Predictors of De Novo Nonalcoholic Fatty Liver Disease After Liver Transplantation and Associated Fibrosis
Abstract
Nonalcoholic fatty liver disease (NAFLD) can occur de novo in patients undergoing liver transplantation (LT) for indications other than NAFLD, and it has been increasingly recognized as a complication in the post-LT setting. This study aims to better characterize de novo NAFLD after LT by identifying risk factors for its development, describing incidence and extent of fibrosis, assessing the diagnostic utility of noninvasive serum fibrosis algorithms, and comparing survival to those without NAFLD. This was a retrospective single-center analysis of de novo NAFLD in a post-LT cohort. Those whose primary indication for LT was nonalcoholic steatohepatitis (NASH) were excluded. Risk factors were analyzed by univariate and multivariate analyses. De novo NAFLD and fibrosis were assessed on posttransplant liver biopsies, and noninvasive fibrosis scores were calculated from concomitant blood tests. After applying the exclusion criteria, 430 for-cause post-LT biopsies were evaluated; 33.3% (n = 143) had evidence of de novo steatosis and/or NASH at a median of 3.0 years after transplant. On multivariate analysis, body mass index (BMI; odds ratio [OR], 1.12; P < 0.001), diabetes mellitus (OR, 3.01; P = 0.002), hepatitis C virus (OR, 4.61; P < 0.001), weight gain (OR, 1.03; P = 0.007), and sirolimus use (OR, 3.11; P = 0.02) were predictive of de novo NAFLD after LT. Significant fibrosis (≥F2) was present in almost 40% of the cohort. Noninvasive serum fibrosis scores were not useful diagnostic tests. There was no significant difference in the short-term or longterm survival of patients who developed de novo NAFLD. In conclusion, diabetes, BMI, weight gain after LT, and sirolimus-based immunosuppression, in keeping with insulin resistance, were the only modifiable factors associated with development of de novo NAFLD. A significant proportion of patients with de novo NAFLD had fibrosis and given the limited utility of noninvasive serum fibrosis algorithms, alternative noninvasive tools are required to screen for fibrosis in this population. There was no significant difference in the short-term or longterm survival of patients who developed de novo NAFLD.
Abbreviations
-
- AAR
-
- aspartate aminotransferase-to-alanine aminotransferase ratio
-
- AIH
-
- autoimmune hepatitis
-
- ALT
-
- alanine aminotransferase
-
- APRI
-
- aspartate aminotransferase-to-platelet ratio index
-
- AST
-
- aspartate aminotransferase
-
- BMI
-
- body mass index
-
- CNI
-
- calcineurin inhibitor
-
- CRN
-
- clinical research network
-
- DM
-
- diabetes mellitus
-
- FIB-4
-
- fibrosis index based on 4 factors
-
- HbA1C
-
- hemoglobin A1c
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- IR
-
- insulin resistance
-
- IS
-
- immunosuppression
-
- LR
-
- likelihood ratio
-
- LT
-
- liver transplantation
-
- mTOR
-
- mammalian target of rapamycin
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NFS
-
- nonalcoholic fatty liver disease fibrosis score
-
- NODAT
-
- new-onset diabetes after transplant
-
- OR
-
- odds ratio
-
- PBC
-
- primary biliary cirrhosis
-
- PSC
-
- primary sclerosing cholangitis
-
- TE
-
- transient elastography
The prevalence of nonalcoholic fatty liver disease (NAFLD) is increasing in parallel with the global obesity and diabetes epidemics, and it has become the leading indication for liver transplantation (LT) in the United States.1 Following LT, the universal use of immunosuppressant drugs such as corticosteroids, calcineurin inhibitors (CNIs), and mammalian target of rapamycin (mTOR) inhibitors is known to significantly impact the metabolic balance and is associated with the development of insulin resistance (IR), diabetes, hypertension, obesity, and hyperlipidemia.2-5 Many posttransplant patients fulfill the criteria for the metabolic syndrome. Given that NAFLD is the liver manifestation of the metabolic syndrome, it is not surprising that both recurrent and de novo NAFLD occur after LT.6 NAFLD encompasses a histological spectrum ranging from simple liver steatosis to severe nonalcoholic steatohepatitis (NASH). Because of ongoing injury, fibrosis and ultimately cirrhosis may develop with the potential for subsequent decompensation of the graft and/or development of hepatocellular carcinoma (HCC).
