A Comparison between splenic fossa and subhepatic fossa auxiliary partial heterotopic liver transplantation in a porcine model
This work was supported by Zhejiang Provincial Medical Science Research Foundation (No. 2009A179) and Hangzhou Civic Science and Technology Planning Project (No. 20090833B41).
Potential conflict of interest: Nothing to report.
Abstract
To test the alternative possible locations for the placement of a liver graft and the relevant surgical technique issues, we developed a porcine model of auxiliary partial heterotopic liver transplantation (APHLT) and evaluated the difference between 2 styles of liver transplantation, either subhepatic fossa or splenic fossa APHLT, by comparing survival and biochemical indexes. Thirty-eight miniature pigs were randomly divided into 2 groups. A left hemihepatic graft without the middle hepatic vein (HV) was procured from the living donor. In group A (n = 9), an 8 mm diameter polytetrafluoroethylene (PTFE) graft approximately 2.5 cm long was connected to the left HV while another PTFE graft of the same size was connected to the left portal vein (PV). The liver graft was implanted in the right subhepatic fossa following splenectomy and right nephrectomy. In group B (n = 10), a PTFE graft of the same size was connected to the left HV while the liver graft was implanted in the splenic fossa following splenectomy and left nephrectomy. Survival rate and complications were observed at 2 weeks after transplantation. Data were collected from 5 animals in group A and 6 animals in group B that survived longer than 2 weeks. The liver function and renal function of the recipients returned to normal at 1 week after surgery in both groups. Eighty-eight percent (14/16) of the PTFE grafts remained patent at 2 weeks after surgery, but 44% of the PTFE grafts (7/16) developed mural thrombus. No significant differences in the survival rate and biochemistry were found between the 2 groups. In conclusion, the splenic fossa APHLT can achieve beneficial outcomes similar to the subhepatic fossa APHLT in miniature pigs, although it also has a high morbidity rate due to hepatic artery thrombosis, PV thrombosis, and PTEF graft mural thrombus formation. Liver Transplantation 22 812–821 2016 AASLD.
Abbreviations
-
- ALF
-
- acute liver failure
-
- ALT
-
- auxiliary liver transplantation
-
- APHLT
-
- auxiliary partial heterotopic liver transplantation
-
- APOLT
-
- auxiliary partial orthotopic liver transplantation
-
- AST
-
- aspartate aminotransferase
-
- BDD
-
- bile duct drainage
-
- BUN
-
- blood urea nitrogen
-
- Cr
-
- creatinine
-
- GL
-
- graft liver
-
- H & E
-
- hematoxylin-eosin
-
- HA
-
- hepatic artery
-
- HCA
-
- hypertonic citrate adenine solution
-
- HV
-
- hepatic vein
-
- IVC
-
- inferior vena cava
-
- LDLT
-
- living donor liver transplantation
-
- LHA
-
- left hepatic artery
-
- NL
-
- native liver
-
- OLT
-
- orthotopic liver transplantation
-
- POD
-
- postoperative day
-
- PR
-
- patency rate
-
- PTFE
-
- polytetrafluoroethylene
-
- PV
-
- portal vein
-
- SA
-
- splenic artery
-
- SD
-
- standard deviation
-
- SEM
-
- standard error of the mean
-
- SV
-
- splenic vein
-
- TBil
-
- total bilirubin
Auxiliary liver transplantation (ALT) is a well-established method developed in the early days of liver transplantation to treat acute liver failure (ALF) when regeneration of the native liver (NL) is possible or to reduce the risk of metabolic disorders; it is also used for small-for-size liver transplantation.1-3 This type of transplantation can be performed using either an orthotopic or heterotopic procedure. Goodrich et al.4 were the first to describe the successful use of a heterotopically placed auxiliary graft in a canine experiment. Auxiliary partial orthotopic liver transplantation (APOLT) was first developed in 1985 by Bismuth and Houssin.5 Although APOLT has the advantage over auxiliary partial heterotopic liver transplantation (APHLT) in lower outflow pressures,6 many disadvantages have limited the wide application of this procedure in clinics, including it being time-consuming, technically complex, and having a higher risk of primary nonfunction and vascular complication, as well as biliary complication.7 Also, APOLT requires a liver resection from a critically ill patient with severe coagulopathy, which may put the patient in danger of postoperative bleeding and the restricted mass of transplantable tissue. Moreover, native hepatectomy may be extremely challenging and may cause life-threatening complications in some patients, especially for patients who have previously undergone abdominal surgery and peritonitis.
