Survival of children after liver transplantation for hepatocellular carcinoma
René Adam was on the speakers' bureau of Merck-Serono and Sanofi-Aventis. Christophe Duvoux advises and received grants from Novartis, Astellas, and Chiesi. Stefan Schneeberger consults for Astellas.
Abstract
Hepatocellular carcinoma (HCC) in childhood differs from adult HCC because it is often associated with inherited liver disease. It is, however, unclear whether liver transplantation (LT) for HCC in childhood with or without associated inherited disease has a comparable outcome to adult HCC. On the basis of data from the European Liver Transplant Registry (ELTR), we aimed to investigate if there are differences in patient and graft survival after LT for HCC between children and adults and between patients with underlying inherited versus noninherited liver disease, respectively. We included all 175 children who underwent LT for HCC and were enrolled in ELTR between 1985 and 2012. Of these, 38 had an associated inherited liver disease. Adult HCC patients with (n = 79) and without (n = 316, matched by age, sex, and LT date) inherited liver disease served as an adult comparison population. We used multivariable piecewise Cox regression models with shared frailty terms (for LT center) to compare patient and graft survival between the different HCC groups. Survival analyses demonstrated a superior longterm survival of children with inherited liver disease when compared with children with HCC without inherited liver disease (hazard ratio [HR], 0.29; 95% CI, 0.10-0.90; P = 0.03) and adults with HCC with inherited liver disease (HR, 0.27; 95% CI, 0.06-1.25; P = 0.09). There was no survival difference between adults with and without inherited disease (HR, 1.05; 95% CI, 0.66-1.66; P = 0.84). In conclusion, the potential survival advantage of children with an HCC based on inherited disease should be acknowledged when considering transplantation and prioritization for these patients. Further prospective studies accounting for tumor size and extension at LT are necessary to fully interpret our findings. Liver Transplantation 24 246–255 2018 AASLD.
Abbreviations
-
- A1ATD
-
- α-1-antitrypsin deficiency
-
- BSEP
-
- bile salt export pump
-
- CI
-
- confidence interval
-
- ELTR
-
- European Liver Transplant Registry
-
- GSD
-
- glycogen storage disease
-
- HCC
-
- hepatocellular carcinoma
-
- HR
-
- hazard ratio
-
- IQR
-
- interquartile range
-
- LT
-
- liver transplantation
-
- TTI
-
- tyrosinemia type I
-
- UNOS
-
- United Network for Organ Sharing
-
- UW
-
- University of Wisconsin
Hepatocellular carcinoma (HCC) in children is rare and accounts for 0.5%-2.0% of all pediatric tumor cases.1-4 Only 0.5%-1.0% of HCC cases occur in patients under the age of 20, with the majority presenting between 10 and 14 years of age. The clinical and scientific perception of HCC focuses therefore on adult patients who account for more than 99% of all HCC cases.5-7 The majority of childhood HCCs worldwide occurs secondary to chronic hepatitis B infection. In highly endemic areas for hepatitis B, the incidence of HCC in children and adolescents ranges from 0.09 to 0.45 cases per million per year and decreases with successful implementation of vaccination programs.8 In countries where hepatitis B prevalence is low, most childhood HCC cases appear to be associated with inherited liver disease.9, 10 Metabolic inherited conditions such as tyrosinemia type I (TTI),11 bile salt export pump (BSEP) deficiency,12 α-1-antitrypsin deficiency (A1ATD),13, 14 or glycogen storage disease (GSD)15 are known risk factors for malignant transformation with or without prior cirrhosis. It has been suggested that HCCs arising in patients with inherited disease form a distinct clinical entity given that the pathophysiology behind these tumors differs considerably from other HCC types.16-18 Evidence to support this hypothesis is, however, scarce. It is still unknown whether HCC in inherited metabolic liver disease is induced via a common specific mechanism (ie, by inflammatory or toxic injury, nuclear factor kappa B activation [TTI], impaired autophagy, and gain-of-function mechanisms [A1ATD]), or via a combination of these mechanisms.18-21
Curative therapy of childhood HCC requires complete surgical resection. In nonresectable tumors, liver transplantation (LT) may be considered.4 Although several case series indicated a favorable outcome of LT in childhood HCC,22-25 there are no studies available comparing survival rates of pediatric and adult HCC patients taking into account the underlying cause of HCC. A systematic assessment of the outcome of children who underwent LT for HCC was reported in 2006 for the United Network for Organ Sharing (UNOS) database26 and updated to an extended cohort27 in 2010 showing a 5-year patient survival rate of 53.5%. In this study, however, no information on underlying disease was reported so that HCCs based on inherited liver disease and acquired noninherited liver disease could not be differentiated.