Recurrent NAFLD after LT is common, but the literature quotes a wide recurrence range. The recurrence rates for hepatic steatosis and steatohepatitis at 5 years after transplant are reported as ranging from 10.0% to 100.0% and 4.0% to 28.0%, respectively.7-9 There is, however, a paucity of studies characterizing de novo NAFLD (NAFLD that occurs in patients who underwent transplantation for indications other than NASH) in the post-LT population.10, 11 Dumortier et al. studied the development of de novo NAFLD and reported that de novo liver steatosis developed in 31.0% and steatohepatitis in 3.8% of 421 recipients at 3.3 years. Obesity, dyslipidemia, diabetes mellitus (DM), arterial hypertension, tacrolimus treatment, alcoholic cirrhosis as indication for LT, and donor graft steatosis were identified as risk factors for de novo NAFLD. The presence of an increasing number of concurrent risk factors was associated with a higher prevalence of steatosis.11 Seo et al. suggested that de novo steatosis and NASH occurred in 18.0% and 9.0% of LT recipients, respectively, and that a >10.0% increase in body mass index (BMI) after LT was a risk factor.10
Despite the surging trend in NAFLD, transplant physicians do not have an appreciation of the burden or impact of de novo disease in the transplanted population. Fibrosis and cirrhosis were reported to occur in 29.0% and 2.2% of the post-LT de novo NAFLD population.11 This obviously has significant implications in terms of disease progression and ultimately risk of decompensation and/or development of HCC. Even if these patients do not develop cirrhosis, they remain at a high risk of cardiovascular and renal morbidity and mortality.9, 12
The incidence, risk factors, and progression of post-LT NAFLD-related fibrosis have not been well elucidated to date. It is important to gauge fibrosis progression over time in order to ensure early identification of complications. Liver biopsy is the “gold standard” test to diagnose fibrosis. However, it has numerous limitations, including cost, patient acceptability, sampling error, interobserver and intraobserver variability, and the risk of complications.13 To overcome the disadvantages associated with liver biopsy, numerous noninvasive tools have been developed to detect fibrosis including transient elastography (TE),14 magnetic resonance elastography,15 and multiple serum marker algorithms, including the aspartate aminotransferase-to-alanine aminotransferase ratio (AAR),16 the aspartate aminotransferase-to-platelet ratio index (APRI),17 the BARD score,18 the fibrosis index based on 4 factors (FIB-4) score,19, 20 and the nonalcoholic fatty liver disease fibrosis score (NFS; Supporting Table 1).21 To date, none of these clinical tools have been validated in the post-LT NAFLD cohort but have been validated in other cohorts. In pre-LT NAFLD, FIB-4 has been shown to have the best diagnostic accuracy for advanced fibrosis (area under the receiver operating characteristic curve [AUROC], 0.86), followed by the AAR (AUROC, 0.83) and NFS (AUROC, 0.81).22 The use of these fibrosis scores in the general post-LT setting has been less promising (AUROCs: APRI, 0.68; FIB-4, 0.72; and NFS, 0.75).23
| Value (n = 430) | |
|---|---|
| Age, years | 49.7 ± 11.4 |
| Sex, male | 273 (63.5) |
| BMI | 27.0 ± 5.6 |
| Diabetes | 135 (31.4) |
| Hypertension | 119 (27.7) |
| Transplant indication | |
| Hepatitis C | 182 (42.3) |
| PBC/PSC/AIH | 109 (25.3) |
| Alcoholic liver disease | 42 (9.8) |
| Hepatitis B | 26 (6.0) |
| Other | 71 (16.5) |
| IS regimen | |
| Tacrolimus | 208 (48.4) |
| Cyclosporine | 218 (50.7) |
| Sirolimus | 26 (6.0) |
| Mycophenolate mofetil | 101 (23.5) |
| Prednisone | 125 (29.1) |
NOTE:
- Data are given as n (%) or mean ± standard deviation.
- Identify risk factors for the development of de novo NAFLD in the posttransplant population.
- Describe the incidence and extent of fibrosis in patients with biopsy-proven de novo NAFLD after LT and investigate the diagnostic utility of simple noninvasive serum fibrosis scores in identifying advanced fibrosis.
- Compare survival to those without NAFLD.
Patients and Methods
Study Design
This was a retrospective single-center study that analyzed demographic and clinical characteristics to assess for development of de novo NAFLD in a post-LT cohort and investigated the potential utility of noninvasive serum fibrosis scores to detect fibrosis or cirrhosis. The study received a priori approval from the Research and Ethics Committe of University Health Network, University of Toronto.
Study Population
- NAFLD group: All posttransplant liver biopsies that showed evidence of steatosis or NASH, with at least 6-month follow-up, excluding those enumerated in the exclusion criteria below.
- Comparison group: All posttransplant liver biopsies that showed no evidence of steatosis or NASH, excluding those enumerated in the exclusion criteria below.