- To analyze the feasibility and practicality of a splenic fossa APHLT model using the left hemihepatic graft without the middle hepatic vein (HV) from a living donor in a miniature pig.
- To undertake a comparative study of splenic fossa and subhepatic fossa APHLT.
- To assess the utility of artificial interposition graft in an APHLT model.
Materials and Methods
Animals
All animal experiments were performed according to the guidelines set by the US National Institutes of Health (1985). The Animal Care and Use Committee of Zhejiang Chinese Medical University approved this experimental protocol. Eighteen female Guangxi Bama miniature pigs weighing between 16 and 22 kg were used in the subhepatic fossa APHLT model (group A). Twenty female Guangxi Bama miniature pigs weighing between 14 and 22 kg were used in the splenic fossa APHLT model (group B). An additional animal was used as a whole blood donor for each donor and recipient pair. Animals were followed for 2 weeks, at which time they were killed.
Animals were fed with a standard diet and fasted for 24 hours before surgery. Ketamine hydrochloride (20 mg/kg) and atropine sulfate (0.1 mg/kg) were administered intramuscularly, followed by endotracheal intubation and administration of an anesthetic gas composed of isoflurane (1%-1.5%), oxygen (2 L/minute), and room air (2 L/minute) under mechanical ventilation. Anesthesia was supplemented by the addition of pentobarbital sodium (30 mg/kg). Catheters were introduced through an ear lateral marginal vein for intravenous infusions, drug administration, and blood sampling. Ringer's solution was given for hemodynamic support as needed. Depending on the amount of blood loss, 1-3 IU of whole blood (200 mL each) was given in the operation.
Donor Operation
After laparotomy, the donor liver was skeletonized from all surrounding ligaments. After hilar dissection at the base of the hepatoduodenal ligament, the left hepatic artery (LHA) and the left PV branch supplying the left lateral and left medial lobes were identified and isolated. The left bile duct branch was transected, and a polyethylene tube was inserted for external bile drainage (Fig. 1A). The LHA and the left PV were temporarily occluded with vascular clamps in order to reveal the demarcation line between the left and right hemiliver. The demarcation line is an improved Taira line,12 deviated to the left of the middle HV. Parenchymal transaction was initiated with Peng's multifunctional operative dissector and a harmonic scalpel (Generator 300, Ethicon Endo-Surgery LLC, Johnson-Johnson Co., New Brunswick, NJ) by scoring the liver surface just to the left of the gallbladder fossa at the demarcation line (Fig. 1B and Fig3A). The small penetrating vessels and biliary radicles were sutured and ligated as required. The left HV was encircled at the junction to the inferior vena cava (IVC). During these procedures the vessels remained unclamped. After an intravenous injection of 2 mg/kg of heparin sodium, the left hemihepatic graft without the middle HV was removed and preserved in cold hypertonic citrate adenine solution (HCA), which is an improved Ross solution. Intravenous injection of 1 mg/kg of protamine sulfate was used to reverse the previous heparinization. After thorough hemostasis, abdominal closure was performed.
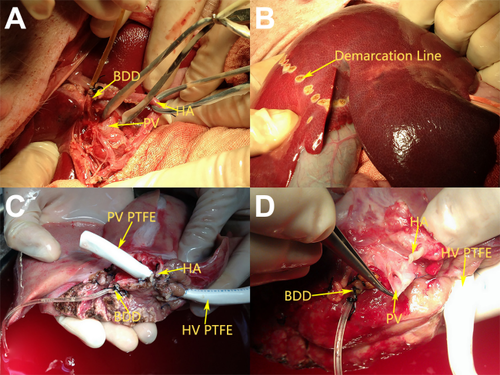
(A) Donor procedure: Isolation of the left hepatic duct, left PV, and LHA. (B) Donor procedure: The demarcation line between the left and right hemiliver. (C) Back-table procedure: Preparation of the liver graft in group A. (D) Back-table procedure: Preparation of the liver graft in group B.
Back-table Operation
The harvested liver was immersed in a sterile bag containing 4°C HCA solution. At the back table, 1 L of 4°C HCA solution was flushed via the left PV. The biliary system was washed with 4°C HCA solution. The liver graft comprised approximately 40% of the whole liver weight.
In group A, an 8-mm, internal diameter, thin-walled polytetrafluoroethylene (PTFE) graft (GORE-TEX, W.L. Gore & Associates, Inc., Newark, DE) approximately 2.5 cm long was connected to the left HV. Then, another 8-mm diameter PTFE graft approximately 2.5 cm long was connected to the left PV (Fig. 1C). In group B, an 8-mm diameter PTFE graft approximately 2.5 cm long was connected to the left HV (Fig. 1D).