The aim of our study was to use the advantages of the large European Liver Transplant Registry (ELTR) to analyze if there are differences in survival after LT for HCC between children and adults on the one hand and between patients with underlying inherited and noninherited liver disease on the other hand. We hypothesized that childhood HCC arising from underlying inherited disease may have superior graft and patient survival after LT when compared with HCC in children with noninherited liver disease or to HCC in adults. We further hypothesized that LT for HCC arising from underlying inherited metabolic disease yields a better outcome than in sporadic HCC regardless of age.
Patients and Methods
ELTR DATABASE
Established in 1985, the ELTR is a voluntary collaboration of 168 European Liver Transplant Centers in 31 countries.28 For ELTR, a prespecified set of variables is collected for each LT patient in the participating centers after transplantation. Moreover, follow-up data are provided by contributing centers at each follow-up visit so that retransplantations as well as deaths can be recorded. All data are monitored by ELTR staff at regular intervals. Quality of the data is controlled by annual site visits at randomly chosen participating centers.29 Coverage of LT patients in Europe within ELTR has been shown to be as high as 97% in 2012. Data obtained from the ELTR database can thus be described as highly representative for Europe. None of the participating centers’ donor organs were at any point obtained from executed prisoners or other institutionalized persons. This study was approved by and performed under the auspices of the Board of the European Liver and Intestine Transplant Association (ELITA), the governing society of the ELTR.
STUDY POPULATION
Pediatric HCC Patients
All children and adolescents under the age of 18 years having undergone LT for HCC between 1985 and 2012 and being registered in ELTR were included in this study, regardless of potential additional or underlying diagnoses resulting in a total sample size of 175 patients. This group was further divided into patients with the additional diagnosis of an inherited liver disease (PinHCC, n = 38) and those without a code for underlying inherited diseases (pHCC, n = 137; Table 1). Patients with the incidental posttransplant finding of HCC in the hepatectomy specimen were not considered for inclusion because they were not transplanted for HCC making them inherently different.
| Children (n = 38) | Adults (n = 79) | |
|---|---|---|
| Inherited | ||
| Wilson's disease | 2 (5.3) | 18 (22.8) |
| Cystic fibrosis | 0 (0.0) | 2 (2.5) |
| Byler's disease | 8 (21.1) | 1 (1.3) |
| Others | 4 (10.5) | 16 (20.3) |
| Antitrypsin deficiency | 2 (5.3) | 29 (36.7) |
| GSD | 0 (0.0) | 8 (10.1) |
| Tyrosinemia | 22 (57.9) | 5 (6.3) |
| Children (n = 137) | Adults (n = 316) | |
| Noninherited | ||
| Primary biliary cirrhosis | 0 (0.0) | 5 (1.6) |
| Primary sclerosing cholangitis | 1 (0.7) | 3 (0.9) |
| Secondary biliary cirrhosis | 2 (1.5) | 0 (0.0) |
| Cholestatic disease—others | 2 (3.3) | 2 (0.6) |
| Alcoholic cirrhosis | 0 (0.0) | 79 (25.0) |
| Other cirrhosis | 0 (0.0) | 1 (0.3) |
| Cryptogenic (unknown) cirrhosis | 14 (10.2) | 12 (3.8) |
| Autoimmune cirrhosis | 0 (0.0) | 7 (2.2) |
| Virus B–related cirrhosis | 10 (7.3) | 61 (19.3) |
| Virus C–related cirrhosis | 0 (0.0) | 115 (36.4) |
| Virus BD–related cirrhosis | 0 (0.0) | 4 (1.3) |
| Virus BC–related cirrhosis | 0 (0.0) | 7 (2.2) |
| Virus-related cirrhosis—other | 1 (0.7) | 2 (0.6) |
| Cirrhosis-combined virus C and alcoholic cirrhosis | 1 (0.7) | 13 (4.1) |
| Cirrhosis-combined virus B and alcoholic cirrhosis | 0 (0.0) | 1 (0.3) |
| Budd-Chiari | 0 (0.0) | 4 (1.3) |
| Unspecified and other | 105 (76.6) | 0 (0.0) |
- NOTE: Data are given as n (%).