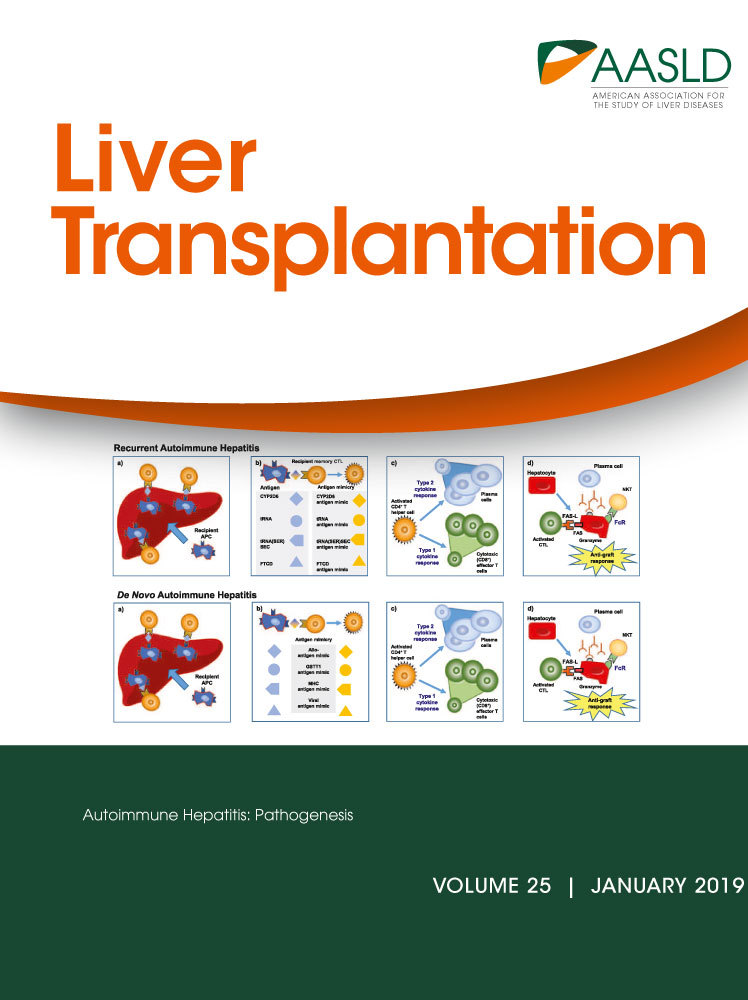
Patients transplanted for NASH or cryptogenic cirrhosis were excluded. Patients younger than 18 years of age, who underwent transplantation at another center, or who underwent multiorgan transplant were excluded. All biopsies performed within the first 12 months of LT were excluded. Biopsies from patients with genotype 3 hepatitis C virus (HCV) were excluded. In patients who had multiple biopsies, the first biopsy that showed evidence of NAFLD or the first biopsy 12 months after LT (in patients who had no NAFLD on any biopsy) were included and subsequent biopsies were excluded (Fig. 1).
Indication for Liver Biopsy
Liver biopsies were performed for multiple reasons, most commonly to investigate a cause for elevated liver enzymes. Other indications included fibrosis staging or to investigate radiological findings.
Immunosuppression Regimen
All patients received intravenous methylprednisolone followed by an oral course of prednisone and tacrolimus/cyclosporine immediately following LT. The oral prednisone dose followed a standard tapering regimen and terminated at 3 months after LT. Tacrolimus was considered the first-line CNI since approximately 1995. Tacrolimus was substituted with cyclosporine in case of tacrolimus intolerance or neurotoxicity or HCV treatment drug-drug interactions.
Mycophenolate mofetil was added if CNI dose reduction was required. Sirolimus was considered in those who were intolerant to CNIs or had evidence of impaired renal function or who underwent transplantation for HCC. Sirolimus was not started in the first 6 weeks after LT.
Steatosis and Fibrosis Scoring
Liver biopsies were deemed adequate when at least 15 mm long and/or having >11 fully sampled portal tracts. Tissues were fixed for a minimum of 2 hours in 10% formalin-buffered solution and embedded in paraffin. Sections were cut at 4-5 µm, and all were routinely stained with hematoxylin-eosin as well as Masson’s trichrome stains. Each biopsy report was subsequently reviewed and classified as having no steatosis, simple steatosis, or steatohepatitis. Any steatosis >5% was regarded as significant, whereas steatosis with evidence of cell injury characterized by ballooning and inflammation constituted the definition for steatohepatitis. The fibrosis score was assigned according to modified METAVIR (Laennec) stage 0-4 to enable comparison with the non-NAFLD (control) group.13 On this scale, NAFLD fibrosis grades 1a, 1b, or 1c fibrosis on the Kleiner-Brunt scale (ie, perivenular or periportal fibrosis with no bridging) were apportioned stage 1 out of 4 on the Laennec scale.24 Subsequently, the presence of bridging fibrosis and complete and incomplete nodules were assessed using the Laennec scale.
Data Collection
Data were extracted from the patient’s medical records. Where many results for the same variable were available, the result that was measured closest to the date of biopsy was recorded. Only blood results that were measured within 6 months of biopsy date were considered valid. Data were deidentified for analyses. The following variables were recorded from both the active and comparison groups: age; sex; height; weight at time of transplant and biopsy; BMI; primary diagnosis; secondary diagnosis; date of transplant; date of biopsy; presence of diabetes (no diabetes/diabetes pre-LT/new-onset diabetes after transplant [NODAT]); on insulin (yes/no); presence of dyslipidemia (any patient whose past medical history includes this diagnosis or who uses/has used lipid-lowering drugs); presence of hypertension (any patient whose past medical history includes this diagnosis or who uses/has used antihypertensives); immunosuppression (IS) such as steroids, tacrolimus, cyclosporine, sirolimus, and mycophenolate mofetil (yes/no for each); HCV genotype; steatosis in donor liver (>5%, yes; ≤5%, no); donor age; donor BMI; and biochemical parameters, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, bilirubin, platelets, international normalized ratio, cholesterol, triglycerides, and hemoglobin A1c (HbA1C). Before LT, the use of hypoglycemic agents was used to identify patients with diabetes. After LT, NODAT was diagnosed based on the 2003 International Consensus Guidelines25 in LT recipients who were not diabetic before transplantation.