Recipient Operation
Two consecutive series of transplantations were performed. In group A (n = 9), animals received an auxiliary partial liver transplant in the subhepatic fossa. All animals in group B (n = 10) received a splenic fossa heterotopically placed partial liver graft.
Group A
The exposure was provided through a long median incision. A segment of the PV was isolated and quickly cleared. After the splenectomy and right nephrectomy were performed, the splenic artery (SA) was divided as far as possible and led through the mesenteric root into the right subhepatic space. The right renal vein was divided as far distally as possible. The liver graft was implanted in the right subhepatic space. The left HV of the liver graft was anastomosed end-to-end to the right renal vein of the recipient (via the PTFE graft); the left PV of the liver graft was anastomosed end-to-side to the PV of the recipient (via the PTFE graft); and the LHA of the liver graft was anastomosed end-to-end to the SA of the recipient (Fig.2A and Fig.3B). At this moment, care was taken to ensure that neither vein was twisted. The polyethylene tube inserted into the left bile duct of the graft was led through the abdominal wall as an external biliary drainage. On the distal site of the PV anastomosis, the diameter of the recipient PV was banded to half of its original size. After thorough hemostasis, abdominal closure was performed.
Group B
The abdomen of the recipient was opened through a midline incision. After splenectomy and left nephrectomy, a segment of the splenic vein (SV) as well as the adjacent SA were quickly cleared and tailored. The left renal vein was divided as far distally as possible. The liver graft was placed in the splenic fossa. The left PV of the liver graft was anastomosed end-to-end to the SV of the recipient. The anastomosis of the left HV, LHA, and biliary drainage were performed in the same fashion as in group A (Fig.2C and Fig.3C). Great care was taken to ensure that neither vein was twisted. The diameter of the recipient PV was banded to half of its original size. After thorough hemostasis, abdominal closure was performed.
Postoperative Care
Intravenous therapy consisted of 1500 mL of 5% dextrose and saline containing 4 million units of penicillin G and 100 mg of ranitidine daily until postoperative day (POD) 3. No immunosuppressant was administered. The animals were fed with a standard diet from POD 1. The recipients were given 500 mL of dextran-40 during the operation and daily until POD 3. Then warfarin (1.25 mg) was mixed with oral feeding daily thereafter.
Animal Survival
Surviving pigs were killed on POD 15, and all pigs that died before POD 15 were further studied for the cause of death. At death or autopsy, both the NL and the graft were carefully dissected, and all vessels and anastomoses were examined for patency.
Biochemistry
Blood samples were taken before the operation and at 1, 3, 7, and 14 days after operation. Serum aspartate aminotransferase (AST), total bilirubin (TBil), blood urea nitrogen (BUN), and creatinine (Cr) levels were determined with an autoanalyzer (type 7020, Hitachi Co., Ltd., Tokyo, Japan).
Histological Examination
All animals were autopsied, and hepatic specimens for light microscopy were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin-eosin (H & E) for histological examination.
Statistical Analysis
All values were expressed as the mean ± standard deviation (SD). Statistical analysis of the values in both groups was made by 1-way analysis of variance and Student t test. Survival curves were constructed with the Kaplan-Meier method. The log-rank test for trends was used when survival curves were compared across groups. P value < 0.05 was considered statistically significant. All statistical procedures were performed on a commercially available computer program (SPSS for Windows 23.0, SPSS Inc., Chicago, IL).
Results
Survival and Clinical Course
Table 1 depicts the average donor, recipient, and transplant characteristics. The miniature pigs were usually awake in 3 or 4 hours following the operation and began food intake the next morning. Kaplan-Meier survival curves for group A and group B can be seen in Fig.4.
| Group A (n = 9) | Group B (n = 10) | |
|---|---|---|
| Donor body weight, kg | 17.1 ± 1.1 | 17.2 ± 1.9 |
| Recipient body weight, kg | 18.6 ± 1.9 | 19.7 ± 1.5 |
| Donor operative time, minutes | 156.7 ± 17.3 | 160 ± 17 |
| Donor blood transfusion, mL | 355.6 ± 194.3 | 380 ± 198.9 |
| Recipient operative time, minutes | 165.6 ± 11.3 | 161 ± 9.9 |
| Recipient blood transfusion, mL | 488.9 ± 176.3 | 520 ± 193.2 |
| Hot ischemia time, minutes | 6.2 ± 1.0 | 6.5 ± 1.1 |
| Cold ischemia time, minutes | 184.4 ± 15 | 178 ± 9.2 |
| Donor postoperative survival time, days | 11.2 | 10.6 |
| Recipient postoperative survival time, days | 8.5 | 9.0 |
- NOTE: Values are presented as mean ± SEM. No significant difference was found in any parameter between group A and group B.
