Living donor liver transplantation with a left trisegmental graft from a donor with anomalous branching of the portal vein
Potential conflict of interest: Nothing to report.
Abbreviations
-
- 3D
-
- 3-dimensional
-
- CT
-
- computed tomography
-
- GRWR
-
- graft-to-recipient weight ratio
-
- INR
-
- international normalized ratio
-
- LDLT
-
- living donor liver transplantation
-
- LPV
-
- left portal vein
-
- POD
-
- postoperative day
-
- PT
-
- prothrombin time
-
- PV
-
- portal vein
-
- RA
-
- right anterior bile duct
-
- RAHA
-
- right anterior hepatic artery
-
- RAPV
-
- right anterior portal vein
-
- RAS
-
- right anterior segment
-
- RP
-
- right posterior bile duct
-
- RPHA
-
- right posterior hepatic artery
-
- RPS
-
- right posterior segment
-
- T-Bil
-
- total bilirubin
-
- UP
-
- umbilical portion
Anatomical variations of the donor liver can affect graft selection for living donor liver transplantation (LDLT). The right anterior portal vein (RAPV) arising from the umbilical portion (UP) of the left PV is a rare variant and an obstacle to donating a hemiliver graft. Here, we present a case of successful LDLT using a left trisegmental graft from a donor with this anatomical variation.
Case Report
A 45-year-old male patient with liver cirrhosis due to nonalcoholic steatohepatitis applied to our center for liver transplantation. He was registered on the waiting list for deceased donor liver transplantation. However, 2 years later, his condition gradually deteriorated due to ascites and edema, and he and his family wished for LDLT instead of waiting for a deceased donor. His body weight, height, and body mass index were 173 cm, 87 kg, and 29.0 kg/m2, respectively. He had massive ascites but no episodes of encephalopathy. His total bilirubin (T-Bil) level, albumin level, and prothrombin time (PT; %) were 2.6 mg/dL, 3.0 g/dL, and 45%, respectively, resulting in a Child-Pugh score of 10 (grade C) and a Model for End-Stage Liver Disease score of 15.
His 44-year-old wife was evaluated as a donor. Her computed tomography (CT) scan demonstrated that the RAPV branched off from the UP (Fig. 1A,B) and the right anterior hepatic artery (RAHA) also arose from the left hepatic artery (Fig. 1C). Drip infusion CT cholangiography demonstrated that the common hepatic duct trifurcated into the left, anterior, and posterior ducts (Fig. 2A). CT volumetry showed that the left lobe graft or the posterior segment graft did not fulfill our criteria for the minimum graft volume (0.6% of the recipient body weight; Table 1).1 The right lobe graft was abandoned because of the technical difficulties in reconstruction of PVs2 as well as the arteries. A left trisegmental graft was selected because CT volumetry showed sufficient volumes for both the graft and the remnant (Table 1). Simple reconstructions with a single orifice were expected for the PV and the hepatic artery.
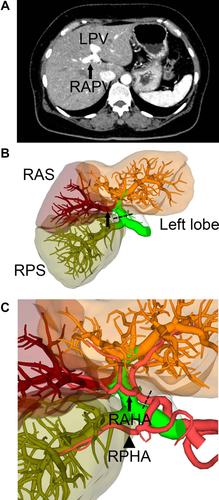
Vascular anatomy of the donor. (A) An axial CT image revealed that the RAPV (arrow) arose from the UP of the LPV. (B) A 3D image also demonstrated that the right anterior branch of the portal vein (arrow) diverged from the UP of the LPV. The dashed line indicates the portal vein cutting line. (C) A 3D image of the artery clearly showed the RAHA (arrow) originating from the left hepatic artery whereas the RPHA (arrowhead) arose from the gastroduodenal artery. The dashed line indicates the cutting line of the artery.
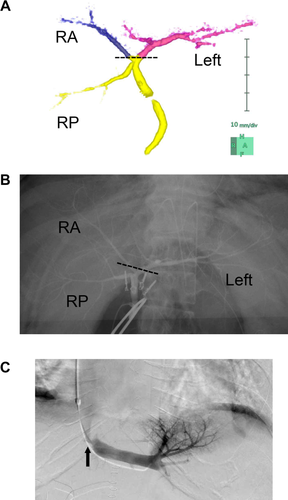
Biliary anatomy of the donor and postoperative hepatic vein stenosis of the recipient. (A) A 3D image of the bile duct shows the common hepatic duct trifurcated to the left (purple), the right anterior (blue), and the right posterior branches. The dashed line indicates the cutting line of the bile duct. (B) The intraoperative cholangiogram with marking clips confirmed the biliary anatomy. (C) A postoperative hepatic venography of the recipient showed hepatic vein stenosis (arrow), which was amenable to balloon dilatation therapy.