Noninvasive Scoring Panels
Fibrosis scoring panels including AAR, APRI, BARD score, NFS, and FIB-4 were calculated based on the standard formulae.16-21 These scores can have more than 1 threshold depending on the diagnostic use. Formulas and thresholds from the literature are available in Supporting Table 1.
Statistical Analysis
For all analyses, SAS 9.4 (SAS Institute, Inc., Cary, NC) or SPSS (SPSS for Windows 20.0, Chicago, IL) was used. We considered differences statistically significant when P values were <0.05. Nominal 2-sided P values were used.
Continuous data are reported as mean or median values. Student t or nonparametric tests were used to compare continuous variables, as appropriate. Categorical data are reported as proportions/percentages, and comparisons were made using either Fisher’s exact test or chi-square test, as appropriate. Variables that were significant on univariate analysis for predicting de novo NAFLD were entered into a stepwise logistic regression analysis. The P value for addition or elimination from the models was P < 0.05. AUROCs were used to determine the performance of noninvasive fibrosis scores for predicting fibrosis in de novo NAFLD, with values close to 1.0 showing high diagnostic accuracy. An AUROC of <0.7 was considered a poor test. Test scores in the indeterminate range were excluded prior to assessing performance, ie, APRI (F2-4), scores 0.5-1.5 excluded; APRI (F4), scores 1-2 excluded; FIB-4 (F3-4), scores 1.45-3.25 excluded; and NFS (F3-4), scores 1.455-0.676 excluded. To evaluate the overall accuracy of the scores, the sensitivity, specificity, and positive and negative likelihood ratios (LRs) were also calculated. The optimal thresholds were chosen to maximize the sum of sensitivity and specificity (AAR for F4). Kaplan-Meier analysis (log-rank test) was undertaken to evaluate patient survival between the 2 groups. The rate of fibrosis progression was calculated by dividing fibrosis stage by time from transplant to biopsy (median fibrosis units/year).
Results
Study Population
There were 2422 adult patients who underwent 2574 single-organ LTs during the study period. Patients transplanted for NASH/cryptogenic cirrhosis (n = 161) were excluded. Of the remaining patients (n = 2261), 1937 patients had 8530 posttransplant biopsies performed. After applying the exclusion criteria, 430 biopsies were included (Fig. 1).
Baseline Characteristics
The majority of the cohort was male (61.2%) with a mean age of 49.7 years. At the time of biopsy, almost one-third had diabetes and/or hypertension, and the majority were overweight (mean BMI, 27.0 kg/m2). The most common indication for LT was HCV-related cirrhosis (42.4%) followed by primary sclerosing cholangitis (PSC)/primary biliary cirrhosis (PBC)/autoimmune hepatitis (AIH)–related cirrhosis (25.3%) and alcohol-related cirrhosis (9.8%). At the time of the biopsy, most patients were on a CNI, and almost 25.0% were on mycophenolate mofetil, almost 30% on steroids, and 6.0% on sirolimus (Table 1).
De Novo Post-LT NAFLD
Among the 430 patients, 143 (33.0%) had at least 1 biopsy that showed evidence of de novo NAFLD. The time from LT to biopsy was 3.0 years in the NAFLD group and 3.2 years in the control (no NAFLD) group, and this difference was not significant.
The risk factors for development of de novo NAFLD were analyzed (Table 2). Among the pre-LT variables, older age, male sex, and HCV as an indication for LT were significantly higher in the NAFLD group. Older donor age and higher donor BMI were significantly associated with NAFLD, although complete donor data were not available (Table 2). The NAFLD group tended to have more initial graft steatosis, but this was not significantly different between the NAFLD and control groups, although a report was not available in the majority of patients. Among the post-LT risk factors, a higher BMI at the time of biopsy, weight gain (from transplant to biopsy), a higher HbA1C, and the presence of diabetes were significantly associated with NAFLD. Patients who were on insulin, sirolimus, or cyclosporine at the time of biopsy were also significantly more likely to be in the NAFLD group. Significantly fewer patients in the NAFLD group were on steroids at the time of biopsy (Table 2).