Adult HCC Patients
All adult patients (aged 18 years or above at date of first LT) registered in ELTR and transplanted for HCC with an additional code for an inherited liver disease (AdinHCC, n = 79) were extracted and included in this study. In order to create a control cohort, adult HCC patients with a defined code for a noninherited underlying disease (aHCC) were individually matched 1:4 (n = 316) by age (within 5 years), year of transplantation (within 5 years), and sex to each adult HCC patient with underlying inherited liver disease. Again, patients with incidental posttransplant findings of HCC in the hepatectomy specimen were not included.
STUDY DESIGN AND ANALYSIS PLAN
We conducted a historical cohort study based on prospectively collected data from ELTR. In order to investigate the main hypotheses of this study, a 3-stage data analysis plan was developed. This was necessary as age at transplantation and underlying liver disease causing HCC are highly collinear to each other and are difficult to separate in an overall analysis, so that stratified analyses needed to be performed. In a first step, PinHCC patients were compared with pHCC patients to investigate if there was a difference in survival rates between these 2 types of HCC in childhood. Because the underlying cause of LT in the group of pediatric patients without inherited diagnosis was often missing or unspecific, we performed a sensitivity analysis comparing PinHCC with pHCC patients with a definite noninherited cause of HCC only (n = 18; Table 1). Second, PinHCCs were compared with AdinHCC to investigate whether there was an effect of age on survival in patients with inherited HCC. In a third step, AdinHCC patients were compared with aHCC patients to investigate if there was a difference between the HCC types in adult age.
Data Management and Statistical Analyses
The data set extracted for this study was checked for plausibility by ELTR staff and by the authors of this manuscript. In case of implausible or missing data (eg, unspecified cause of transplantation), the transplant center was recontacted by ELTR staff (V.K.). Missing data were obtained as far as possible, and errors were corrected accordingly. Follow-up for each individual included the time from first LT to last recorded visit at the transplant center or death. Patient survival was defined as the time between first LT and death. Graft survival was defined as the time from first LT to retransplantation or death (whatever happened first). Censoring due to being lost to follow-up for the transplant center was assumed to happen completely at random.
All data analyses were performed using Stata, version 12 (StataCorp, College Station, TX). Baseline characteristics were compared between groups using chi-square tests, Fisher's exact tests, and Wilcoxon rank sum tests as appropriate. Survival times were displayed using Kaplan-Meier plots and were compared between groups using univariate and multivariate shared frailty Cox regression models (adjusted for sex, age, year of transplantation, and allowing for clustering of outcomes by transplantation center by using a shared frailty term). Proportional hazards assumptions were assessed using Schoenfeld residuals. In case of nonproportional hazards, piecewise regression models were used.
Results
In total, 570 patients undergoing LT for HCC with a number of associated liver diseases were included (Table 1). Type of associated liver disease differed considerably by age in those HCC patients with an associated inherited liver disease. Patients suffering from BSEP received liver transplants in childhood at a median age of 2.4 years, in contrast to patients with alpha-1-antitrypsin deficiency who were adults aged 59.4 (median) when undergoing first liver transplantation (Fig. 1).
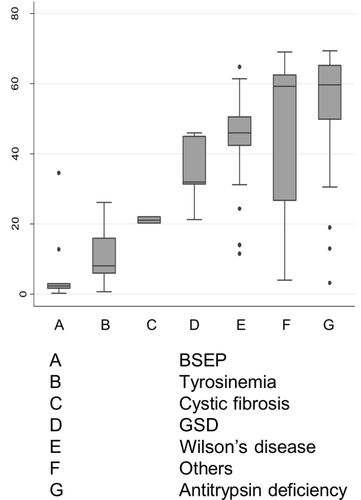
Age at transplantation of patients with HCC and underlying inherited liver disease. Displayed are box plots (box, IQR; line, median; whiskers, 1.5 times the IQR).