In group A, 7 of the 9 donors survived more than 14 days. The remaining 2 donors died from intraperitoneal hemorrhage within 24-48 hours postoperatively. Four of the 9 recipients survived less than 14 days. Table 2 depicts the survival times and causes of death in the group A recipients. In 5 recipients that survived for 2 weeks, neither the size of the liver graft nor the size of the host liver had changed at autopsy. The liver grafts of 2 recipients were heavily mottled with patches of necrosis and abscess formation. Hepatic artery thrombosis was present in those 2 animals. The remaining 3 liver grafts appeared to be normal macroscopically; all vascular channels were found to be patent and free of thrombosis at autopsy.
| Pig Number | Group A (n = 9) | Group B (n = 10) | ||||
|---|---|---|---|---|---|---|
| Survival (days) | Cause of Death | Patency in Anastomosis | Survival (days) | Cause of Death | Patency in Anastomosis | |
| 1 | 1 | Abdominal hemorrhage | * | 1 | Abdominal hemorrhage | * |
| 2 | 14 | Killed | + | 14 | Killed | — |
| 3 | 14 | Killed | + | 14 | Killed | + |
| 4 | 14 | Killed | — | 3 | Liver graft twisted | * |
| 5 | 14 | Killed | — | 1 | Abdominal hemorrhage | * |
| 6 | 3 | Liver graft twisted | * | 14 | Killed | + |
| 7 | 14 | Killed | + | 14 | Killed | — |
| 8 | 1 | Abdominal hemorrhage | * | 14 | Killed | — |
| 9 | 2 | Liver graft twisted | * | 1 | Abdominal hemorrhage | * |
| 10 | 14 | Killed | + | |||
- NOTE: *Uncounted. + patency in anastomosis. —with vascular thromboses.
In group B, 7 of the 10 donors survived more than 2 weeks. One donor died on POD 5 of an abdominal infection. The remaining 2 donors died from intraperitoneal hemorrhage on PODs 1 and 2. Table 2 depicts the survival times and causes of death in the group B recipients. In 6 recipients that survived for 2 weeks, neither the size of the liver graft nor the size of the host liver had changed at autopsy. The liver grafts of 3 recipients were heavily mottled with patches of necrosis and abscess formation. Thrombosis of vascular anastomoses occurred in those 3 animals, thrice in the hepatic artery (HA) and once in the PV. The remaining 3 liver grafts appeared to be normal macroscopically; all vascular channels were found to be patent and free of thrombosis at autopsy.
Patency Rate (PR) of the PTFE Graft
Overall, the 2-week PR of the PTFE graft was 88% (14/16), but the 2-week mural thrombus formation rate of the PTFE graft was as high as 44% (7/16). In group A, 2 liver grafts were dysfunctional. Anastomotic and luminal stenoses were found in 2 PV PTFE grafts and 1 HV PTFE graft; more than half of each PTFE graft lumen was filled with thrombus. Another HV PTFE graft was thrombotic. In group B, 3 liver grafts were dysfunctional. Anastomotic and luminal stenoses were found in 2 HV PTFE grafts, and more than half of each PTFE graft lumen was filled with thrombus. Another HV PTFE graft was thrombotic.
Biochemistry
Five animals in group A and 6 animals in group B survived longer than 14 days after transplantation; the data of these animals were used for analysis. The preoperative and serial postoperative measurements of serum TBil, serum AST, BUN, and Cr are shown in Table 3.