| Characteristic | NAFLD Group (n = 143) | Control Group (n = 287) | P Value |
|---|---|---|---|
| At time of LT | |||
| Age, years | 54.0 (49.0-58.0) | 50.0 (39.0-58.0) | 0.001* |
| Sex, male | 102 (71.3) | 161 (56.1) | 0.002* |
| Donor steatosis, % | 0.11 | ||
| Yes | 14 | 7.7 | |
| No | 7 | 8.4 | |
| No report | 79 | 84 | |
| Donor age, years† | 47.0 (33.0-57.8) | 41.0 (22.0-54.5) | 0.02* |
| Donor BMI‡ | 25.3 (23.1-28.6) | 24.7 (22.2-27.7) | 0.07* |
| HCV, % | 65.7 | 24.0 | 0.001* |
| At time of liver biopsy | |||
| Age, years | 57.0 (52.0-61.0) | 55.3 (46.1-62.5) | 0.07 |
| BMI, kg/m2 | 29.4 (26.3-34.0) | 24.9 (22.3-28.0) | <0.001* |
| Weight gain, transplant to biopsy (kg) | +2.2 (–6.9 to +10.4) | –1.4 (–7.2 to +3.3) | 0.001 |
| Time to biopsy, years | 3.0 (1.9-5.5) | 3.2 (1.5-7.9) | 0.59 |
| Diabetes, % | 49.7 | 28.9 | <0.001* |
| No diabetes | 50.3 | 71.1 | |
| Diabetes before LT | 18.2 | 10.1 | |
| NODAT | 31.5 | 18.8 | |
| Total cholesterol, mmol/L | 3.9 (3.3-4.8) | 4.3 (3.5-5.2) | 0.09 |
| Triglycerides, mmol/L | 1.5 (1.2-2.1) | 1.5 (1.0-2.0) | 0.16 |
| HbA1C, g/dL | 0.06 (0.05-0.06) | 0.05 (0.04-0.06) | 0.02* |
| On insulin | 26.6 | 14.3 | 0.002* |
| Hypertension | 25.8 | 28.9 | 0.56 |
| IS regimen | |||
| Tacrolimus | 48.3 | 48.4 | 0.97 |
| Cyclosporine | 58.7 | 46.9 | 0.02* |
| Sirolimus | 9.8 | 4.2 | 0.02* |
| Mycophenolate | 22.4 | 24.0 | 0.70 |
| Steroids | 19.6 | 33.8 | 0.002* |
| Era of transplant | <0.001* | ||
| 1986-1995 | 0.7 | 20.6 | |
| 1996-2005 | 37.8 | 40.4 | |
| 2006-2015 | 61.5 | 39.0 |
NOTE:
- Data are given as n (%) and median (interquartile range).
- a Significant at level of P < 0.05.
- b Missing data on 147 patients (NAFLD, n = 33; control, n = 114).
- c Missing data on 159 patients (NAFLD, n = 36; control, n = 123).
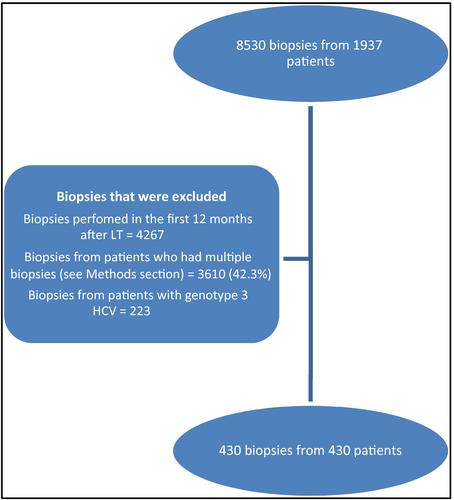
Using stepwise logistic regression, BMI at time of biopsy, weight gain, diabetes, HCV as primary indication for transplant, and sirolimus-based regimen were significant risk factors for development of de novo NAFLD after LT (Table 3). If none of these factors were present, NAFLD occurred only in 5 out of 93 patients (5.4%). If 1, 2, 3, 4, or 5 factors were present, the incidence of NAFLD increased to 26/140 (18.6%), 58/127 (45.7%), 40/54 (74.1%), 13/15 (86.7%), and 1/1 (100.0%), respectively (Fig. 2).
| Variable | OR (95% CI) | P Value |
|---|---|---|
| BMI | 1.12 (1.06-1.17) | <0.001 |
| DM | 3.01 (1.51-6.01) | 0.002 |
| Hepatitis C | 4.61 (2.57-8.29) | <0.001 |
| Weight change | 1.03 (1.01-1.06) | 0.007 |
| On sirolimus | 3.11 (1.19-8.18) | 0.02 |
De Novo Post-LT NAFLD Excluding Patients Who Were Transplanted for HCV
Given that HCV was found to be a risk factor for de novo NAFLD and that patients with a history of HCV made up 42.3% of the total cohort, we repeated the univariate and multivariate analyses after excluding the patients transplanted for HCV to look for any differences. On univariate analysis, similar to the cohort as a whole, we found that male sex, diabetes, and BMI predicted de novo NAFLD. Interestingly, once HCV was excluded, tacrolimus use predicted de novo NAFLD. On multivariate analysis, BMI and tacrolimus use remained significant.
Era of Transplant
The study included patients transplanted over 3 decades (1986-2015). NAFLD occurred significantly more often in those patients transplanted in the third decade (2006-2015). Of the 143 patients with de novo NAFLD, 0.7% occurred in patients transplanted in 1995-1996, 37.8% in patients transplanted in 1996-2005, and 61.5% in patients transplanted in 2006-2015 (Table 2). We also noted a significantly higher median BMI according to transplant era (era of transplant: 25.0 kg/m2 for 1986-1995; 26.3 kg/m2 for 1996-2005; 26.9 kg/m2 for 2006-2015; P = 0.04) and a higher incidence of diabetes, although not statistically significant (presence of DM [n = 154] versus no DM [n = 276]; era of transplant: 10.4%, 44.1%, and 45.5% versus 15.9%, 36.9%, and 47.1% for 1986-1995, 1996-2005, and 2006-2015, respectively; P = 0.58).