COMPARISON PINHCC VERSUS PHCC
Pediatric patients with underlying inherited liver disease (PinHCC, n = 38) were younger (P < 0.001), more likely to be urgently transplanted (P = 0.007), and more likely to receive a partial graft (P = 0.01) than children without an underlying inherited cause (pHCC, n = 137; Table 2). Overall 5-year patient (57.6%; 95% confidence interval [CI], 47.9%-65.1%) and graft survival rates (56.3%; 95% CI, 39.4%-56.3%) of pediatric HCC patients were very similar to those reported by the US-based UNOS registry on pediatric HCCs (n = 58; Fig. 2; Table 3). In the multivariate survival analyses, we found evidence that PinHCC showed a better longterm patient survival than pHCC (hazard ratio [HR], 0.29; 95% CI, 0.10-0.90; P = 0.03, adjusted for sex, year of transplantation, and using a shared frailty term for transplantation center). Age at LT showed almost distinct distributions between both groups (median ages 7 versus 11 years, with the inherited HCC patients being clearly younger). It was therefore not possible to fully differentiate the effect of age and underlying disease. Survival rates increased with increasing age in pHCC patients (HR per year, 1.11; 95% CI, 1.04-1.18; P = 0.001), whereas there was no age effect in PinHCC patients (HR per year, 0.97; 95% CI, 0.81-1.16; P = 0.71). When adjusting the multivariate Cox regression model for age, the protective effect of underlying inheritable disease became slightly smaller (HR, 0.45; 95% CI, 0.16-1.31; P = 0.15). Effect estimates did not change considerably in the sensitivity analysis restricting pHCC patients to those with a defined secondary diagnosis (HR, 0.54; 95% CI, 0.16-1.83; P = 0.32). Results for graft survival were generally comparable with the ones for patient survival (Supporting Information).
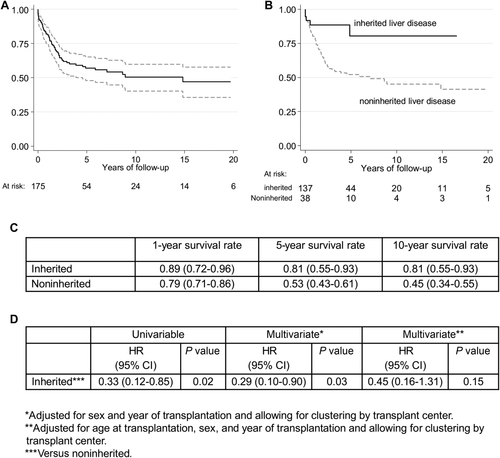
Patient survival in pediatric HCC patients after LT. (A) Overall survival function (n = 175) with 95% CIs and (B) survival function stratified by underlying disease displayed as Kaplan-Meier plots. (C) 1-year, 5-year, and 10-year survival rates by underlying disease. (D) Results of the univariate and multivariate Cox regression models comparing pediatric HCCs with inherited liver disease to those without (P values are obtained via likelihood ratio tests).
| Children (n = 175) | Adults (n = 395) | P Values | |||||
|---|---|---|---|---|---|---|---|
| Noninherited (n = 137) | Inherited (n = 38) | Inherited (n = 79) | Noninherited (n = 316) | Noninherited Versus Inherited Children | Inherited Children Versus Inherited Adults | Noninherited Versus Inherited Adults | |
| Age in yearsa | 11 (8-15) | 7 (3-11) | 52 (41-61) | 53 (43-62) | <0.001 | <0.001 | 0.36 |
| Sexb | 0.79 | 0.09 | 0.99 | ||||
| Male | 76 (55.5) | 22 (57.9) | 58 (73.4) | 232 (73.4) | |||
| Female | 61 (44.5) | 16 (42.1) | 21 (26.6) | 84 (26.6) | |||
| Type of donorb, c | 0.34 | 0.11 | 0.67 | ||||