| Parameter | Group | Preoperative Level | POD 1 | POD 3 | POD 7 | POD 14 |
|---|---|---|---|---|---|---|
| Mean TBil (µmol/L) | A (n = 5) | 1.3 ± 0.6 | 4.0 ± 2.8 | 4.6 ± 4.0 | 3.3 ± 2.0 | 1.7 ± 0.6 |
| 1.5 (n = 3), | 3.2 (n = 3)a | 3.6 (n = 3)a | 4.3 (n = 3)a | 1.8 (n = 3)a | ||
| 1.1 (n = 2)b | 5.3 (n = 2)b | 6.2 (n = 2)b | 1.6 (n = 2)b | 1.6 (n = 2)b | ||
| B (n = 6) | 1.4 ± 0.6 | 3.6 ± 1.6c | 3.6 ± 2.1c | 1.9 ± 1.8 | 1.7 ± 0.9 | |
| 1.4 (n = 3)a | 3.1 (n = 3)a | 2.4 (n = 3)a | 2.5 (n = 3)a | 2.3 (n = 3)a | ||
| 1.3 (n = 3)b | 4.0 (n = 3)b | 4.7 (n = 3)b | 1.3 (n = 3)b | 1.1 (n = 3)b | ||
| Mean serum AST (µ/L) | A (n = 5) | 28.4 ± 6.9 | 628.2 ± 170.0d | 275.0 ± 212.9 | 84.5 ± 73.0 | 65.9 ± 23.1c |
| 25.7 (n = 3)a | 645.5 (n = 3)a | 328.2 (n = 3)a | 111.2 (n = 3)a | 72.3 (n = 3)a | ||
| 32.5 (n = 2)b | 602.2 (n = 2)b | 194.4 (n = 2)b | 44.6 (n = 2)b | 54.7 (n = 2)b | ||
| B (n = 6) | 26.5 ± 7.4 | 667.5 ± 106.6d | 448.3 ± 290.5c | 102.9 ± 84.5 | 72.6 ± 24.4c | |
| 28.3 (n = 3)a | 628.0 (n = 3)a | 447.4 (n = 3)a | 143.7 (n = 3)a | 86.0 (n = 3)a | ||
| 24.7 (n = 3)b | 707.0 (n = 3)b | 449.2 (n = 3)b | 62.1 (n = 3)b | 59.2 (n = 3)b | ||
| Mean BUN (mmol/L) | A (n = 5) | 1.92 ± 1.0 | 6.4 ± 1.2c | 4.2 ± 0.7c | 3.3 ± 1.0 | 2.9 ± 0.4 |
| 2.1 (n = 3)a | 6.3 (n = 3)a | 4.0 (n = 3)a | 3.1 (n = 3)a | 2.7 (n = 3)a | ||
| 1.7 (n = 2)b | 6.7 (n = 2)b | 4.5 (n = 2)b | 3.6 (n = 2)b | 3.1 (n = 2)b | ||
| B (n = 6) | 1.8 ± 0.9 | 5.6 ± 1.5c | 5.2 ± 2.0c | 3.0 ± 1.5 | 2.8 ± 0.8 | |
| 1.5 (n = 3)a | 6.7 (n = 3)a | 5.2 (n = 3)a | 2.3 (n = 3)a | 3.2 (n = 3)a | ||
| 2.2 (n = 3)b | 4.5 (n = 3)b | 5.2 (n = 3)b | 3.6 (n = 3)b | 2.4 (n = 3)b | ||
| Mean Cr (µmol/L) | A (n = 5) | 71.2 ± 16.3 | 138.7 ± 19.5d | 102.6 ± 18.0d | 82.5 ± 8.5 | 78.6 ± 13.8 |
| 67 (n = 3)a | 128.0 (n = 3)a | 100.0 (n = 3)a | 81.5 (n = 3)a | 79.7 (n = 3)a | ||
| 77.5 (n = 2)b | 154.7 (n = 2)b | 107.0 (n = 2)b | 84.2 (n = 2)b | 77.0 (n = 2)b | ||
| B (n = 6) | 68.2 ± 16.3 | 144.015 ± 34.822c | 109.5 ± 24.428c | 86.583 ± 9.772 | 90.23 ± 18.131 | |
| 68.0 (n = 3)a | 125.9 (n = 3)a | 108.4 (n = 3)a | 84.4 (n = 3)a | 93.5 (n = 3)a | ||
| 68.3 (n = 3)b | 162.1 (n = 3)b | 110.6 (n = 3)b | 88.8 (n = 3)b | 86.9 (n = 3)b |
- Note: Postoperative evolution of TBil, AST, BUN, and Cr. No significant alteration between group A and group B at all time points.
- a Anastomosis patent.
- b Anastomosis clotted.
- c Compared with the preoperative value, P < 0.05.
- d Compared with the preoperative value, P < 0.01.