Biochemical and Histological Features of the NAFLD Cohort
Biochemical Features
Among the 143 patients with de novo NAFLD on biopsy, the mean AST, ALT, and total bilirubin were 82 IU/L, 94 IU/L, and 22 µmol/L, respectively; 18% (n = 26) patients had completely normal liver tests (AST and ALT ≤40 IU/L and bilirubin ≤22 µmol/L).
Histological Features
Of the 143 recipients with biopsy-proven de novo NAFLD, steatosis only was present in 48.3% (n = 69) of patients and steatohepatitis in 51.7% (n = 74) of patients. Significant fibrosis (F2-4) and advanced fibrosis/cirrhosis (F3-4) were present in 39.9% and 20.3% of the cohort, respectively (Supporting Fig. 1; Table 2). On univariate analysis, the only modifiable factor that was associated with the development of significant fibrosis was insulin use.
Diagnostic Utility of Simple Noninvasive Fibrosis Scores in De Novo NAFLD
On the basis of the AUROCs, even after excluding indeterminate values, APRI and BARD were not useful diagnostic tests to predict advanced fibrosis (Table 4). The diagnostic utility of the FIB-4 score and the NFS was only modest based on AUROCs (AUROC, 0.75 and 0.74, respectively; Table 4). Only 9 (6.0%) patients of the cohort had cirrhosis, and all had an AST > ALT resulting in a high sensitivity for this test. Using an optimal threshold of >1.625 resulted in high specificity without compromising maximal sensitivity (AUROC, 0.99).
| Outcome | Prevalence | Sensitivity | Specificity | Positive LR | Negative LR | AUROC | Indeterminate* | |
|---|---|---|---|---|---|---|---|---|
| APRI | F2-F4 | 0.39 | 0.63 | 0.59 | 1.55 | 0.62 | 0.57 | 0.41 |
| APRI | F4 | 0.06 | 1.0 | 0.42 | 1.72 | 0 | 0.67 | 0.26 |
| FIB-4 | F3-F4 | 0.20 | 0.92 | 0.61 | 2.36 | 0.13 | 0.75 | 0.46 |
| NFS | F3-F4 | 0.20 | 0.73 | 0.68 | 2.28 | 0.39 | 0.74 | 0.48 |
| BARD | F3-F4 | 0.20 | 0.65 | 0.61 | 1.70 | 0.56 | 0.64 | — |
| AAR | F4 | 0.06 | 1.0 | 0.71 | 3.35 | 0 | 0.99 | — |
| AAR† | F4 | 0.06 | 1.0 | 0.96 | 26.8 | 0 | 0.99 | — |
- a Optimal threshold chosen to maximize the sum of sensitivity and specificity.
- b Indeterminate results excluded.
Fibrosis Progression
We estimated the annual progression of fibrosis in de novo NAFLD to be 0.40 (interquartile range, 0.20-0.70) stages per year based on an approximation of fibrosis stage in relation to the number of years after transplant.
Survival of the NAFLD Cohort Compared with the Control Cohort
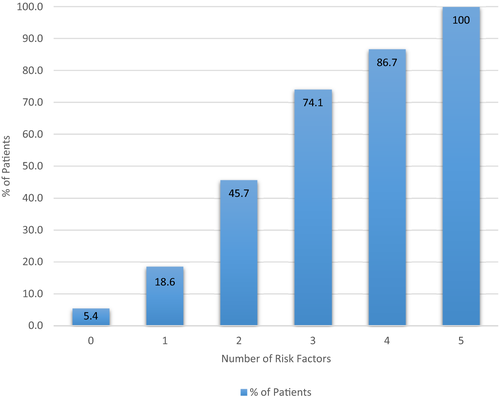
There was no significant difference in the short-term or longterm survival (up to 15 years) of the patients who developed de novo NAFLD compared with those who did not develop NAFLD, although there was a trend toward a shorter longterm survival in the NAFLD cohort (log-rank, 0.54; Fig. 3; Table 5). Similarly, there was no difference in survival in the de novo NASH cohort (log-rank, 0.18). Although, again, there was a trend toward shorter longterm survival.
| Survival | NAFLD group (n = 143) | Control group (n = 287) |
|---|---|---|
| 1 year | 99.3% | 99.7% |
| 5 years | 93.6% | 92.0% |
| 10 years | 80.0% | 83.4% |
| 15 years | 60.3% | 63.4% |
Discussion
Our study represents the largest to date to characterize de novo NAFLD, including determination of risk factors, assessment of fibrosis and review of overall survival. Weight gain, diabetes, dyslipidemia, and hypertension are all relatively common adverse effects of IS in the longer term and represent significant risk factors for pretransplant NAFLD.26, 27 Our study identified diabetes, weight gain, BMI, HCV, and sirolimus-based IS as being predictive of de novo NAFLD. If none of these factors were present, NAFLD occurred in only 5.4% of patients, and if all 5 factors were present, NAFLD occurred in 100% of patients. All of these factors are associated with IR and suggest that this may be at the root of development of de novo NAFLD. This is in keeping with the pathogenesis of pretransplant NAFLD, but it has not been described in de novo NAFLD after LT.28 We also established that de novo NAFLD is associated with significant fibrosis and that currently employed simple serum fibrosis algorithms are not useful to detect fibrosis in this cohort of patients. Finally, we found that, although not statistically significant, there was a trend toward a shorter longterm survival in the de novo NAFLD cohort.