| Deceased | 117 (85.4) | 30 (79.0) | 70 (89.7) | 289 (91.5) | |||
| Living | 20 (14.6) | 8 (21.0) | 8 (10.3) | 27 (8.5) | |||
| Urgency (yes)b, c | 5 (4.1) | 6 (17.7) | 4 (5.9) | 7 (2.2) | 0.007 | 0.08 | 0.09 |
| Days on waiting lista, c | 17 (5-51) | 33 (11-78) | 123 (32-230) | 158 (48-288) | 0.22 | <0.001 | 0.27 |
| Number of retransplantationsb | 0.86 | 0.45 | 0.50 | ||||
| 0 | 122 (89.0) | 34 (89.5) | 74 (93.7) | 288 (91.1) | |||
| 1 | 13 (9.5) | 4 (10.5) | 5 (6.3) | 28 (8.9) | |||
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 3 | 2 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Encephalopathyb, c | 0.54 | 0.28 | 0.06 | ||||
| Grade III-IV | 0 (0.0) | 1 (5.0) | 3 (6.7) | 2 (1.2) | |||
| Grade I-II | 1 (2.3) | 0 (0.0) | 5 (11.1) | 31 (19.1) | |||
| No | 42 (97.7) | 19 (95.0) | 37 (82.2) | 129 (79.6) | |||
| Ascitesb, c | 0.38 | 0.048 | 0.02 | ||||
| Refractory | 1 (2.9) | 1 (5.0) | 5 (12.5) | 8 (4.9) | |||
| Controlled | 4 (11.8) | 0 (0.0) | 8 (20.0) | 69 (41.8) | |||
| No | 29 (85.3) | 19 (95.0) | 27 (67.5) | 88 (53.3) | |||
| Ischemia timea, c | 465 (330-613) | 450 (213-570) | 510 (348-625) | 466 (342-600) | 0.28 | 0.08 | 0.30 |
| Preservation liquidb, c | 0.06 | >0.99 | 0.68 | ||||
| UW | 91 (75.8) | 19 (59.4) | 41 (59.4) | 203 (64.2) | |||
| Other | 29 (24.2) | 13 (40.6) | 28 (40.6) | 109 (35.8) | |||
| Bypassb, c | 0.06 | <0.001 | <0.001 | ||||
| Extracorporal | 10 (10.2) | 0 (0.0) | 16 (32.7) | 102 (35.3) | |||
| Vena cava | 16 (16.3) | 2 (6.7) | 10 (20.4) | 125 (43.9) | |||
| None | 72 (73.5) | 28 (93.3) | 23 (46.9) | 58 (20.3) | |||
| Type of graftb, c | 0.01 | <0.001 | 0.53 | ||||
| Full size | 72 (54.9) | 12 (31.6) | 73 (92.4) | 284 (89.9) | |||
| Partial | 59 (45.1) | 26 (68.4) | 6 (7.6) | 32 (10.1) | |||
- NOTE: Data are given as n (%) and median (IQR).
- a Wilcoxon rank sum test.
- b Fisher's exact test or chi-square test as appropriate.
- c Number of missing values for type of donor (n = 1), urgency (n = 30), days on waiting list (n = 438), encephalopathy (n = 292), ascites (n = 311), ischemia time (n = 35), bypass (n = 108), and type of graft (n = 6).
| ELTR (n = 175) | UNOS (n = 58) | |
|---|---|---|
| Sex, male, n (%) | 98 (56.0) | 30 (51.7) |
| Age at transplantation, yearsa | 10.2 (6.0-14.3) | 10.5 |
| Patient survival, %b | ||
| 1-year survival | 81.2 (74.2-86.5) | 85.2 |
| 2-year survival | 68.3 (60.5-75.8) | 68.8 |
| 5-year survival | 57.6 (47.9-65.1) | 53.5 |
| Graft survival, %a | ||
| 1-year survival | 73.6 (66.0-79.7) | 83.7 |
| 2-year survival | 58.5 (50.0-66.0) | 60.6 |
| 5-year survival | 56.3 (39.4-56.3) | 42.8 |
- NOTE: See Guiteau et al.27 (2010).
- a For ELTR median and IQR are reported; for UNOS only data for the median were available.
- b For ELTR survival rates are presented together with 95% CIs; this was not possible for UNOS data as no uncertainty measures were reported in the original publication and no information on lost to follow-up was given.
