration to patients with decompensated cirrhosis, either included on the waiting list for LT or not. In the specific setting of the waiting list, the reduced incidence of severe life-threatening complications would not only enhance the patient’s chance to reach LT, but would also attenuate the impact of specific complications, such as renal dysfunction and hyponatremia, on LT outcome. Moreover, lowering the need of hospitalizations would protect patients against the risk of nosocomial infections, which are very often caused by multidrug-resistant bacteria and are burdened by a high mortality.28, 29