Pretransplant NAFLD is characterized by hepatic and peripheral IR, leading to insufficient inhibition of hepatic gluconeogenesis, increased lipid accumulation, and decreased glycogen synthesis.29 This leads to increased circulating free fatty acids, which can further promote inflammation and endoplasmic reticulum stress. This in turn aggravates and maintains the insulin-resistant state, constituting a vicious cycle.28 Both diabetes and obesity are linked to IR and pretransplant NAFLD. More than 70.0% of obese adults with type 2 diabetes have NAFLD,30 and 88.0% of patients with NASH have been found to have features of the metabolic syndrome, including diabetes and/or obesity.31 Similarly, in our study, almost 50.0% of the NAFLD group had diabetes, and the majority of the NAFLD cohort was overweight with a median BMI of 29.4 kg/m2 (range, 26.3-34.0 kg/m2). We also established, on univariate analysis, that a higher HbA1C and being on insulin were significantly associated with de novo NAFLD. This suggests that poor glycemic control causing greater IR, and not only diabetes alone, is predictive of de novo NAFLD. We observed that more patients with a history of HCV, even after excluding HCV genotype 3 patients, had de novo NAFLD (65.7% of the NAFLD group versus 24.0% of the control group). In the pre-LT population, HCV-associated NAFLD occurs in up to 55% of infected individuals.32 The molecular basis for this phenomenon is that HCV is known to induce an insulin-resistant state, with decreased adiponectin and heightened tumor necrosis factor α production, ultimately causing oxidative stress and hepatic fat accumulation.33, 34 Finally, although the total numbers were low, sirolimus-based IS was also a predictor of de novo NAFLD. We postulate that this also has a pathogenetic basis in IR on the basis of previous literature. Acute sirolimus use inhibits the main mTOR, mTOR multiprotein complex 1, and prevents nutrient-induced IR. However, the chronic use of this immunosuppressant has been shown to inhibit both mTOR complexes 1 and 2 in vivo, leading to IR especially in the liver alongside activation of hepatic gluconeogenesis.35 It also inhibits insulin-mediated inactivation of hepatic glycogenolysis,36 causes a reduction in human pancreatic ductal cells, and inhibits glucose-stimulated insulin secretion.37 There are a number of studies describing the risk factors for development of recurrent NAFLD, and postulated risk factors include an increased BMI, posttransplant hypertriglyceridemia, steroid use, metabolic syndrome, and insulin use.9, 38 Proposed risk factors for de novo NAFLD include diabetes and elevated BMI.10, 11 Similar to the findings of our study, these are all linked to an insulin-resistant state and further validate our observations.
In contrast to previous literature, sirolimus and not tacrolimus was associated with development of de novo NAFLD in the total cohort.11 Once patients transplanted for HCV were excluded, tacrolimus was associated with de novo NAFLD. We postulate that there may be a synergistic effect of HCV and sirolimus on IR through their effects on the mTOR pathway, whereas tacrolimus in and of itself may induce de novo NAFLD in the non-HCV cohort.
One-third of the post-LT biopsies included in the study showed evidence of de novo NAFLD at a median time to biopsy of 3 years. Given that these were not protocol biopsies, it is not possible to comment on the actual incidence of de novo NAFLD in the total post-LT cohort. Approximately half of these patients had simple steatosis (48.2%) and half had NASH (51.8%) with an overall incidence of simple steatosis or NASH of 15.9% and 17.2%, respectively. This incidence of NASH is higher than has been reported in previous studies. Seo et al. reported 18% steatosis and 9% NASH in a similar cohort at a median of 28 months.10 Dumortier et al. reported similar rates of NAFLD (31.1%) but much lower rates of NASH (5.3%) at a median of 40 months.11 Our cohort included older patients (54, 52, and 48 years) at time of transplant, more diabetic patients (46.2%, 37.4%, and 38%), and patients with a higher BMI (29.4 kg/m2, 28.3 kg/m2, and value not given) than the Dumortier et al. and Seo et al. cohorts, respectively,10, 11 which may help to explain the higher incidence of NASH seen in our cohort.
There was a significant increase in the number of biopsies with NAFLD as we moved forward in decades from 1986 to 2015. They are not protocol biopsies and therefore do not tell us about the incidence of the disease. However, it was not particularly surprising given that we also noted an increase in BMI and an increase in the incidence of diabetes over this time period.
We postulate that the impact of de novo NAFLD is more important than previously understood, and it may significantly affect graft survival by promoting fibrosis and cirrhosis. We determined that almost 40.0% of de novo NAFLD patients had a biopsy that showed significant fibrosis (F2-4). Additionally, cirrhosis was evident in >5.0% of patients with de novo NAFLD. Noninvasive serum biomarkers are a simple means of performing serial follow-up and have already been validated in pre-LT NAFLD. However, they were not useful in this post-LT population. Almost 50% of the FIB-4 and NFS score results were in the indeterminate range (46% and 48%, respectively) and even after excluding these, the AUROCs were modest at best. AAR detected cirrhosis with high sensitivity and specificity. We chose an optimal threshold of 1.625 to maximize sensitivity and specificity, and this had a positive LR of 26.8 to detect cirrhosis. This is much higher than the cutoff of 1 that is suggested in the literature to detect cirrhosis.16 Further studies are needed to test the validity of this threshold, given the low number of patients with cirrhosis in the cohort.