COMPARISON PINHCC VERSUS ADINHCC
In the second step of our analysis, we compared children (PinHCCs, n = 38) and adults (AdinHCCs, n = 79) with HCC and associated inherited liver disease. Baseline characteristics revealed that adult patients (AdinHCC) were less likely to receive a partial graft (P < 0.001; Table 2) and appeared to have more advanced liver disease. They were more often diagnosed with encephalopathy and ascites (P = 0.048), were more likely to be treated with a bypass (P < 0.001), and were longer on the waiting list (P < 0.001; Table 2). Although there was almost no difference in patient survival in the first 2 years after LT between both groups (HR, 1.22; 95% CI, 0.55-2.70; P = 0.63), patients with PinHCCs showed a better longterm survival (HR, 0.27; 95% CI, 0.06-1.25; P = 0.09, piecewise Cox regression model; Fig. 3). Graft survival analyses results were similar to those for patient survival (Supporting Information).
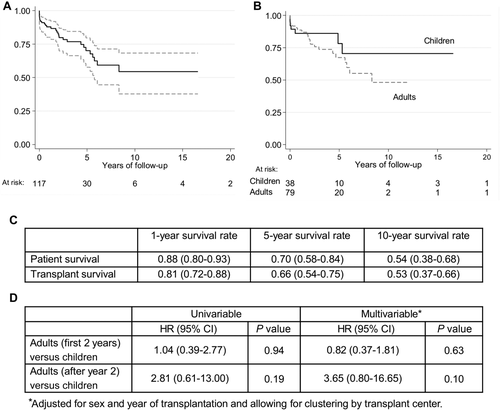
Patient survival in HCC patients with inherited liver disease after LT. (A) Overall survival function (n = 117) with 95% CIs and (B) survival function stratified by age displayed as Kaplan-Meier plots. (C) 1-year, 5-year, and 10-year survival rates by age at transplantation. (D) Results of the univariable and multivariable Cox regression models comparing pediatric HCCs with inherited liver disease to adult HCCs with inherited liver disease (P values are obtained via likelihood ratio tests).
COMPARISON ADINHCC VERSUS AHCC
In the last step of our analysis, we compared AdinHCC patients (n = 79) with their matched aHCC patients (n = 316) with defined other underlying diseases (Table 1). AdinHCC patients were less likely to suffer from ascites (P = 0.02) and less likely to be treated with a bypass (P < 0.001). In contrast, there was almost no difference in patient survival (HR, 1.05; 95% CI, 0.66-1.66; P = 0.84, aHCC as reference group; Fig. 4) and graft survival (HR, 1.04; 95% CI, 0.68-1.15; P = 0.85; Supporting Information) in the multivariate survival analyses.
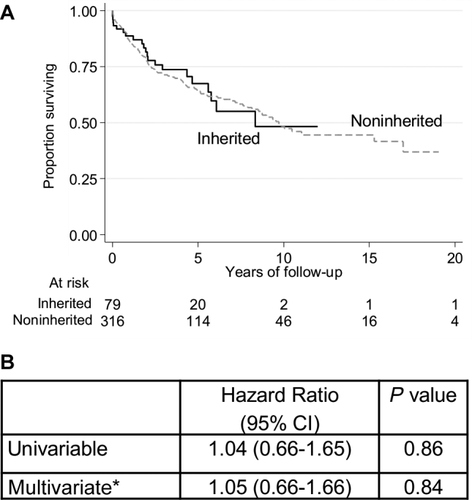
Patient survival in adult HCC patients after LT. (A) Survival function stratified by underlying disease (inherited, black line; noninherited, gray line) displayed as Kaplan-Meier plots. (B) Results of the univariable and multivariable Cox regression models comparing adult HCCs with inherited liver disease to those without (P values are obtained via likelihood ratio tests).
Recurrence of HCC did not account for graft loss leading to retransplantation in any of the 4 groups (Supporting Information). The incidence of death due to HCC recurrence was higher in children (38.0 deaths [95% CI, 26.4-54.7] per 1000 patient years) than in adults (24.2 deaths [95% CI, 17.9-32.7] per 1000 patient years). No death due to HCC recurrence occurred in patients with underlying inherited liver disease.
Discussion
In this study, we investigated the hypothesis that children transplanted for HCC with associated inherited liver disease show better LT outcomes than other HCC patients. For this purpose, we compared these children with 2 other HCC groups, children with sporadic HCC, not based on inherited liver disease, and adults with HCC and inherited liver disease. Contrary to previous expectations, most childhood HCCs registered in ELTR were not associated with inherited liver disease. We showed that children with HCC based on inherited disease had a better longterm survival than children with a noninherited HCC or adult patients with an inherited HCC. Although not all comparisons were significant on a 5% level due to the small sample size in the groups of pediatric HCCs with associated inherited liver disease, the hazard of death was 3 to 4 times smaller in pediatric HCC patients with inherited liver disease than in their comparison groups.