| Graft Type | Graft Volume, mL (% of the Whole Liver) | Remnant, mL (% of the Whole Liver) | GRWR |
|---|---|---|---|
| Right lobe | 696 (60%) | 458 (40%) | 0.80% |
| Left lobe | 429 (37%) | 725 (63%) | 0.49% |
| RPS | 464 (40%) | 690 (60%) | 0.53% |
| Left trisegment | 661 (57%) | 493 (43%) | 0.76% |
Operative Procedures
An 8-cm upper midline incision was made, followed by a 5-mm umbilical incision for laparoscopy. A Gelport (Applied Medical, Rancho Santa Margarita, CA) was placed at the 8-cm incision, and a 5-mm port was placed in the right lateral upper abdomen under the pneumoperitoneum. The right liver was mobilized under hand-assisted laparoscopic surgery to enable liver parenchymal transection under the midline incision. The left triangular ligament and the coronary ligament were transected. The 8-cm midline incision was extended to 14 cm, and a wound protector was applied. The common trunk of the left and middle hepatic veins was encircled with a Penrose drain. The gallbladder was removed, and the hepatic arteries were identified and isolated. The common trunk of the left and right anterior branches of the portal vein (PV) was isolated. The artery and the PV were temporarily clamped to visualize the demarcation line between the posterior segment and the anterior segment. An intraoperative cholangiogram with marking clips was performed to confirm the anatomy of the bile ducts (Fig. 2B), followed by liver parenchymal transection with a water jet toward the root of the anterior duct. The anterior and left hepatic ducts were encircled together, and the caudal end of the Penrose drain was repositioned to the cranial side of the ducts to facilitate the remaining parenchymal dissection under the liver-hanging maneuver. After parenchymal transection was completed, the bile duct was transected following intraoperative cholangiography. As expected, the anterior duct and the left hepatic duct appeared on the stump, with a short distance between the orifices. The artery, PV, and hepatic vein were transected, and the graft was harvested. The operative time was 417 minutes, and the blood loss was 970 g. The postoperative course of the donor was uneventful, and she was discharged on postoperative day (POD) 13. The serum T-Bil level and PT–international normalized ratio (INR) peaked at 4.0 mg/dL and 1.46, respectively, on POD 1 and decreased thereafter.
In the recipient operation, vascular anastomoses were performed in a routine fashion. A duct-to-duct biliary reconstruction was feasible because of the short distance between the orifices in the graft. The recipient suffered from clinically suspected acute cellular rejection that responded to methylprednisolone and mild hepatic vein stenosis that was amenable to balloon dilation therapy (Fig. 2C). The serum T-Bil level and PT-INR peaked at 6.8 mg/dL and 2.58, respectively, on POD 1 and decreased thereafter. He was discharged on POD 60 in good health.
Discussion
In this report, we present a case of LDLT using a left trisegmental graft from a donor with a significant PV anomaly, in which the right anterior branch originated from the UP of the left portal vein (LPV). This anatomical variation is topologically similar to a relatively frequent variation, with the right posterior PV arising as the first branch of the PV. However, one must distinguish between these variants with caution when planning a donor operation. In the latter, the right lobe graft is applicable because the anterior and posterior branches of the PV can be transected with a sufficient margin, thus enabling the unification of the double orifices with a Y-graft interposition during a back-table procedure. In contrast, in the present case, the anterior PV diverged from the UP of the LPV, which made it difficult to ensure adequate margins for anastomosis without disturbing portal flow to the remnant. Cheng et al. classified PV branching patterns into 4 categories.3 The right posterior PV arising as the first branch of the PV corresponds to type III, which are amenable to venoplasty using a Y-graft, whereas the RAPV arising from the UP of the LPV (the present case) applies to type IV, which is considered a contraindication to the right lobe graft.2 Sureka et al. examined PV anatomy in 967 patients based on CT scans and demonstrated that type III anomaly represented 48 patients (4.96%) and type IV represented only 2 patients (0.2%).4
In addition to the anatomy of the PV, anatomical variations of the artery and the bile duct must be thoroughly evaluated to ensure feasible reconstructions. In the present case, there was an anatomical variation of the artery as well, which was unfavorable for a right lobe graft but favorable for a left trisegmental graft. The right anterior artery arose from the left hepatic artery, whereas the right posterior artery arose independently from the gastroduodenal artery, which allowed for a simple arterial reconstruction in the recipient with a single orifice while preserving the arterial supply for the donor remnant. Type IV portal anomalies are so infrequent that it is unknown whether a type IV anomaly frequently accompanies this type of arterial anomaly or if it was just a coincidence in this particular case. A 3-dimensional (3D) fusion image of the artery in conjunction with the PV helped us understand this rare anatomical variation with confidence.
Another important issue for this procedure is the volume of the donor remnant. Leelaudomlipi et al. performed a volumetric analysis of 155 living donors and reported that the ratio of the right posterior segment (RPS) to the whole liver averaged 30%.5 Because the criteria for the minimum liver remnant for the donor ranges from 30% to 35% in most institutions, more than half of the donor candidates are expected to not meet the criteria. However, type III portal anomalies appear to be associated with a larger posterior segment volume than usual (35% on average in our unpublished data). Although there are no data on the posterior segment volume in type IV variation because of its extreme rarity, if the same applies to type IV, the donor candidates with type IV variation may be favorable to a trisegmental graft from the volumetric point of view. Indeed, the liver remnant of the present case was 43%, and no sign of posthepatectomy liver failure was encountered.
This unusual operative procedure would not have been realized without several chance events: anatomical variations of both the PV and artery that were convenient for the left trisegmental graft and a favorable proportion of the volume between the graft and the remnant. We acknowledge that this procedure is indicated only for limited cases. Nevertheless, we should keep in mind that the left trisegmental graft may be a valid option for donors with a type IV portal variant because a recipient usually has only a few living donor candidates and because overlooking this possibility can result in missing the only chance for a cure for the recipient. We would like to emphasize that a thorough examination of the donor's anatomy is important, in order to safely perform LDLT as well as to avoid missing valuable chances for recipients, who may have no other treatment options.
-
Kojiro Taura, M.D., Ph.D.
-
Toshimi Kaido, M.D., Ph.D.
-
Takayuki Anazawa, M.D., Ph.D.
-
Shintaro Yagi, M.D., Ph.D.
-
Hideaki Okajima, M.D., Ph.D.
-
Shinji Uemoto, M.D., Ph.D.
-
Department of Surgery
-
Graduate School of Medicine
-
Kyoto University
-
Kyoto, Japan