Quantification of hepatocellular injury using the serum level of TBil and AST did not demonstrate any differences between group A and group B at any time point. The TBil serum level peaked at POD 3, gradually declined afterward, and nearly normalized by POD 14. Compared with the preoperative TBil (1.3 ± 0.6 μmol/L), TBil on POD 1 (4.0 ± 2.8 μmol/L), TBil on POD 3 (4.6 ± 4.0 µmol/L), TBil on POD 7 (3.3 ± 2.0 μmol/L), and TBil on POD 14 (1.7 ± 0.6 μmol/L) did not demonstrate any statistically significant differences in group A. However, in group B, TBil on POD 1 (3.6 ± 1.6 µmol/L) and TBil on POD 3 (3.6 ± 2.1 µmol/L) were significantly higher, which compared to preoperative TBil (1.4 ± 0.6 µmol/L). The AST serum level sharply peaked at POD 1, gradually declined afterward, and nearly normalized by POD 7. Compared with preoperative serum AST, AST on POD 1 and AST on POD 14 were significantly higher in both groups.
Serum level of BUN and Cr peaked at POD 1, gradually declined afterward, and nearly normalized by POD 7. Compared with preoperative BUN, BUN on POD 1 and BUN on POD 3 were significantly higher in both groups. Cr serum level evolution exhibited a similar pattern. Still, at each time point, there was no statistically significant difference between the 2 groups in terms of BUN and Cr serum level.
Pathology
The autopsy demonstrated 10 normal NLs, with mild sinusoidal congestion and slight edema of the liver cells (Fig. 5A,C,G). Only 1 shows a dilated hepatic central vein with the normal liver architecture (Fig. 5E).
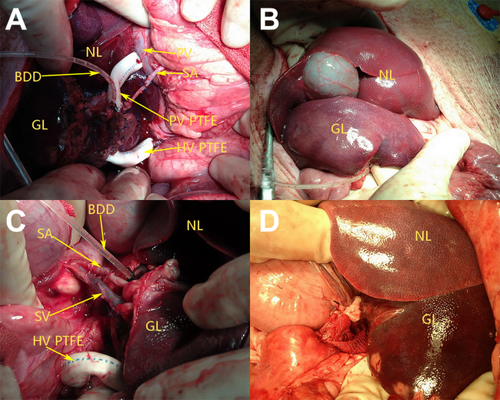
(A) Intraoperative photograph taken after the completion of vascular reconstruction in group A. (B) After implantation in group A, we observed the liver graft below the NL. (C) Intraoperative photograph taken after the completion of vascular reconstruction in group B. (D) After implantation in group B, we observed the liver graft below the NL.
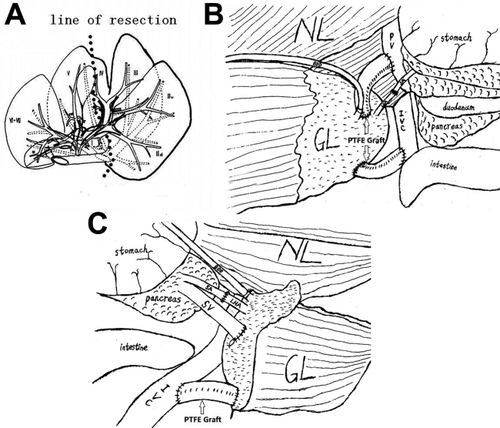
(A) Schematic representation of the line of resection in a donor pig's liver. (B) Schematic representation of the anastomosis after reconstruction in group A. (C) Schematic drawing of the anastomosis after reconstruction in group B.
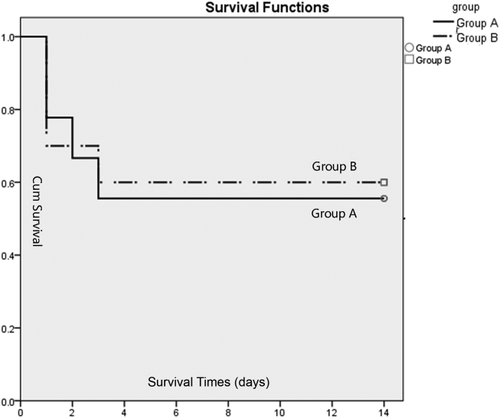
Kaplan-Meier survival curves. There was no difference in survival between group A and group B (log-rank test, P = 0.87).