One explanation for the better outcome of pediatric HCC patients with inherited liver disease might be that they are generally followed up in regular intervals, which may lead to timely HCC detection and earlier LT compared with sporadic HCC patients. This explanation is supported by the finding that adult patients (and especially those with no underlying inherited disease) were generally sicker, had more ascites at transplantation, and had longer waiting times before LT. However, these findings could also be explained by longer waiting times for adult LT due to a higher scarcity of organs for adults. Our results suggest that younger age is a prognostic factor for better survival rates after LT for HCC. However, some of the effect of inherited liver disease in children remained apparent (HR, 0.45) after adjusting for age so that inherited liver disease can be seen as an additional prognostic factor for better survival rates in pediatric HCC. A survival advantage based on associated inherited disease was not seen in adults, where HCC patients with and without inherited liver disease showed similar survival rates. This might be attributable to the different types of inherited liver diseases, which are associated with HCC in children and in adults (Fig. 1).
This is the first study which assessed comprehensively the competing effect of pediatric HCC and underlying inherited liver disease on patient and graft survival after LT. Crude overall survival rates were thereby in line with the largest previously published patient cohort from the UNOS database.27 Data on pediatric HCC patients undergoing LT are otherwise sparse.
Because of small numbers of patients with childhood HCC, there is little evidence regarding optimal treatment for this group of patients. Children with HCC are therefore evaluated for LT extrapolating experience from adult patients by applying the conventional Milan criteria. The criteria have been modified repeatedly, and these and other more liberal criteria like the University of California, San Francisco criteria have been a matter of ongoing debate.30, 31 The ELTR data presented here provide the most comprehensive cohort of children who underwent liver transplantation for HCC. Our results suggest that prognosis of a child with underlying inherited liver disease is generally better than anticipated.
However, our analyses and the underlying data set have several limitations. One major limitation is the lack of characterization of the individual HCC with respect to the histological and radiological stages and tumor volume. In the majority of patients (more than 85%), there were no data on tumor stage or grade for the various classifications such as conventional Milan criteria, Barcelona score, or the pediatric pretreatment extension score Pretreatment Extent of Disease. Moreover, no data on individual cirrhosis status or chemotherapy for HCC were available, limiting the interpretability of study results. Another drawback of our analysis originates from the nature of the source data. Several selection biases may apply to registry data, ie, the methods of diagnosing (or labeling) a patient in the source data, the methods of transplantation eligibility, selection on who was entered in the registry, and how complete follow-up was in the participating centers of ELTR. We tried to account for this by including the transplantation center as a shared frailty term in our multivariate models, so that our models accounted for heterogeneities between LT centers. However, given the high proportion of patients lost to follow-up after the first 5-10 years (especially in pediatric patients who were transferred to adult care), longterm survival estimates need to be interpreted with caution. Moreover, the group of patients with pediatric HCC but no underlying inherited disease was poorly characterized, and it cannot be excluded that some of these patients suffered from undocumented inherited liver disease. We accounted for this by performing a sensitivity analysis including only those patients with a confirmed underlying noninherited cause for HCC and sufficient follow-up information. Results remained virtually unchanged reassuring us that the original analysis showed valid results. The potential longterm benefits observed for pediatric HCC patients with underlying inherited disease in comparison to their adult peers might at least partially be due to the relative survival advantages of children when compared with adults. We did not account for this in the analysis of this study (eg, by using relative survival) because we were explicitly interested in the true survival rates of the respective patient groups.
In summary, we were able to analyze the largest ever reported cohort of pediatric HCC patients treated with LT. We have shown that pediatric HCC patients with underlying inherited liver disease have a survival advantage when compared with pediatric HCC patients with no underlying inherited disease and to adult HCC patients. We suggest that our results are taken into consideration for future transplant organ allocation. However, studies with more detailed information on tumor characteristics, waiting times, and disease severity are necessary to fully interpret our findings.