Although we understand fibrosis progression to be nonlinear, we estimated the annual progression of fibrosis in de novo NAFLD to be a meaningful 0.4 stages per year based on a proximate evaluation of fibrosis stage in relation to the number of years after transplant. This corresponds to 1 stage of fibrosis progression over 2.5 years. This is significantly faster than what has been described in NAFLD patients in the pre-LT setting. In a systematic review and meta-analysis, Singh et al. described 1 stage of progression over 14.3 years for patients with NAFLD and 7.1 years for patients with NASH.39 We postulate that there are a number of reasons for the accelerated fibrosis progression after LT, compared with before LT, including rapid weight gain after LT, new-onset metabolic syndrome, and adverse effects associated with IS, including hypertension, dyslipidemia, IR, and diabetes.40-42 In addition, all patients with de novo NAFLD underwent transplantation for other indications, and there is the possibility of recurrent disease (HCV or autoimmune liver diseases) contributing to fibrogenesis. Our study suggests the need for validated noninvasive tools to identify earlier-stage fibrosis, when the ability to effectively intervene may have a greater impact on slowing disease progression.
Epidemiological, familial, and twin studies provide evidence for a strong genetic component of NAFLD susceptibility.43 Genome-wide association studies have identified some genetic modifiers of disease severity and progression, eg, patatin-like phospholipase domain-containing 3.44 More recently, epigenetics, specifically DNA methylation, has been shown to affect IR and severity of NAFLD.45 Dumortier et al. found the presence of donor steatosis to be an important predictor of de novo NAFLD, indicating a genetic basis conferred by the donor liver.11 Less than 20% of the patients in our study had a donor biopsy report available, and although there was a trend to suggest that donor steatosis might be associated with de novo NAFLD, this was not statistically significant. We also found that older age and higher BMI in the donors were associated with NAFLD, although data on approximately one-third of donors were not available. In recent years, we have been systematically collecting donor information and preimplantation biopsies, which will enable us to continue to study this area in the future.
Longterm prognosis in patients with de novo NAFLD has not been described. Prior studies have shown that patients with recurrent NAFLD are at increased risk of cardiovascular events and renal impairment.9, 12 On the basis of the shared metabolic risk factors, post-LT patients with de novo NAFLD are likely to be at increased risk of cardiovascular death, in addition to progression to liver cirrhosis and subsequent complications of portal hypertension. Our study showed a trend toward poorer longterm survival outcomes in de novo NAFLD patients, although this was not statistically significant. Larger studies and longer follow-up times are needed to accurately understand the implication of increased cardiovascular and renal risks.
Our study admittedly has limitations by virtue of using for-cause and not protocol liver biopsies to diagnose NAFLD.
Nonetheless, this is a limitation that is common with other prior studies.9, 10 A systematic longitudinal approach to diagnosis of de novo NAFLD would potentially increase the number of patients demonstrated to have hepatic steatosis after transplant. In addition, the retrospective nature of this study is a natural limitation, due to the risk of bias inherent to study design when compared with a prospective trial. Nonetheless, our study represents the largest available experience at a single center of de novo NAFLD after LT and allowed determination of risk factors for this condition. With respect to fibrosis determination, we employed the METAVIR classification rather than the NASH Clinical Research Network (CRN) system,46 though the semiquantitative assessment of fibrosis in these systems is comparable. We acknowledge that other factors, such as disease recurrence or previous rejection, can contribute to the development of fibrosis within the NAFLD cohort. This study represents a real-world cohort, including those with previous rejection or disease recurrence, and suggests that de novo NAFLD is associated with significant fibrosis. However, dedicated systematic and comprehensive studies are needed to establish the exact relationship between post-LT NAFLD and fibrosis. Given the retrospective nature of this study, other limitations include heterogeneity of patients, variable IS, and no control for lipid-lowering medications or diabetes severity. In addition, we could not control for diet and physical activity of patients, which may contribute to development of NAFLD. Finally, noninvasive radiological tools, such as TE, have been validated in the pre-LT cohort, but this was not routinely performed in our retrospective cohort and so was not included in the analysis.
In conclusion, our study is the largest to characterize de novo NAFLD, a condition that is being increasingly recognized in the post-LT population. An improved understanding of de novo NAFLD is likely of significance to all solid organ transplantation, given that it arises in the common metabolic environment engendered by IS. We observed that diabetes, weight gain, BMI, HCV, and sirolimus-based IS were the factors associated with the development of de novo NAFLD, and they were all associated with IR. The development of fibrosis impacts a significant proportion of patients with de novo NAFLD. Given the limited utility of noninvasive serum fibrosis tools, alternative validated fibrosis tools are needed to screen for fibrosis in de novo NAFLD. Early recognition and targeted modification of identified risk factors could potentially reduce the risk of de novo NAFLD and associated complications.
Potential conflict of interest
Nothing to report.