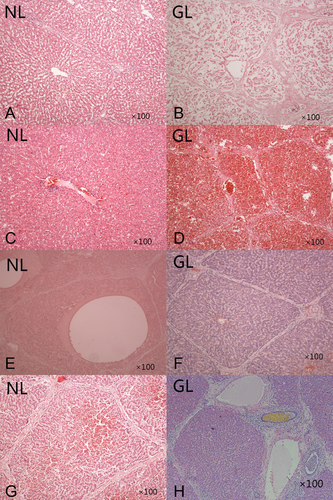
Histology results by H & E staining. (A and B) A section of a liver transplant with vascular thrombosis at POD 15 autopsy in group B. (A) NL shows mild sinusoidal congestion and morphological changes with normal lobular architecture integrity. (B) GL shows necrotic coagulation of liver cells. (C and D) A section of a liver transplant with patency of all vascular anastomoses at POD 15 from an autopsy in group B. (C) NL shows slight edema of liver cells with normal liver architecture. (D) GL shows severe hemorrhage necrosis of liver cells. (E and F) A section of a liver transplant with patency of all vascular anastomoses at POD 15 autopsy in group A. (E) NL shows a dilated hepatic central vein with normal liver architecture. (F) GL shows a normal architecture without rejection or inflammatory changes. (G and H) A section of a liver transplant with patency of all vascular anastomoses at POD 15 from an autopsy in group B. (G) NL shows mild degenerated liver cells with sinusoidal congestion. (H) GL shows mild hepatitis with portal inflammation.
The autopsy demonstrated that the 5 deceased liver grafts had thrombosis of the vascular anastomoses, and all patients showed necrotic coagulation of the liver cells (Fig. 5B). The autopsy demonstrated that the 6 vital liver grafts had patency of all vascular anastomoses and that all but 1 patient in group B showed severe hemorrhage necrosis of the liver cells (Fig. 5D). None showed evidence of changes due to rejection. Only slight amounts of lymphocyte, plasma cell, and other mononuclear cell infiltration were present in the portal tracts and interlobular septa (Fig. 5H).
Discussion
The optimal surgical indication of APHLT is still a controversial issue. Theoretically, APHLT is an attractive alternative for OLT in patients with certain acute and chronic liver diseases in which a complete or partial resection of the liver is unnecessary or even contraindicated. Because of a poor longterm survival record, this procedure is only applied for carefully selected patients.10 It is particularly applicable for patients who are contraindicated for removing the NL. Another promising indication of APHLT is ALF. At present, donor shortage is a major issue in liver transplantation. If the NL regenerates, then the liver graft can be reused rather than removed or left to atrophy. When a permanent liver graft is required due to liver regeneration failure, autotransplantation of the heterotopic graft to the orthotopic position could be an attractive choice.
Since the successful partial heterotopic liver transplantations in canine and swine by Terpstra et al.,13 many research groups have published their articles dealing with methodology.14-16 They basically follow the modified techniques developed by Terpstra et al.,13 which involve a reduced-size liver being placed in the right subhepatic space adjacent to the diaphragm, with both arterial and portal inflow and venous drainage through the suprahepatic vena cava of the graft into the recipient's infrahepatic vena cava. Because of a progressively increasing gap between the organ demand and donation, reusing allografts from recipients comes up as a possible strategy for expanding the donor pool.17 Ringers et al.11 tested a new method of reusing auxiliary liver grafts in a secondary recipient, but their heterotopic auxiliary left liver with renal vein–PV anastomosis was deprived of splanchnic blood. Because the hepatotrophic factor is the prime factor for preserving hepatic integrity,18 the procedure from Ringers et al.11 may put the liver graft in danger of progressive atrophy. Another important factor for a promising surgical procedure is that the remaining native hepatic vessel is structurally intact. From this perspective, the splenic fossa may be an ideal alternative location for heterotopically implanting the liver graft.
In 1968, Calne and Williams19 accomplished the first splenic fossa auxiliary heterotopic whole liver transplantation in a human, but the recipient died of bleeding soon after the operation. In swine, Kesen et al.20 reported an animal model of splenic fossa auxiliary heterotopic whole liver transplantation, but the survival rate of their model was disappointingly low. A higher risk of venous outflow obstruction and multiple anastomoses being kinked or compromised are believed to be the main drawbacks of this procedure. The first successful splenic fossa ALT was performed by Fourtanier et al.21 in a patient with a PV thrombosis. Recently, Dou et al.22 reported that a patient with Wilson's disease has been symptom-free during a 5-year follow-up after receiving an auxiliary partial liver graft in splenic fossa. However, limited knowledge is available regarding the pathophysiology of splenic fossa APHLT due to the small number of patients and the short follow-up periods. Therefore, an examination of splenic fossa APHLT with an animal model will help to better understand the pathophysiology and to lay a solid scientific foundation for clinical practice.
Our experience shows that more time is required for liver reperfusion in the splenic fossa group than the subhepatic fossa group, but no significant differences were found in the survival and biochemistry change between the 2 groups. The possible explanation is that both groups used a similar method to reconstruct the HA and hepatic venous outflow with minor differences in the PV. Splenic fossa APHLT has been favored over subhepatic fossa APHLT because the splenic fossa is anatomically more convenient for a partial liver graft after excising the native spleen. This procedure solves the issue of finding space for the graft and makes the blood vessel anastomosis easier. In addition, this procedure preserves the native hepatic vessel architecture well, which makes it more easy to perform a possible future OLT. Because of the limited availability of splenic vessels under normal conditions in our procedure, we trimmed the main bifurcate of SV and SA to obtain a larger orifice, and we performed blood vessel anastomosis with loupe magnification. Many technical issues of the splenic fossa APHLT still need to be well addressed in the future to prevent vascular complications, such as the anastomosis at an obtuse angle between the spleen vein–PV and the long distance of venous return. In order to facilitate vessel reconstruction, we strongly suggest using a pig weighing more than 40 kg in future animal studies.
Functional competition for portal blood flow between the graft and NL is an inherent issue in ALT. Many procedures have been tested to solve this problem, including ligation of the PV of the NL in APOLT,3 PV arterialization, or renal vein–PV anastomosis in APHLT with an untouched hepatic ligament.23, 24 In an animal study on correcting inborn errors of metabolism, the best results were obtained with constriction or split-flow of the recipient's own PV.25 In our study, we prevented graft atrophy and alleviated the NL-relevant detrimental effects related to sufficient portal blood inflow by banding the native PV to 50% of its original diameter following the methods previously described.26, 27 The graded hemiportal banding technique from Rela et al.28 was also considered in this study by using a small-for-size liver graft in APHLT to bypass the space constraints of the abdominal cavity and alleviate the aforementioned functional competition issue.
Another concern of APHLT was elevated venous back pressure. However, Chan et al.29 recently reported using a left liver graft heterotopically implanted on the right side in living donor liver transplantation (LDLT) with a good result. And Haberal et al.10 reported longterm survival after heterotopic segmental ALT in a 17-year-old female; the HV was anastomosed to infrarenal IVC. This means caval anastomosis placement as adjacent as possible to the right atrium was not always the determining factor of longterm survival. In the swine, however, a large number of short hepatic miniveins penetrate into the retrohepatic IVC, making it very difficult to separate a section of IVC adjacent to the right atrium while preserving the retrohepatic IVC. Therefore, we applied the subhepatic IVC in our procedure by connecting the unilateral renal vein with the interposition graft as an efferent vessel to keep a free and unimpeded outflow from the liver graft. Our study showed that the recipient's renal function returned to normal after about a week and that the renal vein could be ligated while preserving the kidney in clinical practice.
A LDLT procedure usually requires additional vein and artery grafts, but either cryopreserved or autologous grafts are hardly available.30, 31 The indications for prosthetic vessel grafts for the splanchnic venous system are particularly strict because the slow blood flow is highly thrombogenic. The rationale for PTFE grafts used in APHLT for ALF is as follows: if it can maintain patency for at least 6-12 months until the NL regenerates, then the liver graft can be reused rather than be removed or left to atrophy as before.
Although a beneficial effect has been reported in the reconstruction of the middle HV with PTFE grafts under certain circumstances,31, 32 the effects of thin-walled PTFE grafts on PR in LDLT are inconsistent.32 We adapted a similar procedure in this study by using the thin-walled PTFE grafts, but the outcome is not good, as shown by the 2-week PR (88%) and the 2-week mural thrombus formation rate (44%). The possible explanation is that the thin-walled PTFE graft is vulnerable to extrinsic compression, is easily bent and collapsed, and thus leads to luminal thrombus formation. Meanwhile, Hwang et al.31 reported that the 6-month PRs of ringed PTFE grafts combined with small vessel patches used in LDLT were 76.6%.
In conclusion, we compared the splenic fossa APHLT and the subhepatic fossa APHLT in a controlled animal study. We found that the splenic fossa APHLT has a similar survival rate and biochemical change to that of the subhepatic fossa APHLT but that it is better at maintaining the integrity of the native hepatic vessel structure and is easier to perform. Thus, the surgical knowledge from this experimental study enables us to initiate another study in the swine ALF model to evaluate the longterm effects of splenic fossa APHLT with small-for-size liver grafts with a ringed PTFE graft as the interposition graft. Undoubtedly, many issues, such as the HA thrombosis and PV thrombosis, need to be well addressed to obtain a better survival rate and lower morbidity rate in further studies.